Abstract
Background:
Oxaliplatin-associated shock (referred to as shock) is a rare but life-threatening adverse event.
Objectives:
This pioneering cohort study aimed to quantitatively investigate the association between oxaliplatin use and shock in patients with stage III colorectal cancer (CRC), identify potential independent risk factors for shock, and assess the cycle-to-shock during oxaliplatin treatment.
Design:
The study utilized a nested case–control (NCC) design to assess the association between oxaliplatin and shock and employed a case-crossover approach to address unmeasured confounders.
Methods:
All newly diagnosed stage III CRC patients were identified from the CRC Health Database (2012–2016). Conditional logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CIs) for oxaliplatin’s link to shock incidence.
Results:
Among 6932 oxaliplatin recipients, 331 suffered shock. In all, 3309 controls were selected via risk-set sampling for the shock cases. Oxaliplatin use is associated with a doubled risk of shock (adjusted OR: 2.08, 95% CI: 1.23–3.52). Two independent risk factors were male sex (adjusted OR: 1.33, 95% CI: 1.05–1.69) and heart diseases (adjusted OR: 1.65, 95% CI: 1.17–2.32). The case-crossover analysis revealed a more than fourfold risk (OR: 4.4, 95% CI: 1.67–11.62). In total, 22 of 331 shock cases were exposed to oxaliplatin within 2 days of shock onset, with a median cycle-to-shock time at the seventh cycle.
Conclusion:
Oxaliplatin use significantly increased shock risk in stage III CRC patients. Male sex and heart disease are two independent risk factors.
Keywords: adverse event, colorectal cancer, oxaliplatin, pharmacovigilance, shock
Plain language summary
This pioneering study identified potential independent risk factors and the cycle-to-shock of oxaliplatin-associated shock which is a rare but life-threatening adverse event
Why was the study done? Oxaliplatin-induced anaphylactic shock (referred to as shock) is a rare but life-threatening adverse event which is a harmful and undesirable experience associated with medical care in a patient.
What did the researchers do? This pioneering cohort study aimed to quantitatively investigate the association between oxaliplatin use and shock in patients with stage III colorectal cancer (CRC), identify potential independent risk factors for shock and assess the cycle-to-shock during oxaliplatin treatment. All newly diagnosed stage III CRC patients were identified from the CRC Health Database (2012–2016). The study utilized a nested case-control (NCC) design to assess the association between oxaliplatin and shock and employed a case-crossover approach to address unmeasured confounders. Conditional logistic regression was used to quantify the association between oxaliplatin and shock incidence.
What did the researchers find? Among 6,932 oxaliplatin recipients, 331 suffered shock. 3,309 controls were selected via risk-set sampling for the shock cases. Oxaliplatin use is associated with a doubled risk of shock. Independent risk factors were male sex and heart diseases. The risk of shock was 33% higher for males and 65% higher for people with heart diseases compared to females and those without heart diseases. The case-crossover analysis revealed a more than four-fold risk of shock of oxaliplatin. Twenty-two of 331 shock cases were exposed to oxaliplatin within two days before the shock onset. The median cycle-to-shock time is at the seventh cycle.
What do the findings mean? Oxaliplatin use significantly increased shock risk in stage III CRC patients. Male sex and having heart diseases are two independent risk factors.
Introduction
Oxaliplatin, a third-generation platinum compound, is more efficacious and has fewer adverse events than cisplatin and carboplatin, even when combined with other cancer chemotherapeutic agents. Surgical operations are mainly performed in stages I and II of colorectal cancer (CRC) patients. After surgery, adjuvant chemotherapy will be added when the cancer progresses to stage III. Oxaliplatin is widely used to treat stage III and stage IV CRC patients but is not used for stage I to stage II patients following the National Cancer Comprehensive Network (NCCN) guidelines.1,2 However, stage IV patients with multiple metastases and poor physical conditions might administer more chemotherapy agents. Therefore, the current study focused on stage III CRC patients with oxaliplatin treatment.
Oxaliplatin, very short terminal half-life of 14.1 min using an infusion time of 2 h, is rapidly hydrolyzed in vivo to produce platinum, a bioactive derivate forming DNA-platinum adducts, and oxalate, a metabolite.3,4 Accumulating oxalate, a chelator reacting with calcium ions, contributes to cause neurotoxicity. 4
Oxaliplatin-associated common adverse events include alopecia, diarrhea, nausea, vomiting, and late effects, such as multiple neuropathies. 1 Hypersensitivity reactions (HSR) and anaphylaxis may damage the nervous system and occasionally be fatal.2,4 The incidence of HRS due to oxaliplatin is increasing and ranges from 8% to 20%. 2
Oxaliplatin-associated anaphylactic shock is a rare but life-threatening adverse event. According to previous clinical trials, oxaliplatin-associated anaphylactic shock incidence rate is less than 1%. 2 Descriptive statistics have recently published several case reports and case series regarding adverse events in grades 1–3 adverse events (Common Terminology Criteria for Adverse Events, CTCAE, grades). However, the shock events (CTCAE grade 4) increase in real-world clinical circumstances. The incidence rate, risk factors, and mechanisms still need to be clarified and debatable. 2 Severe life-threatening anaphylactic shock, which is more likely to be idiosyncratic, is a rare but severe and fatal complication. 5 Acute anaphylactic shock may be associated with immune-mediated reactions. 6 These idiosyncratic reactions may be associated with dose accumulations and genetic polymorphisms in enzymes, transporters, or receptor proteins, which could lead to altered clearance of toxic metabolites. 7 Previous studies revealed that long-term oxaliplatin accumulation might damage the dorsal root ganglia (DRG), inducing chronic peripheral neuropathy, autonomic dysfunction, and alteration of cardiovascular and respiratory regulations, leading to blood pressure and neurogenic shock.8–11 In addition, although debatable, diabetes mellitus (DM) and other metabolic complications, such as hyperlipidemia and hyperuricemia, might also affect the severity of peripheral neuropathy.12–16 Therefore, the current study aimed to investigate the incident rate and risk factors using real-world data.
