Abstract
Background:
Biologic agents have demonstrated efficacy in treating ulcerative colitis (UC); however, treatment failure to tumor necrosis factor inhibitors (TNFi) is common in the real world. Data on preferential sequencing in clinical practice after failure remain limited.
Objectives:
This study aimed to evaluate real-world outcomes of patients cycling to TNFis or switching to non-TNFi biologics following first-line failure with TNFis.
Design:
Retrospective cohort study in Germany.
Methods:
Adult patients with UC were identified using administrative claims data from 1 May 2014 to 30 June 2022 provided by a statutory sickness fund. Patients newly initiating first-line therapy with TNFis and then switching to another agent were identified. Patients were defined as within-class switched (WCS), if they cycled to another TNFi, or outside-class switchers (OCS), if they switched to a non-TNFi biologic [ustekinumab (UST) or vedolizumab (VDZ)] and followed from index (switch date) to death, insurance end, or study end on 30 June 2022. Inverse probability of treatment weighting (IPTW) was performed to adjust for differences in baseline characteristics between groups, and weighted Cox regression models were used to compare primary (time to discontinuation and second treatment switch) and secondary outcomes (corticosteroid-free drug survival).
Results:
We identified 166 patients initiating TNFis and switching to a subsequent treatment (mean age: 42.9 years, 49.4% female). Following IPTW, there were 71 and 76 patients in the WCS and OCS groups, respectively. Compared to OCS, WCS were more likely to discontinue the new therapy [hazard ratio (HR), 1.82, 95% confidence interval (CI), 1.14–2.89, p = 0.012], and switch a second time (HR, 3.46, 95% CI, 1.89–6.36, p < 0.001). Moreover, WCS showed an increased likelihood of initiating prolonged corticosteroid therapy (HR, 1.42, 95% CI, 0.77–2.59, p = 0.260); however, the results were not significant.
Conclusion:
Following first-line TNFi failure, this study suggests that real-world outcomes among patients with UC are less favorable when cycling to another TNFi, compared to switching to a non-TNFi such as UST or VDZ.
Keywords: biologics, claims data, switching, therapy sequence, TNF-cycling, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition affecting the colon. The disease is globally on the rise, with a prevalence of 160–250 per 100,000 in Germany.1,2 UC can significantly impact the patient’s quality of life, potentially resulting in disability and surgery, and subsequently leading to considerable mental morbidity, including depression and anxiety.3,4
To achieve the treatment goal of maintaining steroid-free remission, good health-related quality of life, and minimizing disability, treatment strategies aim to provide rapid relief of clinical symptoms and, where possible, achieve endoscopic healing. 5 Recommendations suggest tailoring therapy selection based on the extent of the disease and guide treatment strategies for different severities, including mild-to-moderately and moderately-to-severely active UC.5–7
The latest guidelines from the German Society for Digestive and Metabolic Diseases recommend treatment with systemic corticosteroids to induce remission in moderately-to-severely active UC. 7 Advanced therapies like tumor necrotic factor inhibitors (TNFis) [i.e. infliximab (IFX), adalimumab (ADA), and golimumab (GOL)], agents targeting leukocyte trafficking (i.e. vedolizumab, VDZ), the anti-p40 (anti-interleukin-12/23) antibody ustekinumab (UST), Janus kinase inhibitors (JAKi) [i.e. tofacitinib (TOF), filgotinib (FIL)] as well as the sphingosine-1-phosphate receptor agonist ozanimod (OZA) are also recommended for the induction of remission and maintenance in patients with moderate-to-severe UC who have inadequate response or intolerance to conventional therapy.5,7,8 Despite the availability of several treatment options, a significant proportion of patients experience primary non-response (10–30%) or secondary loss of response (23–46%) to treatment.6,9 While available strategies following loss of response include dose optimization and subsequently cycling to a different TNFi agent or switching to an agent with an alternative mode of action, there is no clear consensus on the best therapeutic approach. 10 In addition, there is a lack of head-to-head clinical trial evidence on the effectiveness of different biologic agents among patients with inflammatory bowel disease (IBD), particularly after TNFi experience in the first-line setting. 11 We previously conducted a retrospective real-world evidence (RWE) study in Germany comparing outcomes of switching within the TNFi biologics class (within-class switchers, WCS) and switching to a non-TNFi biologic (outside-class switchers, OCS) after initial TNFi treatment failure in Crohn’s disease (CD). 12 The CD study revealed that WCS were more likely to require prolonged corticosteroid therapy, re-switch to a different biologic, or discontinue the index treatment compared to OCS.
To further explore this phenomenon in patients with UC and address the current evidence gap, an extension of the above-mentioned CD study was initiated. This current study investigates real-world outcomes among patients with UC initiating first-line treatment with TNFis and subsequently cycling within the TNFi class (ADA, IFX, or GOL) or switching outside class to a non-TNFi biologic agent with an alternative mode of action (VDZ or UST).
Methods
Study design
This study is a retrospective, non-interventional cohort analysis of patients with UC using anonymized claims data obtained from the regional German sickness fund AOK PLUS. AOK PLUS covers approximately 3.4 million individuals in the states of Saxony and Thuringia, corresponding to 50% of the local population and approximately 4–5% of the overall population of statutory insured patients in Germany, offering a national representation of the healthcare system. Claims data provide a comprehensive record of a patient’s medical and treatment history, encompassing all therapeutic agents prescribed across various healthcare sectors and essential for reimbursement purposes. For this study, demographics, inpatient and outpatient diagnostic, procedural, and treatment information were collected. An overview of all variables and respective definitions is found in Supplemental Tables 1–3.
Study periods and population
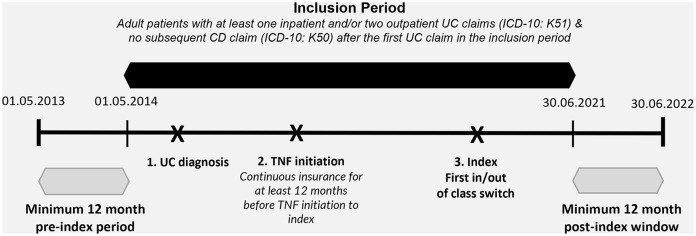
The study period extended from 1 May 2014 to 30 June 2022, and eligibility criteria were assessed within the inclusion period from 1 May 2014 to 30 June 2021, allowing for a 12-month baseline period and a minimum (MIN) 12-month window post-index for patient follow-up (Figure 1). The inclusion period was selected based on the availability of at least one alternative treatment to TNFis (outside-class option) in Germany, specifically VDZ, which received approval in May 2014.
Figure 1.
Study design.
