Abstract
Introduction
Low back and neck pain are common musculoskeletal disorders with multiple treatment options. India’s traditional medical systems, known as Ayush (Ayurveda, Yoga and Naturopathy, Unani, Siddha, Sowa-Rigpa and Homoeopathy) offer range of interventions and are widely used. In view of limited documentation of adverse events following Ayush interventions for lumbar and cervical spondylosis, we synthesized evidence and estimated proportion of studies reporting adverse events.
Methods
We systematically searched all published documents from biomedical and multidisciplinary abstract and citation databases and Ayush-specific repositories from their inception to April 2021. We selected studies as per inclusion criteria and extracted information, adhering to PRISMA guidelines. We systematically reviewed the qualitative evidence form the selected studies.
Results
Majority (94%) of the selected 113 studies were interventional studies and included 77 (68.1%) journal articles and 35 (31%) academic dissertations. Among the Ayush systems, considerable proportion was from Ayurveda (32.7%), followed by Siddha (24.8%), Yoga (22.1%), Unani (15.9%) and Homoeopathy (4.4%). Almost three-fourths of the studies were on lumbar spondylosis (65%; n = 74), followed by cervical spondylosis (31%; n = 35), and the remaining four included both. Thirteen percent of the 113 studies described adverse events [Yoga = 9.7%; Unani = 1.8% and Homoeopathy = 1.8%]. More adverse events were reported among the studies on lumbar (9.7%) than cervical spondylosis (2.7%). The nature of interventions were non-pharmacological (10.6%; n = 12), pharmacological (n = 2; 1.8%) or combined (n = 1; 0.9%).
Conclusions
Only one in eight studies reported any adverse event following Ayush interventions for cervical and lumbar spondylosis. There could be certain degree of underreporting of adverse events and requires further exploration.
PROSPERO Registration ID CRD42020167433.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-01985-3.
Keywords: Adverse reactions, Adverse events, Ayurveda, Yoga and Naturopathy, Unani, Siddha, Homoeopathy, Musculoskeletal disorders
Introduction
In India, other than the conventional system or biomedicine, traditional medicines are widely used [1]. These are defined as “Traditional and Non-conventional systems of health care and healing, which include Ayurveda, Yoga and Naturopathy, Unani, Siddha, Sowa-Rigpa and Homoeopathy (referred by the term: Ayush), etc.” As reported by India’s Ministry of Health and Family Welfare, of the registered doctors, 1.2 million are from the conventional system, and 0.78 million are from the Ayush systems [1]. The utilization of Ayush system is higher among patients with chronic diseases such as skin-related and musculoskeletal ailments [2]. Low back and neck pain are prominent among all musculoskeletal disorders as per the disability burden of India. At the national level, the mean percentage change increase in Disability Adjusted Life Years from 1990 to 2016 for low back and neck pain is 66% [3]. Low back and neck pain has moved from 18th to ninth position in the leading causes of death and disability from 1990 to 2016 [3]. These two conditions are common, and many treatment options are available other than the conventional system, including Ayush, [4, 5]. The use of Ayush treatment modalities for neck and back pain is common due to its easy availability and accessibility [6].
Ayush formulations largely use plants as raw materials. Apart from the plants, resources from metals, minerals, marine and animal origin are also used for the preparation of Ayush system formulations. However, the quality issues and safety concerns of Ayurveda, Siddha, Unani, and Homoeopathy drugs have been raised from various sources [1, 7, 8]. The Ayush medications are considered to be natural molecules and are claimed to produce effects without any adverse events (AE) [9]. In addition to such pharmacological interventions, Ayush systems provide non-pharmacological interventions as an add-on or standalone modality. However, it is indispensable to document adverse events and adverse drug events (ADE) of the Ayush system’s pharmacological and non-pharmacological interventions.