This was the first nationwide cohort study to quantitatively investigate the association between oxaliplatin use and shock while only anecdotal case reports and case series were available. We conducted a cohort design to better address causal inference. The primary aim was to assess the association between oxaliplatin use and shock quantitatively and to identify potential independent risk factors associated with anaphylactic shock. The secondary aim was to describe and examine the oxaliplatin cycle to shock.
Materials and methods
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. 17
National Health Insurance Database and CRC Health Database
To increase the affordability and accessibility of medical care, the Taiwan National Health Insurance (NHI) was launched in 1995 as a government-sponsored single-payer health insurance system. At the end of 2018, approximately 23 million beneficiaries had registered in it, indicating a 99.5% coverage rate. The claim data in this study were obtained from the NHI Research Database, which has been de-identified and cannot be linked to individual medical records and managed by the Health and Welfare Data Science Center of the Ministry of Health and Welfare. NHI Research Database contains the first five main medical diagnosis codes, drugs, medications, and surgical procedures. The CRC Health Database is a disease-specific database derived from the NHI Research Database 18 and has further information about the CRC stage of every patient.
Study cohort
Following NCCN guidelines for colon and rectal cancers and considering patients’ physical conditions, our study cohort comprised all stage III CRC patients who were administered oxaliplatin therapy (any oxaliplatin prescription records identified) between 2012 and 2016. The date of the first oxaliplatin administration was referred to as the oxaliplatin-initiation date. Each oxaliplatin prescription record was regarded as one cycle of oxaliplatin therapy or as having oxaliplatin exposure (exposure versus non-exposure). Each patient in the study cohort was followed up until death, the occurrence of the first shock event, or the end of 2017 (data released to 2017), whichever came first.
Nested case–control design
A nested case–control study was further conducted to select matched controls using risk-set sampling for shock cases. Several baseline clinical characteristics were then compared between cases and controls. The nested case–control design can account for the time-dependent nature of oxaliplatin exposure and minimize immortal time bias. 19
Cases
The study participants were followed up until their first shock occurred. The use of vasopressors defined the shock events. The three vasopressors used in our study were intravenous injections of epinephrine, norepinephrine, or dopamine prescribed in outpatient clinics or during hospitalization. The vasopressor prescription date was regarded as the shock date. To rule out cases of septic shock, we excluded patients with any intravenous antibiotic prescription records before or after 7 days of the shock date. The shock date is the index date in this nested case–control study.
Controls
Each risk set was formed whenever a new shock event occurred. Each risk set included all individuals who were still free of shock (i.e. control candidates). For each shock case that occurred during follow-up, up to 10 controls who were matched on the oxaliplatin-initiation date (within 5 days before or after) were randomly selected from the corresponding risk set. The controls were assigned the same index date as their corresponding case. A future case may be selected as a control for a prior case, and a given participant may be selected as a control for two different cases.
Ascertainment of oxaliplatin exposure
We assessed any prescription records of oxaliplatin within 2 days before the index date for both cases and their matched controls.
Potential risk factors ascertainment of shock
Participants who had at least two morbidities in their diagnostic records in outpatient clinics or at least one disease diagnostic record during hospitalization within 1 year before the stage III CRC diagnosis date were examined further to determine whether they had diabetes, hyperlipidemia, or heart disease (ICD9: 410–414, 425, 426, 427, 428; Supplemental Table S1). Hyperuricemia status was defined as having any prescription records of anti-hyperuricemic agents (allopurinol and its combinations, tisopurine, febuxostat, probenecid, sulfinpyrazone, benzbromarone, isobromindione, lesinurad, colchicine, cinchophen, urate oxidase, or pegloticase) 1 year prior to stage III CRC diagnosis date. Neuropathy was defined as having a diagnosis of neuropathy along with the prescription of vitamin B1, B6, and B12 combinations (thiamine, pyridoxine, and cyanocobalamin) or any prescription records for neuropathic pain relievers (gabapentin, pregabalin, topiramate, carbamazepine, lamotrigine, amitriptyline, nortriptyline, venlafaxine, or duloxetine) within 6 months prior to the oxaliplatin-initiation date.
The Anatomical Therapeutic Chemical (ATC) codes for the drugs described above and relevant ICD-9-CM diagnosis codes are listed in Supplemental Table S1.
Case-crossover design
A case-crossover design was used to investigate the association between oxaliplatin use and shock, a clinically sudden event. In this design, the patients served as their controls – a control referred to the same individual who existed days prior to the occurrence of the shock event. The case-crossover design can account for all unmeasured confounders that are invariant over time, and selection bias in the control groups is avoided. 20 The cases in the case-crossover design were defined the same way as those in the aforementioned nested case–control design. The exposure, that is, receiving oxaliplatin, was assessed in the control period and the hazard period, which was the periods before and up to the shock date.