The base population for this study comprised of adults aged 18 years and older with at least one inpatient and/or two confirmed outpatient diagnoses of UC [International Classification of Diseases and Related Health Problems, 10th revision, German Modification (ICD-10-GM): any K51.-] and without any concomitant inpatient or outpatient diagnoses of CD (ICD-10-GM: K50.-) after the first observable UC diagnosis in the inclusion period. In addition, patients must have newly initiated TNFis as their first-line therapy, defined as no prescriptions of biologics or small molecules for at least 12 months before the first prescription of a TNFi in the inclusion period or longer, conditional on data availability.
Within this population, patients who newly initiated therapy with TNFis in the first line, including ADA, IFX, or GOL, and cycled within-class (hereafter named within-class switchers, WCS) to a different TNFi agent or switched outside-class (hereafter named outside-class switchers, OCS) to VDZ or UST were selected. Due to low sample sizes, patients who switched to JAKi (TOF: n = 9, FIL: n = 0) or OZA (n = 0) were not included in the OCS group, and the investigation focused on switching among biologic agents only. The index date was set to the date of the first switch after first-line TNFi initiation. To ensure completeness of information, all patients were required to be continuously insured for at least 12 months before TNFi (first-line) start until the index date. The baseline period comprised of the 12 months before the index date, and patients were followed from the index date until the earliest of insurance loss, death, or end of the study period on 30 June 2022. Figure 1 provides a graphical outline of the study design and patient inclusion periods.
Outcome measures
The primary outcomes of interest were time to second treatment switch and time to treatment discontinuation. The secondary outcome was corticosteroid-free drug survival. Time to second treatment switch was defined as the time from index to the prescription of a drug different from the index agent, prescribed within 180 days following exhaustion of the supply of the index agent, with no other prescription of the index agent until at least 180 days after supply exhaustion. The presumed end of supply was marked by the number and strength of prescriptions, assuming that patients take the defined daily dose (DDD) as outlined by the World Health Organization. Switching was restricted to the given period to select patients following non-response or loss of response in the real world. Time to discontinuation was defined as the duration from index to the cessation of therapy or switch, marked by the start of a 60-day gap (90-day in sensitivity analyses) without any prescriptions of the index agent after the presumed completion of the drug supply or switching. Notably, to define the presumed end of supply, we accounted for stockpiling and assumed coverage with medication during hospitalization periods. The primary outcomes of this study provide evidence for the treatment persistence of second-line therapy approaches following first-line therapy failure. As a secondary objective, corticosteroid-free drug survival was defined as the time between the index and start of prolonged corticosteroid therapy, determined by at least three consecutive prescriptions of systemic corticosteroids within 12 months, including oral budesonide. In sensitivity analyses, definitions requiring two and four prescriptions were also investigated. Across all outcomes, patients were censored at the earliest of death, insurance end, or end of the study period. Specifically for corticosteroid-free drug survival, we additionally accounted for censoring at discontinuation or switch; however, the results remained consistent (data not shown).
As explorative information, treatment sequences were evaluated among the overall population of continuously insured patients who initiated first-line therapy with advanced therapies and visualized using Sankey diagrams. Treatment lines were determined based on observed switching patterns between advanced therapies, whereby switch was defined as the introduction of a new agent different from an agent used in the prior line at any time point. Notably, in contrast to the time-dependent primary outcome definition for the switch, switching for sequence analyses was not restricted to a particular time period following the index line.
Statistical analysis
Patient characteristics were summarized using descriptive statistics, including frequency distributions for categorical variables and mean, standard deviations (SD), median, and range [MIN, maximum (MAX)] for continuous variables. To assess the differences between WCS and OCS, we used χ2 tests and t-tests. In addition, to account for differences in baseline characteristics between the two groups, we employed inverse probability of treatment weighting (IPTW). Propensity scores were estimated using a logistic regression model with an indicator of whether the patient was WCS or OCS. Relevant confounders included in the model were based on baseline covariates observed in the 12-month pre-index period (Supplemental Table 4). Common support was enforced and stabilized IPTW weights were trimmed at extreme values of the distribution (1st and 99th percentiles; Supplemental Figure 1). The balance of cohorts before and after weighting was assessed by comparing standardized mean differences (SMD) of baseline characteristics. A SMD threshold of >0.2 (absolute value) was used to indicate imbalance.13,14 In a doubly robust sensitivity approach, outcomes were additionally controlled for all covariates with an absolute SMD cutoff of 0.1. Time-to-event analyses were performed using IPT-weighted Kaplan–Meier and Cox proportional hazard regressions. Statistical analyses were conducted using STATA 17 (StataCorp LLC; College Station, TX). 15
To adjust for primary non-response and secondary loss of response at baseline, real-world proxies based on the timing of TNFi initiation and switch were used in the absence of reasons for discontinuation in claims data. Primary non-response was defined based on a switch within 4 months of TNFi initiation (sensitivity: 3 months) and secondary loss of response if the switch occurred after 4 months. A 4-month threshold was used, as the maximal improvement in patients achieving remission in the induction phase is expected to occur within 14 weeks.16,17 Due to low patient numbers in the primary non-response group, results were not stratified by patients with primary non-response or secondary loss of response across all outcomes.
The reporting of this study conforms with the Strengthening the Reporting of Observational Studies in Epidemiology statement for cohort studies 18 (Supplemental Table 7).
Results
Patient selection
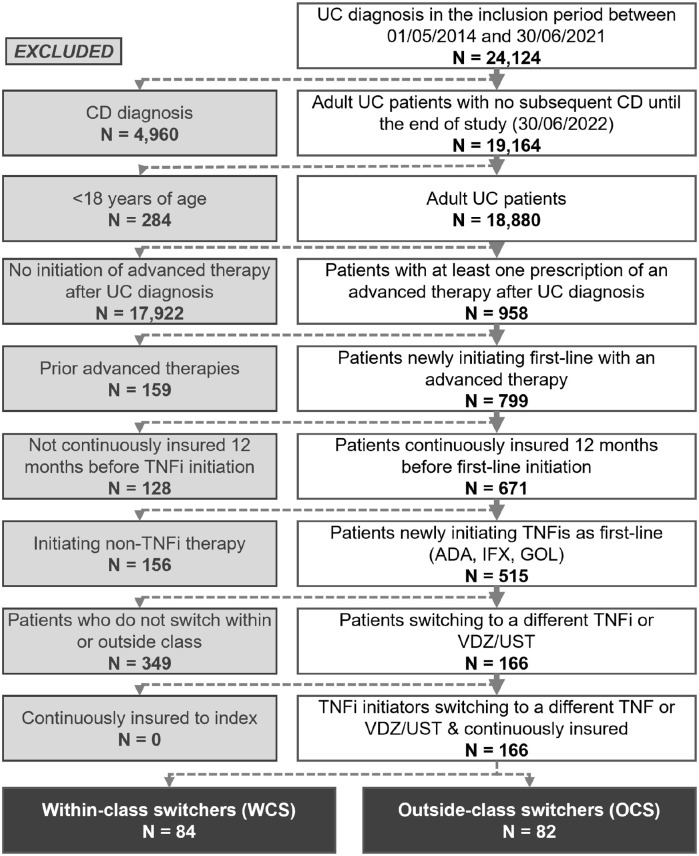
Overall, 18,880 adult patients with UC and no subsequent diagnosis of CD were identified in the AOK PLUS dataset between 1 May 2014 and 30 June 2021 (Figure 2). Among this population, 671 (3.6%) patients were naïve, newly initiating a first-line advanced therapy for UC in the inclusion period and continuously insured for 12 months before the first-line initiation. Among 515 patients who newly initiated first-line therapy with TNFis, 166 comprised the final cohort of patients switching to a second-line biologic after failure on the first-line TNFi. In total, 66 (39.8%), 65 (39.2%), and 35 (21.1%) patients received first-line IFX, ADA, and GOL, respectively.