ADE is defined as “Any untoward medical occurrence that may present during treatment with a pharmaceutical product, but that does not necessarily have a causal relationship with this treatment” [10]. On the other hand, AE is defined as a “Medical occurrence temporally associated with the use of a medicinal product, but not necessarily causally related” [11]. AE is a relatively broader term covering the problems from both pharmacological and non-pharmacological interventions. Detection, documentation, and reporting of AE and ADE are fundamental to pharmacovigilance activities. It is the science of assessing and monitoring the risk/benefit profiles of medications [12, 13]. In the published literature, systematic reviews of such events are available for surgical procedures, drug interventional, and manipulative therapy in the conventional systems of medicine for lumbar and cervical spondylosis [14]. However, there is no systematic review on AE of Ayush interventions for spondylosis. In this context, we aimed to synthesize evidence from the AE attributable to Ayush-based pharmacological and non-pharmacological interventions for lumbar and cervical spondylosis and compare AEs within the Ayush systems of medicine and among those two conditions.
Methods
Screening and study selection
We conducted this systematic review in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The protocol was registered with the international prospective register of systematic reviews (PROSPERO ID: CRD42020167433) [15] and is published elsewhere [16].
We identified the key terms based on the population, intervention, outcome and Ayush-specific terms for cervical and lumbar spondylosis (Supp Table 4). We systematically searched using key terms in PubMed, Embase and Scopus. We searched Ayush-specific platforms such as Ayush research portal, Digital Helpline for Ayurveda Research Articles (DHARA), and Shodhganga, a postgraduate thesis repository. We also checked for the reports and published documents of India’s National Pharmacovigilance Co-ordination Centres (NPvCC), Intermediary Pharmacovigilance Co-ordination Centres (IPvCCs) and Peripheral Pharmacovigilance Co-ordination Centres (PPvCC) of Ayush. Search terms used for different databases and the number of results are given in Supplementary Table 5. We included the articles only in English language and published upto April 2021. According to the inclusion criteria (Supp Table 3), search results were screened for eligibility based on the PICOS strategy. Population (cervical or lumbar spondylosis), Interventions (Ayush-Ayurveda/Yoga and Naturopathy/Unani/Siddha/Homoeopathy), no comparator was considered, outcome (AE), Study design (all types of study designs). We have not considered the different duration of treatments required under each Ayush system of medicines in the present review. Selected studies from the biomedical databases multidisciplinary abstract and citation databases (Pubmed, Embase, Scopus and Google Scholar) were compiled in Zotero reference management software [17] and imported into the Rayyan web application [18] for the title and abstract screening.
Inclusion criteria:
Cervical and lumbar spondylosis patients of any age, irrespective of gender and geographical region.
Ayush interventions (Ayurveda, Yoga and Naturopathy, Unani, Siddha, Sowa-Rigpa, and Homoeopathy) including both pharmacological and non-pharmacological interventions.
Any type of adverse events (AE) attributable to Ayush medications and procedures.
Randomized and non-randomized controlled trials, open clinical trials, case–control studies, cross-sectional studies, quasi-experimental studies, prospective and retrospective cohort studies, case series, and case studies, qualitative studies, dissertation and thesis.
Exclusion criteria:
Musculoskeletal disorders other than cervical and lumbar spondylosis.
Other than the Ayush system of medicine such as Chinese, conventional, massage, physiotherapy, etc.
Ayush studies those don’t have AE information.
Systematic review and meta-analysis, animal studies, books, protocols, and studies with incomplete information.