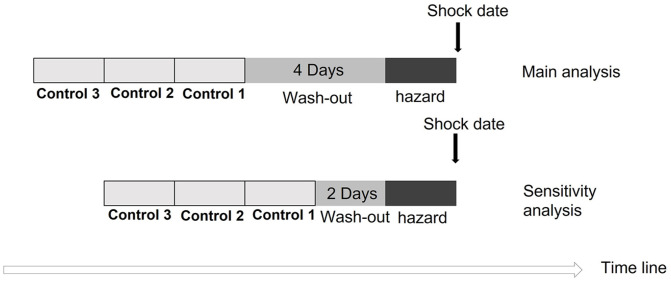
We examined whether participants received oxaliplatin during two specific time intervals: the hazard period, which comprised the days immediately preceding the shock date, and a control period. The time between the hazard and control period was considered the wash-out period. Both the hazard period and the control period spanned 2 days. In addition, we expanded the number of control periods to include two and three intervals. Considering the half-life of oxaliplatin, the wash-out period consisted of a 4-day interval.3,21
Statistical analysis
Data management and analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). Descriptive statistics were used to summarize the characteristics of the study population and shock cases. We depicted the number of shock cases against the number of oxaliplatin cycles after the oxaliplatin-initiation date. In case-crossover analysis, exposures to oxaliplatin were compared between hazard and control periods; in a nested case–control analysis, oxaliplatin exposure odds between shock cases and matched controls were compared. Both nested case–control and case-crossover studies are paired designs; thus, conditional logistic regression, a pair-matched analytic approach, was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) between shock and oxaliplatin use. This method accounts for within-subject correlation, enabling researchers to control for potential confounding factors, examine the effect of oxaliplatin on shock, and explore potential risk factors of oxaliplatin-associated shock.
Sensitivity analysis
In case-crossover analysis, we further tested another wash-out period of 2 days to examine the robustness and consistency of the analysis. The ORs were estimated with an increased matching number (two and three) of control periods. We drew a schematic diagram illustrating the layout of the case-crossover design and its sensitivity analysis (Figure 1).
Figure 1.
The illustration of case-crossover design.
Results
Selection process and baseline characteristics of the study participants
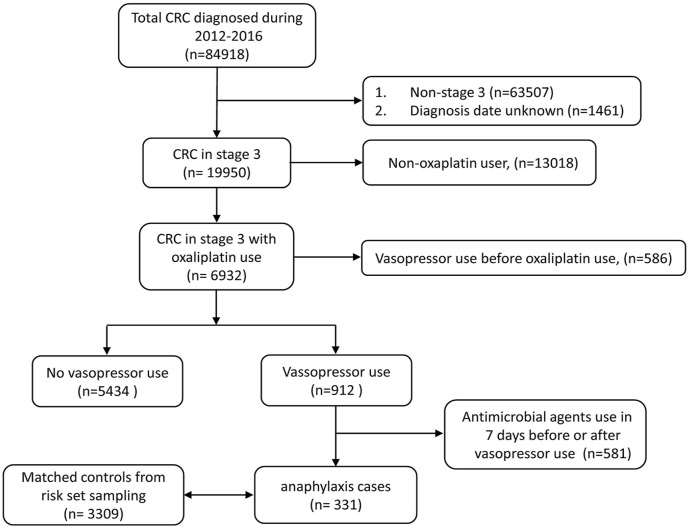
Of the total 84,918 patients diagnosed with CRC between 2012 and 2016, 19,950 patients had stage III CRC. Our study cohort consisted of 6932 patients recruited from 19,950 individuals, all of whom had been administered oxaliplatin alone or in combination as part of their cancer chemotherapy regimen. In all, 912 patients used vasopressors. Of them, 581 patients with a history of using parenteral antimicrobial agents were excluded, while 331 patients were regarded as shock cases (Figure 2).
Figure 2.
The study cohort for the oxaliplatin-associated shock in patients with stage III colorectal cancer.
The proportion of male patients (both approximately 60%) and the Charlson Comorbidity Index (median score: 2) of cases and controls were similar. The median interval from stage III diagnosis to first oxaliplatin use was 54 (41, 112) days. The median diagnosis age was younger among oxaliplatin users (60 in the study cohort versus 66 in total stage III CRC). The proportion of patients with neuropathy (1.67%) was relatively low in our study cohort, whereas the proportions of patients with diabetes (16.27%), heart diseases (10.26%), and hyperuricemia (9.94%) were slightly higher (Supplemental Table S2).
Baseline characteristics of shock cases and matched controls
During follow-up, 331 shock cases were observed in our study cohort, and 3309 controls were selected using risk-set sampling. Twenty-two cases (6.65%) and 125 (3.78%) matched control had oxaliplatin exposure within 2 days before the index date. The median cumulative oxaliplatin cycle at the index date was the 9th and 10th cycles in cases and controls, respectively. A higher proportion of male patients were more likely to develop shock (63.14% versus 55.88%). The distributions of other metabolic comorbidities and demographic data were similar between cases and controls, with a higher proportion of heart disease in shock cases than the matched controls (15.11% versus 9.88%). (Table 1).
Table 1.