Figure 2.
Patient attrition chart.
Among 84 WCS (50.6%) who cycled to a different TNFi, 37 (44.0%), 27 (32.1%), and 20 (23.8%) switched to IFX, ADA, and GOL, respectively. In the OCS group (N = 82, 49.4%), 67 (81.7%) patients switched to VDZ, with an additional 15 (18.3%) switching to UST (note that UST approval was granted in September 2019).
Baseline characteristics
In the overall unweighted cohort, patients displayed a mean (SD) age of 42.9 (13.6) years, with 54.8% of WCS and 43.9% of OCS comprising female patients (Table 1). Based on our proxy, 30.7% of patients were primary non-responders (4-month definition; 19.3% based on 3-month definition). Overall, 80.7% of patients received corticosteroid therapy during the baseline period, and 33.1% received immunomodulators. The most frequently observed comorbidities at baseline were iron deficiency anemia (30.7%), followed by hypertension (28.9%) (Supplemental Table 5). Notably, 25.3% of all patients experienced acute infections of the upper respiratory tract (Supplemental Table 5), while 19.9% of patients presented with any cardiovascular disease (CVD) (Table 1). A greater proportion of WCS presented with an index (switch date) in earlier years (2014–2017, 40.5% WCS versus 12.2% OCS), likely reflecting the slower uptake of the first OCS option (VDZ) in clinical practice in Germany (Table 1). In the overall unadjusted cohorts, the mean duration of follow-up was longer for WCS compared to OCS (46.9 versus 35.2 months).
Table 1.
Patient characteristics during the baseline period, stratified by WCS and OCS.
| Characteristic | All (N = 166) | WCS (N = 84) | OCS (N = 82) |
|---|---|---|---|
| Female, n (%) | 82 (49.40) | 46 (54.76) | 36 (43.90) |
| Age at index, years | |||
| Mean (SD) | 42.87 (13.61) | 43.11 (14.87) | 42.63 (12.27) |
| Median (range) | 42 (20–78) | 42 (20–78) | 43 (20–70) |
| CCI | |||
| Mean (SD) | 0.87 (1.57) | 0.98 (1.83) | 0.77 (1.26) |
| Median (range) | 0 (0–9) | 0 (0–9) | 0 (0–6) |
| Comorbidities, n (%) | |||
| Any cancer | 6 (3.61) | 3 (3.57) | 3 (3.66) |
| Colorectal cancer | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Any CVD | 33 (19.88) | 21 (25.00) | 12 (14.63) |
| Diabetes | 19 (11.45) | 10 (11.90) | 9 (10.98) |
| CKD | 8 (4.82) | 2 (2.38) | 6 (7.32) |
| Hypertension | 48 (28.92) | 29 (34.52) | 19 (23.17) |
| Combined history (CVD, diabetes, hypertension, CKD) | 62 (37.35) | 35 (41.67) | 27 (32.93) |
| Therapy use, n (%) | |||
| Antibiotics | 62 (37.35) | 33 (39.29) | 29 (35.37) |
| Corticosteroids | 134 (80.72) | 70 (83.33) | 64 (78.05) |
| 5-ASA and similar agents | 156 (93.98) | 78 (92.86) | 78 (95.12) |
| Immunomodulators | 55 (33.13) | 31 (36.90) | 24 (29.27) |
| UC-related surgery | 10 (6.02) | 5 (5.95) | 5 (6.10) |
| GP visits | |||
| Mean (SD) | 9.07 (7.42) | 9.77 (8.22) | 8.35 (6.48) |
| Median (range) | 7 (0–41) | 8 (0–36) | 7 (0–41) |
| Gastroenterologist visits | |||
| Mean (SD) | 6.27 (6.14) | 5.02 (5.37) | 7.55 (6.62) |
| Median (range) | 6 (0–25) | 4 (0–23) | 8 (0–25) |
| All-cause hospitalizations | |||
| Mean (SD) | 0.96 (1.39) | 1.05 (1.42) | 0.87 (1.35) |
| Median (range) | 0 (0–6) | 1 (0–6) | 0 (0–6) |
| UC-related hospitalizations (main cause) | |||
| Mean (SD) | 0.58 (0.89) | 0.62 (0.83) | 0.54 (0.95) |
| Median (range) | 0 (0–4) | 0 (0–3) | 0 (0–4) |
| Loss of response, n (%) | |||
| Primary non-response (switch ⩽4 months) | 51 (30.72) | 32 (38.10) | 19 (23.17) |
| Primary non-response sensitivity (⩽3 months) | 32 (19.28) | 19 (22.62) | 13 (15.85) |
| Index year, n (%) | |||
| 2014 | 5 (3.01) | 5 (5.95) | 0 (0.00) |
| 2015 | 9 (5.42) | 4 (4.76) | 5 (6.10) |
| 2016 | 13 (7.83) | 11 (13.10) | 2 (2.44) |
| 2017 | 17 (10.24) | 14 (16.67) | 3 (3.66) |
| 2018 | 29 (17.47) | 14 (16.67) | 15 (18.29) |
| 2019 | 28 (16.87) | 14 (16.67) | 14 (17.07) |
| 2020 | 44 (26.51) | 16 (19.05) | 28 (34.15) |
| 2021 | 21 (12.65) | 6 (7.14) | 15 (18.29) |
| Duration of follow-up, months | |||
| Mean (SD) | 41.13 (21.15) | 46.89 (22.01) | 35.23 (18.57) |
| Median (range) | 36.39 (2.33–96.26) | 44.38 (2.33–96.26) | 29.15 (12.82–87.42) |
5-ASA, 5-aminosalicylic acid; CCI, Charlson Comorbidity index; CKD, chronic kidney disease; CVD, cardiovascular diseases; GP, general practitioner; OCS, outside-class switchers; SD, standard deviation; UC, ulcerative colitis; WCS, within-class switchers.