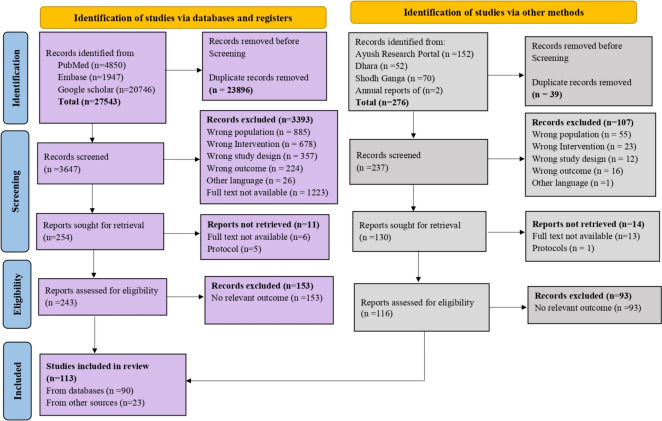
Studies from other Ayush-specific repositories and websites were compiled in Microsoft Excel® before uploading to Rayyan. The authors (ER, JS) reviewed independently and screened all the titles and abstracts for their eligibility to include for the full-text review. Two independent reviewers reviewed the full text of selected articles (ER, JS). During the full-text screening, the reasons for exclusion were recorded and reported (Fig. 1).
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart of selection of studies
Data extraction and management
We used a data extraction form in Microsoft Excel 2016® to collect the necessary information as per the objectives. The form included characteristics on participant (cervical, lumbar spondylosis, age, gender, etc.), study (study design, presence of comparison group, etc.), interventions (type of Ayush system, type of intervention—pharmacological/non-pharmacological, duration of treatment, dose, route of administration, etc.) and outcomes (adverse events, adverse effect, serious adverse events, adverse drug reactions, side effects, etc.). We also recorded the year of publication, publication type, author details, status of the registration of the clinical trials, obtaining informed consent from the participants, and type of blinding for randomized controlled trials. One of the reviewers (JS) extracted the reported AE, and the second reviewer (ER) cross-verified the records. Any disagreement between the reviewers is resolved with the involvement of the third reviewer (BSB) through discussion. The information was then utilized for analysis.
Risk-of-bias assessment
The risk of bias in the selected articles was assessed using a revised Cochrane risk of bias tool (RoB-2 tool) for randomized trials, ROBINS-I tool for non-randomized studies and JBI’s (formerly known as Joanna Briggs Institute) critical appraisal tool for case studies and case series. Judgement on the risk of bias was determined by the signalling questions with responses as ‘yes’, ‘probably yes’, ‘probably no’, ‘no’ and ‘no information’. Two reviewers independently assessed the risk of bias; a consensus was reached through discussion for any disagreement. However, a third reviewer’s opinion was obtained wherever necessary. The overall risk of bias was ascertained as high, some concerns or low for RCT; serious, moderate, low for non-RCT; low, moderate or high risk for case study and case series.
Data synthesis
A qualitative synthesis of the included articles was done based on the population, intervention and outcome. The proportion of AE was summarized based on the study condition (cervical, lumbar), type of Ayush system, study group (treatment, comparison group) and the nature of intervention (pharmacological, non-pharmacological). Ayush interventions used for cervical and lumbar spondylosis were categorized based on the presence of a comparison group. The articles which had reported AE are presented with the number of participants who had experienced AE, description of AE, the total sample size of the study and the type of Ayush system as intervention. Proportion of AE is reported based on the studies that reported AE among all the studies. They were characterized based on the information of research mandates such as registration with Clinical Trials Registry, Approval from Human ethics committee and the information on informed consent process.
Ethics approval
Ethics committee review is exempted for conducting systematic reviews as per India’s National ethical guidelines for biomedical and health research involving human participants [19].
Results
We retrieved 27819 articles through an initial search from databases and other portals. We excluded duplicate articles during the screening. In the course of title and abstract screening, we excluded the articles based on the selection criteria. After the full article screening, we included 113 studies for the final analysis and qualitative synthesis, as per the PRISMA flowchart (Fig. 1).