Baseline clinical characteristics of shock cases and matched controls in nested case–control analysis.
| Baseline clinical characteristics | Shock cases (n = 331) | Matched controls (n = 3309) |
|---|---|---|
| Oxaliplatin use in 2 days before the index date, n (%) | 22 (6.65%) | 125 (3.78%) |
| Cumulative oxaliplatin cycles since the oxaliplatin-initiation date | 9.00 (6.00,12.00) | 10.00 (6.00,12.00) |
| Age at diagnosis, median (Q1, Q3) | 61.00 (52.00, 69.00) | 60.00 (51.00, 68.00) |
| Male, n (%) | 209 (63.14%) | 1849 (55.88%) |
| Days between diagnosis date and first oxaliplatin use, median (Q1, Q3) | 52.00 (39.00,82.00) | 51.00 (39.00,77.00) |
| Charlson Comorbidity Index, median (Q1, Q3) | 2.00 (0.00,3.00) | 2.00 (0.00,3.00) |
| Diagnosis year, n (%) a | ||
| 2012 | 86 (25.98%) | 912 (27.56%) |
| 2013 | 83 (25.08%) | 741 (22.39%) |
| 2014 | 65 (19.64%) | 670 (20.25%) |
| 2015 | 57 (17.22%) | 613 (18.53%) |
| 2016 | 40 (12.08%) | 373 (11.27%) |
| Diabetes, n (%) b | 56 (16.92%) | 541 (16.35%) |
| Hyperuricemia, n (%) c | 37 (11.18%) | 304 (9.19%) |
| Hyperlipidemia, n (%) b | 29 (8.76%) | 336 (10.15%) |
| Neuropathy, n (%) d | 7 (2.11%) | 75 (2.27%) |
| Heart disease, n (%) b | 50 (15.11%) | 327 (9.88%) |
The first diagnosed year of the patients with colorectal cancer.
Subjects who had greater than or equal to two disease diagnostic records in outpatient or greater than or equal to one disease diagnostic record in inpatient settings in 1 year prior to CRC stage III diagnosis date were defined as having those comorbidities. The ICD9 records of diabetes and hyperlipidemia were 250 and 272.4, respectively. The heart diseases were defined as ICD9 410–414, 425, 426, 427, and 428.
Hyperuricemia status was defined by medication prescription records. Subjects with any listed medication prescription records in 1 year prior to the stage III CRC diagnosis date were regarded as having hyperuricemia. Medications used to treat hyperuricemia were allopurinol (and its combinations), tisopurine, febuxostat, probenecid, sulfinpyrazone, benzbromarone, isobromindione, lesinurad, colchicine, cinchophen, urate oxidase, pegloticase. Subjects who were considered to have neuropathy were defined by having a neuropathy diagnosis along with a prescription of vitamin B1, B6, and B12 combinations or any listed medication prescription records in 6 months prior to the oxaliplatin-initiation date.
Subject diagnosis of ICD9 356 along with vitamin B1, B6, and B12 combination treatment (thiamine, pyridoxine, and cyanocobalamin) or any prescription records of gabapentin, pregabalin, topiramate, carbamazepine, lamotrigine, amitriptyline, nortriptyline, venlafaxine, duloxetine.
Oxaliplatin-associated shock
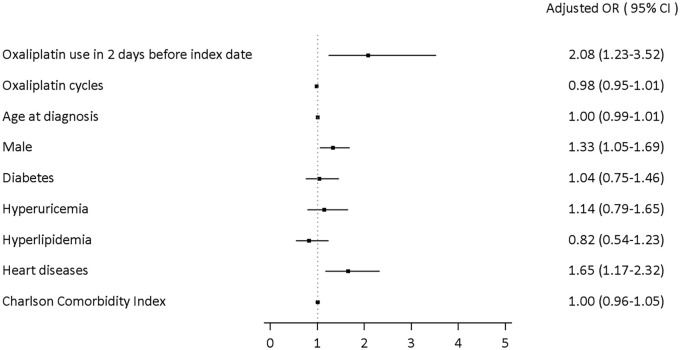
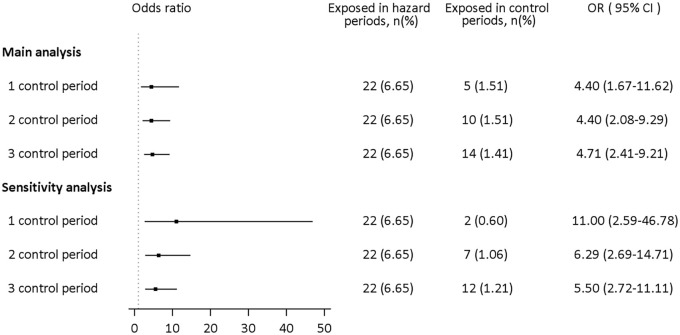
In the nested case–control analysis, oxaliplatin was consistently positively associated with shock (adjusted OR: 2.08, 95% CI: 1.23–3.52). The male population was an independent risk factor for shock. Male patients had more than 30% higher odds of developing shock than female patients (adjusted OR: 1.33, 95% CI: 1.05–1.69). Moreover, stage III CRC patients with heart diseases had 65% higher odds of developing shock (adjusted OR: 1.65, 95% CI: 1.17–2.32). Other metabolic comorbidities were not statistically significant (Figure 3). In the case-crossover analysis, in which all time-invariant within subjects’ confounders were automatically adjusted, oxaliplatin use was associated with a 4.40-fold increase in the risk of shock in the main analysis. Based on sensitivity analysis, the results showed consistency regardless of the number of control periods. Also, when the wash-out period was shortened to 2 days, the risk increased to 11-fold in one control period selected; the risk decreased to 6.29- and 5.50-fold after two and three control periods, respectively. The results from the case-crossover analysis support the findings of the nested case–control analysis, which yielded a more conservative estimate of OR (Figure 4).
Figure 3.
Associations between oxaliplatin use, potential risk factors, and oxaliplatin-associated shock in nested case–control analysis.
CI, confidence interval; OR, odds ratio.
Figure 4.