Prior to IPTW, imbalance (defined as absolute SMD threshold >0.2) between WCS and OCS was observed in a number of patients characteristics, including the proportion of female patients (54.8% WCS versus 43.9% OCS, SMD = 0.217, p = 0.162), occurrence of any CVD (25.0% WCS versus 14.6% OCS, SMD = 0.261, p = 0.094), hypertension (34.5% WCS versus 23.2% OCS, SMD = 0.251, p = 0.138), chronic kidney disease (CKD; 2.4% WCS versus 7.3% OCS, SMD = −0.230, p = 0.107), number of gastroenterology visits (5.0 WCS versus 7.6 OCS, SMD = −0.419, p = 0.008), and proportion of patients with primary loss of response (38.1% WCS versus 23.2% OCS, SMD = 0.326, p = 0.037) (Table 2).
Table 2.
Balance of covariates in the 12-month baseline period among unweighted and weighted cohorts after inverse probability of treatment weighting.
| Unweighted | Weighted | |||||||
|---|---|---|---|---|---|---|---|---|
| WCS (N = 84) | OCS (N = 82) | SMD | p Value | WCS | OCS | SMD | p Value | |
| Female (%) | 54.76 | 43.90 | 0.217 | 0.162 | 47.89 | 50.16 | −0.046 | 0.824 |
| Age at index (mean) | 43.11 | 42.63 | 0.035 | 0.824 | 41.96 | 42.68 | −0.052 | 0.806 |
| CCI (mean) | 0.98 | 0.77 | 0.132 | 0.396 | 0.62 | 0.71 | −0.065 | 0.676 |
| Therapy use (%) | ||||||||
| Antibiotics | 39.29 | 35.37 | 0.081 | 0.602 | 34.61 | 31.98 | 0.055 | 0.772 |
| Corticosteroids | 83.33 | 78.05 | 0.133 | 0.388 | 84.01 | 80.89 | 0.078 | 0.662 |
| Concomitant steroid use | 13.10 | 17.07 | −0.111 | 0.474 | 17.57 | 17.75 | 0.065 | 0.830 |
| 5-ASA and similar agent | 92.86 | 95.12 | −0.095 | 0.540 | 94.96 | 91.06 | 0.171 | 0.503 |
| Immunomodulators | 36.90 | 29.27 | 0.162 | 0.296 | 38.23 | 34.33 | 0.083 | 0.706 |
| Surgeries (yes, %) | 5.95 | 6.10 | −0.006 | 0.969 | 4.32 | 5.66 | 0.055 | 0.697 |
| Comorbidities (%) | ||||||||
| Any cancer | 3.57 | 3.66 | −0.005 | 0.976 | 2.05 | 1.80 | 0.015 | 0.902 |
| Any CVD | 25.00 | 14.63 | 0.261 | 0.094 | 20.28 | 20.05 | 0.006 | 0.979 |
| Diabetes | 11.90 | 10.98 | 0.029 | 0.851 | 10.38 | 8.53 | 0.061 | 0.732 |
| Hypertension | 34.52 | 23.17 | 0.251 | 0.138 | 26.85 | 31.18 | −0.097 | 0.643 |
| CKD | 2.38 | 7.32 | −0.230 | 0.107 | 3.10 | 3.46 | −0.018 | 0.901 |
| Healthcare resource use (mean) | ||||||||
| Number of GP visits | 9.77 | 8.35 | 0.192 | 0.219 | 10.06 | 9.10 | 0.127 | 0.642 |
| Number of gastroenterology visits | 5.02 | 7.55 | −0.419 | 0.008 | 6.63 | 5.93 | 0.117 | 0.624 |
| Number of UC-related hospitalizations | 0.62 | 0.54 | 0.092 | 0.552 | 0.60 | 0.57 | 0.024 | 0.914 |
| Primary loss of response (switch ⩽4 months) (%) | 38.10 | 23.17 | 0.326 | 0.037 | 32.74 | 28.80 | −0.086 | 0.676 |
5-ASA, 5-aminosalicylic acid; CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; CVD, cardiovascular diseases; GP, general practitioner; OCS, outside-class switchers; SMD, standardized mean differences; UC, ulcerative colitis; WCS, within-class switchers.
After trimming extreme weights for IPTW, 71 WCS and 76 OCS remained in the final analytical cohort. The cohorts were well balanced, with no statistically significant differences remaining in baseline confounders (Table 2). In a doubly robust approach, covariates with an absolute SMD > 0.1 were additionally controlled for across all outcomes. Specifically, outcomes were controlled for 5-ASA use and similar at baseline, number of GP visits, and number of gastroenterology visits. Following this adjustment, the results across all outcomes remained consistent (data not shown).
Time to discontinuation and second treatment switch
The primary outcomes of this study were time to discontinuation and a second treatment switch. Overall, 74.6% (N = 53) of WCS and 48.7% (N = 37) of OCS discontinued the index therapy. WCS displayed an 81.8% increased likelihood of discontinuing the index therapy compared to OCS [hazard ratio (HR), 1.82, 95% confidence interval (CI), 1.14–2.89, p = 0.012, Figure 3]. WCS displayed a median time to discontinuation of 9.5 months compared to 23.5 months among OCS. The results were robust in sensitivity analyses, in which discontinuation was defined based on an extended 90-day gap definition after the end of drug supply (HR for WCS, 1.88, 95% CI, 1.18–2.99, p = 0.008, Supplemental Figure 2).
Figure 3.
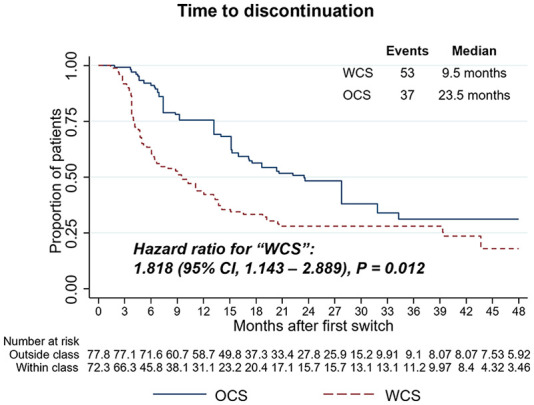
Weighted Kaplan–Meier and Cox proportional hazards models for time to treatment discontinuation (60-day gap definition) comparing WCS and OCS. HRs, 95% CIs, and p values demonstrate statistical differences between cohorts.
CI, confidence interval; HR, hazard ratio; OCS, outside-class switchers; WCS, within-class switchers.