General characteristics of included studies
General characteristics of the included articles such as the year of publication, study population, study design, type of intervention, size of the study sample and basic information on AE type are presented (Table 1). Publication of Ayush studies on cervical and lumbar spondylosis (CS and LS) was from 1976 to 2021. The number of publication is very less from 1976 to 2005. It gradually increased from 2006 and reached a peak in 2012. With a sudden dip in 2014 and an increase in 2015, followed by a reduction after 2019 (Supp Fig. 2). Majority (95%) of the selected 113 studies were interventional studies and included 77 (68.1%) journal articles and 35 (31%) academic dissertations. Among the interventional studies, 52% (n = 59) were RCT, and remaining (43%) were non-RCT studies. Observational studies included, single case series (0.9%) and five (4.4%) case reports. Majority (82%) of the studies were conducted in India. Among the Ayush systems, 32.7% was from Ayurveda, followed by Yoga (22.1%), Siddha (24.8%), Unani (15.9%) and Homoeopathy (4.4%). Almost three-fourths of the studies were on lumbar spondylosis [65%; n = 74], followed by cervical spondylosis (31%; n = 35), and the remaining four included both. Interventional studies included higher number of study participants (range 10–313) than the observational studies (range = 1 and 10). Most of the studies (84.1%) include both gender. About 45 (39.8%) studies used one intervention, 27 (23.9%) two, 11(9.7%) three, 12 (10.6%) four and 18 (15.9%) used more than four interventions for either cervical or lumbar spondylosis (Table 2).
Table 1.
Characteristics of selected articles (n = 113)
| Author, year | Type of publication | Country | Population | Study design | Ayush system | Intervention | Sample size | Age (Mean ± SD/range) | Other medications | Facility/to report AE | AE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Groessl, [23] | JA | USA | LS | RCT | Y | Non-pharma | 152 | 53.4 ± 13.3 | Yes | Yes | Yes |
| Michalsen, [20] | JA | Germany | CS | RCT | Y | Non-pharma | 77 | 18–60 years | NM | Yes | Yes |
| Saper, [28] | JA | USA | LS | RCT | Y | Non-pharma | 30 | 44.0 ± 12.0 | Yes | Yes | Yes |
| Zarekar, [34] | JA | India | LS | RCT | A | Combined | 50 | < 35 years | NM | NM | No |
| Cramer, [21] | JA | Germany | CS | RCT | Y | Non-pharma | 51 | 47.8 ± 10.4 | Yes | NM | Yes |
| Yadaiah, [35] | JA | India | LS | RCT | A | Pharma | 124 | 10–89 years | NM | NM | No |
| Williams, [22] | JA | USA | LS | RCT | Y | Non-pharma | 90 | 48.0 ± 1.2 | Yes | NM | Yes |
| Colgrove, [36] | JA | USA | LS | QET | Y | Non-pharma | 10 | 45.6 ± 14.6 | NM | Yes | No |
| Haldavnekar, [37] | JA | India | LS | QET | Y | Non-pharma | 40 | 44.1 ± 13.3 | NM | NM | No |
| Joshi, [38] | JA | India | LS | OPRT | A | Combined | 32 | 40–70 years | NM | NM | No |
| Williams, [25] | JA | USA | LS | RCT | Y | Non-pharma | 60 | 23–67 years | Yes | NM | Yes |
| Kumar, [39] | JA | Germany | LS | RCT | A | Non-pharma | 64 | 18–70 years | NM | Yes | No |