Association between current oxaliplatin use and shock in case-crossover analysis among our study cohort. Main analysis: case-crossover analysis with a wash-out period of 4 days; sensitivity analysis: case-crossover analysis with a wash-out period of 2 days (Figure 2).
CI, confidence interval; OR, odds ratio.
Oxaliplatin cycle-to-shock
Of the 331 shock cases, 22 had oxaliplatin exposure within 2 days before the shock date. Three out of these 22 cases experienced shock at the first cycle of oxaliplatin administration, while the median cycle-to-shock occurrence was the seventh cycle (Figure 5).
Figure 5.
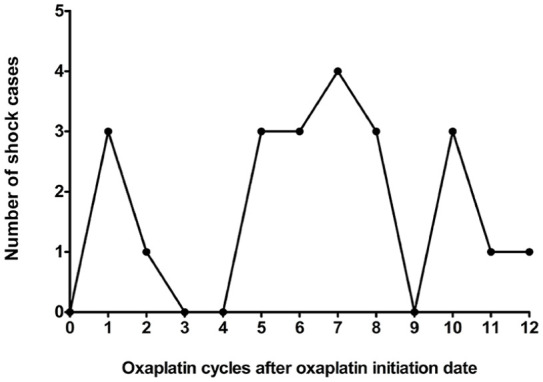
The cycle-to-shock of oxaliplatin. Twenty-two out of 331 shock cases encounter the shock events within 2 days of oxaliplatin treatment. The median cycle was observed at the seventh cycle and most of the shock cases were at the later cycle.
Discussion
The study was the first nationwide study to assess the quantitative association between oxaliplatin and shock. Case-crossover analysis and nested case–control analysis provided consistent positive results. The risk estimated in the nested case–control analysis (Figure 3) was observed to be more conservative compared to the case-crossover analysis (Figure 4). This difference suggests the presence of unmeasured confounders, which were automatically adjusted for in the case-crossover analysis. The consistent results were observed across different durations of the wash-out period.
Male patients were found to be one of the independent risk factors of oxaliplatin-associated shock in the study (adjusted OR: 1.33, 95% CI: 1.05–1.69). The result was similar to previous case reports and case series.2,7,22,23 Furthermore, descriptive statistics have been performed in previous case reports. This first big data analysis with inferential statistics revealed that male CRC patients had a higher risk of suffering shock. In Taiwan, the male population has greater psychosocial factors such as work, family, and economic stresses to enhance potential cardiovascular stress. 24 Oxaliplatin increasing oxidative stress in the cells might enhance cardiovascular stress. Therefore, overall stresses could be one of the potential risks that male CRC patients had a higher risk of shock.
Heart diseases in all sexes were the other independent risk factors of shock in patients with stage III CRC (adjusted OR: 1.65, 95% CI: 1.17–2.32). Heart diseases, including all types of cardiac dysfunctions, had a significant increase in the risk of developing shock. Although the current study could not address the mechanism underlying heart disease on shock events, our analysis showed that baseline heart disease status was one of the independent risk factors for oxaliplatin-associated shock (Figure 3). At the same time, cardiotoxicity of oxaliplatin was not present in any other anecdotal case reports and case series. DM, hyperglycemia, and other metabolic complications might also lead to cardiovascular disorders and chronic peripheral neuropathy.12–14,16 However, we did not find any difference in our analysis (Figure 3). In the current study, the patients with DM and other metabolic syndromes, such as hyperuricemia and hyperlipidemia, were not associated with higher risks of oxaliplatin-associated severe shock.
In our study cohort of 6932 participants, 60 out of 116 patients had neuropathy before the oxaliplatin-initiation date but did not have the neuropathy diagnosis after the oxaliplatin administration. By contrast, 211 out of 6816 patients developed neuropathy after the oxaliplatin initiation date (p < 0.001 after the McNemar test). Oxaliplatin-induced peripheral neurotoxicity (OIPN), a comprehensive adverse reaction, includes acute and chronic neuropathies, such as temperature sensitivity and neuropathic pain in the arms and legs.2,8,25–27 Moreover, chronic OIPN produces autonomic nerve dysfunction in addition to acute symptoms.8,11,27 Cumulative dose of oxaliplatin enhances chronic OIPN.9,16,28,29 The mechanisms of OIPN include neuron-cell nucleotide damage, mitochondrial breakdown with oxidative stress overload, glial activation, and neuroinflammation. 8 Previous studies showed that oxaliplatin accumulated in the DRG and destroyed neuronal mitochondria by forming platinum–mitochondria DNA adducts.8,9 On the other hand, oxalate, the major metabolite of oxaliplatin, reacts with calcium ions and inhibits sodium channel activation in DRG neurons, resulting in nerve hyperexcitability and neurotoxicity. 4 DRG damage caused autonomic nerve dysfunction, resulting in a change in heart rate and blood pressure regulation, an oxaliplatin-associated adverse reaction, and might induce neurogenic shock.10,11,30 In an animal study, long-term exposure to oxaliplatin increased splanchnic sympathetic nerve activity, phrenic nerve frequency, mean arterial pressure, and decreased heart rate and phrenic nerve amplitude, but acute oxaliplatin had no significant effects. Alterations in the central nervous system affecting baroreceptor sensitivity and somato-sympathetic reflex lead to cardiovascular, respiratory functions, and reflexes disorders after chronic oxaliplatin treatment. 11 Previous reports also show that long-term exposure to platinum-based chemotherapy agents also increases cardiotoxicity.31–33 There was no direct evidence that oxaliplatin use was associated with neurogenic shock in the current study. It might be helpful for clinical practitioners to take a closer look at patients who display symptoms associated with autonomic nerve dysfunction.