Following initiation of the index therapy, WCS were additionally statistically more likely to re-switch to a different agent (third-line treatment), with an HR for WCS of 3.46 (95% CI, 1.89–6.36, p < 0.001). Overall, 44 (62.0%) WCS patients and 22 (28.9%) OCS patients switched to another therapy according to the switch definition (Figure 4). Among the 44 WCS, 2 (4.6%) switched to ADA, GOL, and IFX each, 3 (6.8%) switched to TOF, 6 (13.6%) switched to UST, and 29 (65.9%) switched to VDZ. Of the 22 OCS, 8 (36.4%) patients switched to TNFis (4 IFX and 4 GOL), with the remaining 63.6% switching the second time to another mode of action (6 UST, 5 TOF, 2 VDZ, and 1 FIL).
Figure 4.
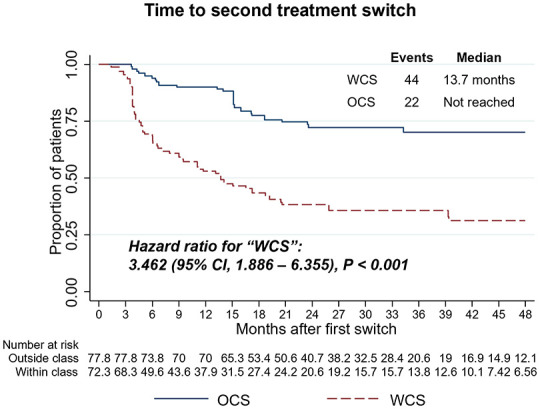
Weighted Kaplan–Meier and Cox proportional hazards models for time to second treatment switch comparing WCS and OCS. HRs, 95% CIs, and p values demonstrate statistical differences between cohorts.
CI, confidence interval; HR, hazard ratio; OCS, outside-class switchers; WCS, within-class switchers.
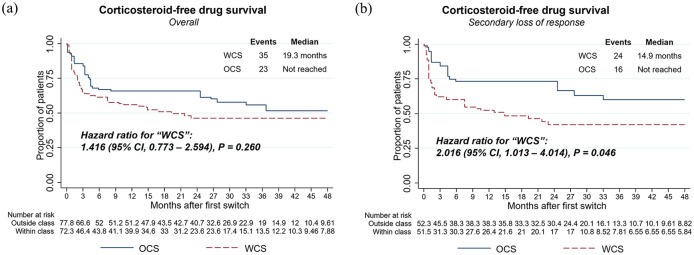
Corticosteroid-free drug survival
WCS was 41.6% more likely to initiate prolonged corticosteroid therapy compared to OCS (HR, 1.42, 95% CI, 0.77–2.59, p = 0.260) but hazards were not significantly different between the cohorts, likely due to a lack of statistical power [Figure 5(a)]. In total, 35 (49.3%) WCS and 23 (30.3%) OCS initiated prolonged corticosteroid therapy. Using sensitivity definitions, whereby prolonged corticosteroid therapy was defined based on the prescription of two or four (main analysis: three) consecutive prescriptions within a 12-month period after the index, we consistently observed an increased tendency for WCS to require CS rescue therapy with an increasing number of CS prescriptions (Supplemental Figure 3). As an additional sensitivity, we further censored patients at their discontinuation or switch; however, the findings remained consistent (data not shown).
Figure 5.
Weighted Kaplan–Meier and Cox proportional hazards models for corticosteroid-free drug survival comparing WCS and OCS among the overall population (a) and the subgroup of patients with secondary loss of response (b) HRs, 95% CIs, and p values demonstrate statistical differences between cohorts.
CI, confidence interval; HR, hazard ratio; OCS, outside-class switchers; WCS, within-class switchers.
Corticosteroid-free drug survival was additionally investigated among patients with secondary loss of response [defined as treatment switch (index) after 4 months of TNFi initiation]. Within this subgroup, we observed statistically significant differences (HR for WCS, 2.02, 95% CI, 1.01–4.01, p = 0.046) in corticosteroid-free drug survival [Figure 5(b)].
Treatment sequences
As explorative information, treatment sequences visualized as Sankey Diagrams were evaluated among the 671 continuously insured patients with UC from Figure 1, who initiated the first line with an advanced therapy (Supplemental Figures 4 and 5). TNFis were the most common agents used as the first-line approach, particularly ADA (39.8%), followed by IFX (24.0%). Among ADA and IFX first-line initiators, VDZ was the most common proceeding treatment approach [15.7% of all ADA initiators, 26.7% of all IFX initiators, Supplemental Figure 5(A) and (B)]. Moreover, following first-line GOL or VDZ therapy, second line with IFX was most commonly observed [23% of all GOL initiators, 10.2% of all VDZ initiators, Supplemental Figure 5(C) and 5(D)].
Discussion
The decision for an effective second-line therapeutic strategy after loss of response to first-line TNFis is challenging in the real world, particularly due to the lack of extensive head-to-head comparative clinical study evidence. To address this evidence gap, we compared real-world outcomes of patients with UC from a large administrative claims dataset in Germany after first-line TNFi exposure, following cycling to a second-line TNFi or switching to a non-TNFi biologic (UST or VDZ).
With the absence of clinical parameters in claims data, our effectiveness endpoints were based on treatment switch, discontinuation (drug survival), and initiation of prolonged corticosteroid therapy (corticosteroid-free drug survival) as indicators of treatment failure. Overall, WCS (TNF-cyclers) were more likely to discontinue therapy (HR, 1.82, 95% CI, 1.14–2.89, p = 0.012), re-switch to another drug (HR, 3.46, 95% CI, 1.89–6.36, p < 0.001), and showed an increased tendency to initiate prolonged corticosteroid rescue therapy (HR, 1.42, 95% CI, 0.77–2.59, p = 0.260). Moreover, we observed significant differences in prolonged corticosteroid therapy initiation among WCS versus OCS with secondary loss of response (HR, 2.02, 95% CI, 1.01–4.01, p = 0.046). The study results remained consistent in unadjusted sensitivity analyses. Treatment switch and discontinuation were defined in accordance with prior real-world definitions, including a preceding study conducted in CD in Germany, whereby results were consistent with the findings of the present study.12,19,20 Given the chronic nature of the disease and limited advanced therapeutic options, clinical effectiveness in the long term without the need for discontinuation or switch is an important aspect of treatment choice in UC. Inadequate therapeutic response, as indicated by discontinuation, switching, or dose adjustments, has been shown to further have a high economic burden and may subsequently affect quality of life.21,22 Moreover, corticosteroid-free drug survival serves as an important treatment goal in UC, and given the association of corticosteroid therapy with various side effects, prolonged use is unsuitable.20,23–26 In this study, initiation of prolonged corticosteroid therapy was defined as at least three consecutive prescriptions of systemic corticosteroids (including oral budesonide) within a period of 12 months in the follow-up. In addition, two and four prescriptions within a 12-month period were tested as a sensitivity analysis. While results were not statistically significant for the overall population, WCS showed an increased tendency to initiate prolonged corticosteroid therapy with an increasing number of prescriptions.