Reports on oxaliplatin-associated HSR and anaphylactic shock have increased in recent years.1,2,34 Current study depicted the cycle-to-shock, in most cases, at later cycles, which implied that the mechanism of oxaliplatin-associated life-threatening severe anaphylactic shock might not be the IgE-mediated HSR (Figure 5). The late-peak pattern was like that reported in previous case reports.2,5,7,34 Anaphylactic shock, an idiosyncratic life-threatening reaction, is rare and hard to predict. 5 The reported incidence rate of oxaliplatin-associated anaphylactic shock with severe and fatal complications is less than 1%. Clinical healthcare professionals established the successful protocol for desensitization to prevent HSR, including the administration of steroids and antihistamines as premedication to prevent IgE-mediated HSR.35–38 Following the desensitization protocol, we found that most patients with CRC in the current study had received steroids and antihistamines as premedication before administering oxaliplatin. Premedication did alleviate immune hyperactivity and reduced HSR. However, the mechanism of oxaliplatin-associated anaphylactic shock remains unclear, and desensitization procedures might not prevent it.2,6
There are limitations to this study. First, we ruled out cases of septic shock by excluding any patients who had been prescribed antimicrobial agents within 7 days before or after the shock date. This may underestimate the true number of shock cases because the specific duration of the antimicrobial use was unavailable, patients without definite (e.g. treated for less than 2 days) infection would be excluded. Furthermore, some patients with very low absolute neutrophil count (ANC), which is routinely checked before the administration of chemotherapeutic agents, may not show any signs of infection. These patients are typically prescribed prophylactic antimicrobials and then receive chemotherapeutic agents until their ANC numbers return to the normal range. Therefore, the association between oxaliplatin and shock may be underestimated in our study. Second, clinical laboratory data of chemistry, immunology, and hematology, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine, IgG, IgE, and IgM levels, were unavailable. Therefore, the molecular mechanism under oxaliplatin-associated shock requires further pharmacological studies. Third, we used three vasopressors as rescue agents to identify shock cases; therefore, we were unable to identify any individual patients with HSR who were not rescued by the listed vasopressors. Fourth, we did not analyze the effect of the desensitization procedure and infusion time of oxaliplatin due to limitations in the study’s data sources. Consequently, we could not rule out the possibility that some cases of shock were prevented by the desensitization procedure and prolonged infusion time. Nevertheless, the desensitization procedure has become the standard protocol for preventing drug hypersensitivity and anaphylaxis in Taiwan. We assume that the influence of the desensitization procedure and infusion time of oxaliplatin on our study’s findings is minimal. Finally, the lack of occupational records prevented us from investigating the patient’s lifetime and occupational exposure to oxaliplatin.
Conclusion
The use of oxaliplatin was positively associated with shock in patients with stage III CRC. The consistent results were observed in both primary and sensitivity analysis. The male population and heart diseases were found to be the independent risk factors of shock. Therefore, prolonged infusion time (4–6 h), diluting the concentration of oxaliplatin (0.2 mg/ml), and strict administration of premedication and supportive care are highly recommended in the male population. Moreover, healthcare professionals need to monitor patients with intolerable neuropathy and functional impairment to decrease neurological dysfunction, especially autonomic nerve dysfunction symptoms such as heart rate and blood pressure changes, dizziness and fainting, sweating, and urinary problems. Further multi-omics studies are required to understand the mechanism underlying oxaliplatin-associated shock.
Supplemental Material
Supplemental material, sj-docx-1-taw-10.1177_20420986241266439 for Oxaliplatin-associated shock in stage III colorectal cancer patients: real-world evidence in Taiwan by Ling-Yi Wang, Hui-Hsia Hsieh, Sung-Chao Chu, Wei-Chuan Chang, Yi-Ting Kuo and Tien-Yuan Wu in Therapeutic Advances in Drug Safety
Supplemental material, sj-docx-2-taw-10.1177_20420986241266439 for Oxaliplatin-associated shock in stage III colorectal cancer patients: real-world evidence in Taiwan by Ling-Yi Wang, Hui-Hsia Hsieh, Sung-Chao Chu, Wei-Chuan Chang, Yi-Ting Kuo and Tien-Yuan Wu in Therapeutic Advances in Drug Safety
Acknowledgments
The authors thank Dr. Yun Yen, MD, PhD, and Dr. Dian-Kun Li, MD, PhD, for their expertise in cancer diagnosis, treatment, and research, and for their thoughtful discussion in the project initiation and manuscript preparation. The authors also thank Dr. I-An Chen at the Department of English, National Taichung University of Education providing language help, writing assistance, or proofreading the article.
Footnotes
ORCID iD: Tien-Yuan Wu  https://orcid.org/0000-0002-5205-4567
https://orcid.org/0000-0002-5205-4567
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ling-Yi Wang, Graduate Institute of Clinical Pharmacy, Tzu Chi University, Hualien, Taiwan; Department of Pharmacy, Buddhist Tzu Chi General Hospital, Hualien, Taiwan.
Hui-Hsia Hsieh, Department of Pharmacy, Taichung Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taichung City, Taiwan.
Sung-Chao Chu, Department of Hematology and Oncology, Buddhist Tzu Chi General Hospital, Hualien City, Taiwan; School of Medicine, Tzu Chi University, Hualien, Taiwan.
Wei-Chuan Chang, Epidemiology and Biostatistics Consulting Center, Department of Medical Research, Buddhist Tzu Chi General Hospital, Hualien City, Taiwan.