While head-to-head clinical trial evidence is limited, several RWE studies comparing TNFis versus biologics with other modes of action among patients who were TNFi experienced have been conducted. As UST was approved more recently in 2019, the evidence is largely based on VDZ; however, this evidence collectively suggests that cycling to another TNFi is not favorable in the real world and an agent with an alternative mode of action may improve outcomes.27–31 In line with our findings, in a cohort study utilizing clinical records from eight Italian IBD centers, the authors observed that among patients who failed IFX, subsequent failure was higher among patients switching to ADA compared to another mode of action (VDZ), particularly among patients with secondary loss of response (48.0% ADA versus 22.4% VDZ, p = 0.035). Aligning with our observations, discontinuation-free survival was significantly higher in patients receiving VDZ rather than those receiving a second TNFi. 27 Hupe et al. 2020 provided insights into UC patients treated in 12 French centers following failure on a first-line TNFi. The authors saw higher rates of remission at week 14 among patients receiving another biologic mode of action (VDZ) compared to IFX [odds ratio (OR), 1.67, 95% CI, 1.08–2.56; p = 0.02], with significantly higher rates of survival without discontinuation and UC-related events. 28 Our findings are further supported by a recent study using data from the UK IBD BioResource, which showed that among patients with UC (n = 301 evaluable) or CD (n = 2539 evaluable) who failed therapy with TNFis (ADA or IFX) due to delayed loss of response or intolerance showed better outcomes (discontinuation or treatment failure based on the occurrence of bowel surgery or physician documentation) when switching to a non-TNFi biologic as opposed to a second TNFi. 32 Moreover, in the Enroll-ex study, which used electronic medical records of patients with moderate to severe IBD in Kuwait, clinical outcomes were compared between patients initiating UST or VDZ in second-line, following first-line TNFi failure. While both outside-class agents were shown to be effective, the study concluded that higher proportions of patients reaching target outcomes were observed among those receiving UST compared to those receiving VDZ. 33 Notably, a systematic literature review and network meta-analysis of randomized trials of patients with moderately-to-severely active UC, treated with TNFis, VDZ, TOF, or UST as first or second-line showed that among patients with prior TNFi treatment, UST and TOF were ranked highest for inducing clinical remission, with a surface under the cumulative probability of 0.87, above VDZ, and ADA (UST versus VDZ: OR, 5.99; 95% CI, 1.13–31.76 and TOF versus VDZ: OR, 6.18; 95% CI, 1.003–8.00; UST versus ADA: OR, 10.71; 95% CI, 2.01–57.20; and TOF versus ADA: OR, 11.05; 95% CI, 1.79–68.41). 29
In contrast to our findings, Rundquist et al. 34 showed in a Swedish Nationwide register study that there were no statistically significant differences in drug survival among patients with first-line TNFi experience, who initiated VDZ compared to another TNFi (69% VDZ versus 62% TNFi, p = 0.30). While their efficacy endpoint was assessed at 12 months, the study differed in that VDZ was the only outside-class option. These results aligned with the VARSITY, phase IIIb, double-blinded trial subgroup analyses comparing patients receiving VDZ or ADA after prior IFX exposure, whereby results did not suggest the choice of ADA was disadvantaged compared to VDZ; however, as this subgroup comprised of MAX 25% of the overall trial population and given defined selection criteria for trial inclusion, results may not reflect the heterogeneity of patients observed in the real world. 35
Importantly, much of the available evidence does not individually or collectively include a comparison of patients initiating UST after TNFi failure, likely due to the recent approval of UST in UC in 2019. As a result of the approval timeline and data availability, a smaller number of UST patients were selected for inclusion in the OCS group in our study. The results from the systematic literature review and network meta-analysis in UC, 29 real-world studies comparing UST to VDZ in CD patients with and without prior exposure to TNFis, whereby UST use was associated with better effectiveness,36–38 as well as the RUN-CD (Real world effectiveness of Ustekinumab in the induction and maintenance therapy for Crohn’s disease) prospective study comparing TNFis versus UST in bio-naïve and experienced patients, 39 highlight the importance of UST use as an outside-class option for contextualizing the results of our study as well as determining a suitable therapeutic strategy following first-line TNFi failure in IBD. Notably, in the unadjusted overall OCS cohort, the time to discontinuation was 25.0 months for UST users and 27.8 months for VDZ users (Supplemental Figure 6). However, due to a smaller sample size, particularly in the UST group, a formal comparison of outcomes between UST and VDZ as outside-class options could not be made in this study. Further research is needed to understand whether outcomes differ between switching to UST or VDZ, and given a potential difference, which clinical factors may be involved.
Overall, the data presented in this study and supported by literature suggest that TNF-cycling may not be beneficial in the real world compared to switching to a non-TNFi outside-class option. Evidently, there were some limitations inherent to the research design of this study. Propensity score methodology was used to adjust for patient characteristics; however, due to the non-randomized nature of the study, unobserved baseline confounders may still exist, particularly with respect to data availability. Propensity score matching was considered an alternative approach; however, adjustment via IPTW was selected to preserve sample sizes given the limited number of patients included in the analysis. Despite this, effect estimates for primary outcomes remained large and robust to sensitivity definitions. Notably, claims data lack clinical information on disease severity indicators, disease duration, as well as information on dosing or therapeutic drug monitoring (TDM). Moreover, the reasons for discontinuation or switch are not available (e.g. primary non-response, secondary loss of response, injection/infusion reaction, infection, patient/clinician preference). Therefore, this study made use of proxies to define the primary non-response or secondary loss of response, based on the time from TNFi initiation to switch. While this study did not evaluate the concomitant use of immunomodulators in WCS and OCS, it is important to note that based on the German guidelines, IFX in particular is recommended to be combined with thiopurines to suppress antibody development. 7 Additional investigations exploring the effects of TDM, dosing, as well as concomitant use with immunomodulators are recommended to further contextualize the study outcomes beyond treatment sequence. While claims data consist of information from routine clinical care and may exhibit a degree of missing or coding errors, particularly with outpatient diagnoses, the data are regarded as valid and of high quality.40–42 Lastly, discontinuation results could only be based on presumed supply days according to DDD, with no information on exact dosing, including body weight-based dosing, which may have impacted prescription gap assumptions. The outpatient data are based on prescriptions filled, and as such, compliance with medication can only be assumed. As a strength, this study used a large administrative statutory health insurance dataset in Germany to select patients with UC, free of study site selection bias. While the dataset is regional, due to uniform healthcare policies and standard practices across Germany, it is representative nationally, and with full capture of treatment as relevant for reimbursement purposes, reflects the therapeutic approaches in routine clinical practice across all healthcare settings.
Conclusion
The findings of this study suggest that patients in UC who are treated with TNFis in the first-line setting may exhibit better outcomes in clinical practice when switching to biologics with other modes of action such as UST or VDZ, rather than cycling to a second TNFi agent. To shape guidelines and define optimal treatment strategies after loss of response to TNFis, further evidence from clinical trials and the real world is needed.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848241262288 for Switching within versus out of class following first-line TNFi failure in ulcerative colitis: real-world outcomes from a German claims data analysis by Evi Zhuleku, Daniel Wirth, Riikka Nissinen, Ivana Bravatà, Despina Ziavra, Andreas Duva, Jennifer Lee, Andreas Fuchs, Sabrina Mueller, Thomas Wilke and Bernd Bokemeyer in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iDs: Evi Zhuleku  https://orcid.org/0000-0002-5407-019X
https://orcid.org/0000-0002-5407-019X
Thomas Wilke  https://orcid.org/0000-0001-8932-6426
https://orcid.org/0000-0001-8932-6426
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Evi Zhuleku, Cytel Inc., Potsdamer Str. 58, Berlin 10785, Germany.
Daniel Wirth, Janssen-Cilag GmbH, Neuss, Germany.
Riikka Nissinen, Janssen-Cilag Oy, Espoo, Finland.
Ivana Bravatà, Janssen-Cilag S.P.A., Milan, Italy.
Despina Ziavra, Janssen-Cilag S.A.C.I., Pefki, Greece.
Andreas Duva, Janssen-Cilag Ltd, High Wycombe, UK.
Jennifer Lee, Janssen-Cilag A/S Denmark, Birkerod, Denmark.
Andreas Fuchs, AOK PLUS, Dresden, Germany.
Sabrina Mueller, Institut Für Pharmakoökonomie Und Arzneimittellogistik e.V., Wismar, Germany.
Thomas Wilke, Institut Für Pharmakoökonomie Und Arzneimittellogistik e.V., Wismar, Germany.
Bernd Bokemeyer, Interdisciplinary Crohn Colitis Centre Minden, Minden, Germany.
Declarations
Ethics approval and consent to participate: This study was a non-interventional and retrospective study, using fully anonymized data. As such, ethics approval and patient informed consent were not required in accordance with the local laws and policies of the participating institutions.
Consent for publication: Not applicable.
Author contributions: Evi Zhuleku: Conceptualization; Formal analysis; Methodology; Project administration; Visualization; Writing – original draft; Writing – review & editing.
Daniel Wirth: Conceptualization; Methodology; Project administration; Supervision; Writing – review & editing.
Riikka Nissinen: Conceptualization; Methodology; Project administration; Supervision; Writing – review & editing.
Ivana Bravatà: Conceptualization; Methodology; Supervision; Writing – review & editing.
Despina Ziavra: Conceptualization; Methodology; Supervision; Writing – review & editing.
Andreas Duva: Conceptualization; Methodology; Supervision; Writing – review & editing.
Jennifer Lee: Conceptualization; Methodology; Supervision; Writing – review & editing.
Andreas Fuchs: Data curation; Methodology; Resources; Writing – review & editing.
Sabrina Mueller: Conceptualization; Methodology; Supervision; Validation; Writing – review & editing.
Thomas Wilke: Conceptualization; Methodology; Supervision; Writing – review & editing.
Bernd Bokemeyer: Conceptualization; Methodology; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Janssen Pharmaceutica NV.
DW, RN, IB, DZ, AD, and JL are employees of Janssen-Cilag. EZ is an employee of Cytel Inc., whose work was funded by Janssen-Cilag. AF participated in this study as part of AOK PLUS. SM and TW are staff members of IPAM. TW has received honoraria from several pharmaceutical/consultancy firms, for example, NovoNordisk, Abbvie, Merck, GSK, BMS, LEO Pharma, Bayer, and Boehringer Ingelheim. BB has received consultancy fees and grants from several pharmaceutical companies, for example, AbbVie, MSD, Ferring, Falk, Takeda, Shield Therapeutics, Pfizer, Biogen, BMS, Janssen, Celltrion, and Galapagos.
Availability of data and materials: Data supporting this study’s findings are not publicly available due to privacy and ethical restrictions related to the use of patient-individual data.
References
- 1. Dignass A, Preiss JC, Aust DE, et al. [Updated German guideline on diagnosis and treatment of ulcerative colitis, 2011]. Z Gastroenterol 2011; 49: 1276–1341. [DOI] [PubMed] [Google Scholar]
- 2. Manthey CF, Reher D, Huber S. [What is confirmed in the treatment of chronic inflammatory bowel diseases]. Internist (Berl) 2021; 62: 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geiss T, Schaefert RM, Berens S, et al. Risk of depression in patients with inflammatory bowel disease. J Dig Dis 2018; 19: 456–467. [DOI] [PubMed] [Google Scholar]
- 4. Viganò CA, Beltrami MM, Bosi MF, et al. Alexithymia and psychopathology in patients suffering from inflammatory bowel disease: arising differences and correlations to tailoring therapeutic strategies. Front Psychiatry 2018; 9: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis 2022; 16: 2–17. [DOI] [PubMed] [Google Scholar]
- 6. Straatmijer T, Biemans VBC, Visschedijk M, et al. Superior effectiveness of tofacitinib compared to vedolizumab in anti-TNF-experienced ulcerative colitis patients: a Nationwide Dutch Registry Study. Clin Gastroenterol Hepatol 2023; 21: 182–191.e2. [DOI] [PubMed] [Google Scholar]
- 7. Kucharzik T, Dignass AU, Atreya R, et al. Updated S3 guideline for ulcerative colitis (version 6.1), Zeitschrift fur Gastroenterologie 2023; 61: 1046–1134. [DOI] [PubMed] [Google Scholar]
- 8. Laredo V, Gargallo-Puyuelo CJ, Gomollón F. How to choose the biologic therapy in a bio-naïve patient with inflammatory bowel disease. J Clin Med 2022; 11: 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016; 7: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fine S, Papamichael K, Cheifetz AS. Etiology and management of lack or loss of response to anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2019; 15: 656–665. [PMC free article] [PubMed] [Google Scholar]
- 11. Macaluso FS, Maida M, Grova M, et al. Head-to-head comparison of biological drugs for inflammatory bowel disease: from randomized controlled trials to real-world experience. Therap Adv Gastroenterol 2021; 14: 17562848211010668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhuleku E, Antolin-Fontes B, Borsi A, et al. Real-world outcomes associated with switching to anti-TNFs versus other biologics in Crohn’s disease patients: a retrospective analysis using German claims data. Therap Adv Gastroenterol 2022; 15: 17562848221130554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 14. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28: 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. StataCorp. Stata statistical software: release 17. College Station, TX: StataCorp LLC, 2021. [Google Scholar]