Yi-Ting Kuo, Department of Pharmacy, Taipei City Hospital Zhongxiao Branch, Taipei City, Taiwan.
Tien-Yuan Wu, Department of Clinical Pharmacy, School of Pharmacy, Taipei Medical University, 250 Wuxing Street, Taipei City 110301, Taiwan; Center for Cancer Translational Research, Tzu Chi University, Hualien City, Taiwan.
Declarations
Ethics approval and consent to participate: The study was exempted from review by the Research Ethics Committee of Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (IRB109-081-C). All the procedures to protect human research subjects complied with the ethical standards of this institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication: The requirement for informed consent was also waived because de-identified data was used.
Author contributions: Ling-Yi Wang: Conceptualization; Data curation; Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Hui-Hsia Hsieh: Conceptualization; Funding acquisition; Resources; Supervision; Writing – original draft; Writing – review & editing.
Sung-Chao Chu: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Wei-Chuan Chang: Data curation; Formal analysis; Methodology; Software; Visualization; Writing – review & editing.
Yi-Ting Kuo: Conceptualization; Validation; Writing – review & editing.
Tien-Yuan Wu: Conceptualization; Data curation; Investigation; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Taipei Medical University (TMU112-AE1-B11); Tzu Chi University (TCMRC-P-109007); the Ministry of Science and Technology, Taiwan (MOST111-2320-B-320-005); and the Ministry of Health and Welfare, Taiwan (MOHW112-TDU-B-222-124013 and MOHW113-TDU-B-222-134013).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data sets generated and/or analyzed during the current study are not publicly available due to institutional restrictions. SAS codes for data development and analysis are available from the authors.
References
- 1. Aliyu UM, Okwonna CO. Delayed hypersensitivity to oxaliplatin in a Nigerian patient with colorectal cancer-A case report. J Oncol Pharm Pract 2021; 27: 1258–1260. [DOI] [PubMed] [Google Scholar]
- 2. Wang JH, King TM, Chang MC, et al. Oxaliplatin-induced severe anaphylactic reactions in metastatic colorectal cancer: case series analysis. World J Gastroenterol 2012; 18: 5427–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ehrsson H, Wallin I, Yachnin J. Pharmacokinetics of oxaliplatin in humans. Med Oncol 2002; 19: 261–265. [DOI] [PubMed] [Google Scholar]
- 4. Yang Y, Zhao B, Gao X, et al. Targeting strategies for oxaliplatin-induced peripheral neuropathy: clinical syndrome, molecular basis, and drug development. J Exp Clin Cancer Res 2021; 40: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsu CW, King TM, Chang MC, et al. Life-threatening severe anaphylactic reactions in patients receiving oxaliplatin chemotherapy. Int J Colorectal Dis 2012; 27: 1679–1680. [DOI] [PubMed] [Google Scholar]
- 6. Song Q, Cai Y, Guo K, et al. Risk factors for oxaliplatin-induced hypersensitivity reaction in patients with colorectal cancer. Am J Transl Res 2022; 14: 2461–2468. [PMC free article] [PubMed] [Google Scholar]
- 7. Bhargava P, Gammon D, McCormick MJ. Hypersensitivity and idiosyncratic reactions to oxaliplatin. Cancer 2004; 100: 211–212. [DOI] [PubMed] [Google Scholar]
- 8. Kang L, Tian Y, Xu S, et al. Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J Neurol 2021; 268: 3269–3282. [DOI] [PubMed] [Google Scholar]
- 9. Warncke UO, Toma W, Meade JA, et al. Impact of dose, sex, and strain on oxaliplatin-induced peripheral neuropathy in mice. Front Pain Res (Lausanne) 2021; 2: 683168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor MP, Wrenn P, O’Donnell AD. Presentation of neurogenic shock within the emergency department. Emerg Med J 2017; 34: 157–162. [DOI] [PubMed] [Google Scholar]
- 11. Rahman AA, Stojanovska V, Pilowsky P, et al. Platinum accumulation in the brain and alteration in the central regulation of cardiovascular and respiratory functions in oxaliplatin-treated rats. Pflugers Arch 2021; 473: 107–120. [DOI] [PubMed] [Google Scholar]
- 12. Gu J, Lu H, Chen C, et al. Diabetes mellitus as a risk factor for chemotherapy-induced peripheral neuropathy: a meta-analysis. Support Care Cancer 2021; 29: 7461–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bano N, Ikram R. Effect of diabetes on neurological adverse effects and chemotherapy induced peripheral neuropathy in advanced colorectal cancer patients treated with different FOLFOX regimens. Pak J Pharm Sci 2019; 32: 125–130. [PubMed] [Google Scholar]
- 14. Wang RY, Lin XL, Xiang ST, et al. Risk factors for oxaliplatin-induced peripheral neuropathy: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 2022; 26: 4028–4043. [DOI] [PubMed] [Google Scholar]
- 15. Kim HD, Ha KS, Woo IS, et al. Tumor lysis syndrome in a patient with metastatic colon cancer after treatment with 5-fluorouracil/leucovorin and oxaliplatin: case report and literature review. Cancer Res Treat 2014; 46: 204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee S, Ma C, Shi Q, et al. Potential mediators of oxaliplatin-induced peripheral neuropathy from adjuvant therapy in stage III colon cancer: findings from CALGB (Alliance)/SWOG 80702. J Clin Oncol 2023; 41: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18: 800–804. [DOI] [PubMed] [Google Scholar]