- 16. Marsal J, Barreiro-de Acosta M, Blumenstein I, et al. Management of non-response and loss of response to anti-tumor necrosis factor therapy in inflammatory bowel disease. Front Med (Lausanne) 2022; 9: 897936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vasudevan A, Gibson PR, van Langenberg DR. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: what should the clinician expect, what should patients be told? World J Gastroenterol 2017; 23: 6385–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 19. Yokoyama K, Yamazaki K, Katafuchi M, et al. A Retrospective Claims Database Study on drug utilization in Japanese patients with Crohn’s disease treated with Adalimumab or Infliximab. Adv Ther 2016; 33: 1947–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubin DT, Mody R, Davis KL, et al. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn’s disease. Aliment Pharmacol Ther 2014; 39: 1143–1155. [DOI] [PubMed] [Google Scholar]
- 21. Bokemeyer B, Picker N, Wilke T, et al. Inadequate response, treatment patterns, health care utilization, and associated costs in patients with ulcerative colitis: Retrospective Cohort Study based on German Claims Data. Inflamm Bowel Dis 2022; 28: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Armuzzi A, Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Dig Liver Dis 2021; 53: 803–808. [DOI] [PubMed] [Google Scholar]
- 23. Cross RK. Safety considerations with the use of corticosteroids and biologic therapies in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis 2017; 23: 1689–1701. [DOI] [PubMed] [Google Scholar]
- 24. Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One 2016; 11: e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin DT, Patel H, Shi S, et al. Assessment of corticosteroid-related quality of care measures for ulcerative colitis and Crohn’s disease in the United States: a claims data analysis. Curr Med Res Opin 2017; 33: 529–536. [DOI] [PubMed] [Google Scholar]
- 26. Loftus EV, Jr, Sands BE, Colombel JF, et al. Sustained corticosteroid-free clinical remission during vedolizumab maintenance therapy in patients with ulcerative colitis on stable concomitant corticosteroids during induction therapy: a post hoc analysis of GEMINI 1. Clin Exp Gastroenterol 2020; 13: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Favale A, Onali S, Caprioli F, et al. Comparative efficacy of Vedolizumab and Adalimumab in ulcerative colitis patients previously treated with Infliximab. Inflamm Bowel Dis 2019; 25: 1805–1812. [DOI] [PubMed] [Google Scholar]
- 28. Hupe M, Riviere P, Nancey S, et al. Comparative efficacy and safety of Vedolizumab and Infliximab in ulcerative colitis after failure of a first subcutaneous anti-TNF agent: a multicentre cohort study. Aliment Pharmacol Ther 2020; 51: 852–860. [DOI] [PubMed] [Google Scholar]
- 29. Singh S, Murad MH, Fumery M, et al. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol 2020; 18: 2179–2191.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lukin D, Faleck D, Xu R, et al. Comparative safety and effectiveness of Vedolizumab to tumor necrosis factor antagonist therapy for ulcerative colitis. Clin Gastroenterol Hepatol 2022; 20: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allamneni C, Venkata K, Yun H, et al. Comparative effectiveness of Vedolizumab vs. Infliximab induction therapy in ulcerative colitis: experience of a real-world cohort at a tertiary inflammatory bowel disease center. Gastroenterol Res 2018; 11: 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kapizioni C, Desoki R, Lam D, et al. Biologic therapy for inflammatory bowel disease: real-world comparative effectiveness and impact of drug sequencing in 13,222 patients within the UK IBD BioResource. J Crohns Colitis 2024; 18: 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alrashed F, Abdullah I, Alfadhli A, et al. Effectiveness of Vedolizumab and Ustekinumab as second biologic agent in achieving target outcomes in tumor necrosis factor antagonists experienced patients with inflammatory bowel disease (enroll-ex study). Front Pharmacol 2023; 14: 1243080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rundquist S, Sachs MC, Eriksson C, et al. Drug survival of anti-TNF agents compared with Vedolizumab as a second-line biological treatment in inflammatory bowel disease: results from nationwide Swedish registers. Aliment Pharmacol Ther 2021; 53: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sands BE, Peyrin-Biroulet L, Loftus EV, Jr, et al. Vedolizumab versus Adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019; 381: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 36. Alric H, Amiot A, Kirchgesner J, et al. The effectiveness of either Ustekinumab or Vedolizumab in 239 patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther 2020; 51: 948–957. [DOI] [PubMed] [Google Scholar]
- 37. Townsend T, Razanskaite V, Dodd S, et al. Comparative effectiveness of Ustekinumab or Vedolizumab after 1 year in 130 patients with anti-TNF-refractory Crohn’s disease. Aliment Pharmacol Ther 2020; 52: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 38. Biemans VBC, van der Woude CJ, Dijkstra G, et al. Ustekinumab is associated with superior effectiveness outcomes compared to Vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther 2020; 52: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bokemeyer B, Plachta-Danielzik S, Deppe H, et al. OP36 real-world evidence on comparative effectiveness of Ustekinumab vs anti-TNF in Crohn’s disease with propensity score adjustment: two-year maintenance phase results from the prospective observational RUN-CD study. J Crohns Colitis 2023; 17(Suppl_1): i50-i. [DOI] [PubMed] [Google Scholar]
- 40. Andersohn F, Garbe E. [Pharmacoepidemiological research with large health databases]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz; 2008; 51: 1135–1144. [DOI] [PubMed] [Google Scholar]
- 41. Hoffmann F. Review on use of German health insurance medication claims data for epidemiological research. Pharmacoepidemiol Drug Saf 2009; 18: 349–356. [DOI] [PubMed] [Google Scholar]
- 42. Schubert I, Koster I, Kupper-Nybelen J, et al. [Health services research based on routine data generated by the SHI. Potential uses of health insurance fund data in health services research]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2008; 51: 1095–1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848241262288 for Switching within versus out of class following first-line TNFi failure in ulcerative colitis: real-world outcomes from a German claims data analysis by Evi Zhuleku, Daniel Wirth, Riikka Nissinen, Ivana Bravatà, Despina Ziavra, Andreas Duva, Jennifer Lee, Andreas Fuchs, Sabrina Mueller, Thomas Wilke and Bernd Bokemeyer in Therapeutic Advances in Gastroenterology