- 18. Lin LY, Warren-Gash C, Smeeth L, et al. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health 2018; 40: e2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Targownik LE, Suissa S. Understanding and avoiding immortal-time bias in gastrointestinal observational research. Am J Gastroenterol 2015; 110: 1647–1650. [DOI] [PubMed] [Google Scholar]
- 20. Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991; 133: 144–153. [DOI] [PubMed] [Google Scholar]
- 21. Shord SS, Bernard SA, Lindley C, et al. Oxaliplatin biotransformation and pharmacokinetics: a pilot study to determine the possible relationship to neurotoxicity. Anticancer Res 2002; 22: 2301–2309. [PubMed] [Google Scholar]
- 22. Larzilliere I, Brandissou S, Breton P, et al. Anaphylactic reaction to oxaliplatin: a case report. Am J Gastroenterol 1999; 94: 3387–3388. [DOI] [PubMed] [Google Scholar]
- 23. Medioni J, Coulon MA, Morere JF, et al. Anaphylaxis after oxaliplatin. Ann Oncol 1999; 10: 610. [DOI] [PubMed] [Google Scholar]
- 24. Weng C-Y, Lin T-K, Chen C-W, et al. Psychosocial risk factors and cardiac rehabilitation. J Intern Med Taiwan 2015; 26: 1–12. [Google Scholar]
- 25. Schulze C, McGowan M, Jordt S-E, et al. Prolonged oxaliplatin exposure alters intracellular calcium signaling: a new mechanism to explain oxaliplatin-associated peripheral neuropathy. Clin Colorectal Cancer 2011; 10: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi J, Kong K, Mozaffar T, et al. Delayed oxaliplatin-associated neurotoxicity following adjuvant chemotherapy for stage III colon cancer. Anticancer Drugs 2006; 17: 103–105. [DOI] [PubMed] [Google Scholar]
- 27. Pachman DR, Qin R, Seisler DK, et al. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 2015; 33: 3416–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zribi A, Nasr SB, Hamdi S, et al. Oxaliplatin-induced peripheral neuropathy risk factors and management in Tunisian population. Pan Afr Med J 2020; 35: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saif MW, Reardon J. Management of oxaliplatin-induced peripheral neuropathy. Ther Clin Risk Manag 2005; 1: 249–258. [PMC free article] [PubMed] [Google Scholar]
- 30. Schmitt LI, Leo M, Kleinschnitz C, et al. Oxaliplatin modulates the characteristics of voltage-gated calcium channels and action potentials in small dorsal root ganglion neurons of rats. Mol Neurobiol 2018; 55: 8842–8855. [DOI] [PubMed] [Google Scholar]
- 31. Ferroni P, Della-Morte D, Palmirotta R, et al. Platinum-based compounds and risk for cardiovascular toxicity in the elderly: role of the antioxidants in chemoprevention. Rejuvenation Res 2011; 14: 293–308. [DOI] [PubMed] [Google Scholar]
- 32. Wachters FM, Van Der Graaf WT, Groen HJ. Cardiotoxicity in advanced non-small cell lung cancer patients treated with platinum and non-platinum based combinations as first-line treatment. Anticancer Res 2004; 24: 2079–2083. [PubMed] [Google Scholar]
- 33. Evans C, Williams M, Mazhar D. Long-term cardiovascular risk following platinum-based chemotherapy for germ cell tumors. Future Oncol 2010; 6: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 34. Lee MY, Yang MH, Liu JH, et al. Severe anaphylactic reactions in patients receiving oxaliplatin therapy: a rare but potentially fatal complication. Support Care Cancer 2007; 15: 89–93. [DOI] [PubMed] [Google Scholar]
- 35. Cakmak ME, Kaya SB, Can Bostan O, et al. Successful desensitization with chemotherapeutic drugs: a tertiary care center experience. Eur Ann Allergy Clin Immunol 2022; 54: 90–94. [DOI] [PubMed] [Google Scholar]
- 36. Chung SJ, Kang SY, Kang RY, et al. A new non-dilution rapid desensitization protocol successfully applied to all-grade platinum hypersensitivity. Cancer Chemother Pharmacol 2018; 82: 777–785. [DOI] [PubMed] [Google Scholar]
- 37. Kidera Y, Satoh T, Ueda S, et al. High-dose dexamethasone plus antihistamine prevents colorectal cancer patients treated with modified FOLFOX6 from hypersensitivity reactions induced by oxaliplatin. Int J Clin Oncol 2011; 16: 244–249. [DOI] [PubMed] [Google Scholar]
- 38. Yoshida Y, Hirata K, Matsuoka H, et al. A single-arm Phase II validation study of preventing oxaliplatin-induced hypersensitivity reactions by dexamethasone: the AVOID trial. Drug Des Devel Ther 2015; 9: 6067–6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taw-10.1177_20420986241266439 for Oxaliplatin-associated shock in stage III colorectal cancer patients: real-world evidence in Taiwan by Ling-Yi Wang, Hui-Hsia Hsieh, Sung-Chao Chu, Wei-Chuan Chang, Yi-Ting Kuo and Tien-Yuan Wu in Therapeutic Advances in Drug Safety
Supplemental material, sj-docx-2-taw-10.1177_20420986241266439 for Oxaliplatin-associated shock in stage III colorectal cancer patients: real-world evidence in Taiwan by Ling-Yi Wang, Hui-Hsia Hsieh, Sung-Chao Chu, Wei-Chuan Chang, Yi-Ting Kuo and Tien-Yuan Wu in Therapeutic Advances in Drug Safety