| Hanan, [40] | JA | Saudi Arabia | LS | QET | U | Non-pharma | 30 | 35.6 ± 8.8 | NM | NM | No |
| Nair, [41] | JA | India | LS | RCT | A | Pharma | 40 | 11–70 years | NM | NM | No |
| Nair, [42] | JA | India | LS | RCT | A | Pharma | 68 | 17–70 years | NM | NM | No |
| Singh, [43] | JA | India | LS | RCT | U | Combined | 40 | 20–60 years | NM | Yes | No |
| Mahanta, [44] | AD | India | LS | Clinical study | A | Combined | 77 | 30–70 years | NM | NM | No |
| Sherman, [27] | JA | America | LS | RCT | Y | Non-pharma | 228 | 48.4 ± 9.8 | Yes | Yes | Yes |
| Rae, [45] | JA | USA | LS | RCT | Y | Non-pharma | 20 | 18–89 years | Yes | NM | No |
| Saper, [28] | JA | USA | LS | RNT | Y | Non-pharma | 320 | 18–64 years | Yes | Yes | Yes |
| Tilbrook, [46] | JA | UK | LS | RCT (parallel group) | Y | Non-pharma | 313 | 18–65 years | Yes | Yes | Yes |
| Stam, [30] | JA | England | LS | RCT | H | Pharma | 161 | 18–65 years | NM | NM | Yes |
| Jadhav, [47] | JA | India | Both | RCT | A | Pharma | 100 | 21–65 years | NM | NM | No |
| Ansari, [48] | JA | India | LS | RCT | U | Combined | 60 | 25–50 years | NM | NM | Yes |
| Aafreen, [49] | JA | India | LS | Controlled CT | U | Pharma | 42 | 37.8 ± 9.3 | NM | Yes | No |
| Lari, [50] | JA | India | LS | RCT | U | Combined | 60 | 20–60 years | NM | NM | No |
| Tekur, [51] | JA | India | LS | RCT | Y | Non-pharma | 80 | 18–60 years | NM | NM | No |
| Smeeta, [52] | JA | India | CS | RCT | A | Combined | 30 | 35–45 years | NM | NM | No |
| Tekur, [53] | JA | India | LS | RCT | Y | Non-pharma | 80 | 18–60 years | NM | NM | No |
| Neyaz, [29] | JA | India | LS | RCT | Y | Non-pharma | 70 | 18–55 years | Yes | NM | Yes |
| Michalsen, [26] | JA | Germany | LS | RCT | Y | Non-pharma | 68 | 18–75 years | Yes | Yes | Yes |
| Monro, [54] | JA | India | LS | RCT | Y | Combined | 61 | 20–45 years | Yes | NM | No |
| Sharma, [55] | JA | India | LS | RCT | A | Pharma | 60 | 36–45 years | NM | NM | No |
| Tausif, [56] | JA | India | CS | RCT | U | Non-pharma | 50 | 46.6 ± 8.9 | NM | NM | No |
| Deepak, [57] | JA | India | LS | RCT | A | Pharma | 40 | 20–60 years | NM | NM | No |
| Morone, [58] | JA | USA | LS | RCT | Y | Non-pharma | 40 | > 65 years | Yes | NM | No |
| Sharma, [59] | JA | India | LS | RCT | A | Pharma | 30 | 20–70 years | NM | NM | No |
| BethHighland, [60] | JA | USA | LS | Pilot RCT | Y | Combined | 68 | 44.3 ± 12.7 | Yes | NM | No |
| Hepburn, [61] | AD | South Africa | CS | CCT | H | Pharma | 50 | 36.1 years | Yes | NM | Yes |
| Kumar, [62] | JA | India | LS | Open randomized Parallel group trial | A | Combined | 38 | 25–65 years | NM | NM | No |
| Sheeraz, [63] | JA | India | LS | OPRT | U | Non-pharma | 40 | 31–70 years | No | NM | No |
| Sawarkar, [64] | JA | India | CS | PRT | A | Pharma | 30 | 20–60 years | Yes | NM | No |
| Kumari, [65] | JA | India | LS | RCT | A | Pharma | 34 | 20–60 years | NM | NM | No |
| Nandini, [66] | JA | India | CS | PRT | Y | Combined | 60 | 24–56 years | NM | NM | No |
| Telles, [67] | JA | India | LS | PRT | Y | Non-pharma | 40 | 20–45 years | Yes | Yes | No |
| Baig, [68] | JA | India | CS | RCT | U | Combined | 33 | 21–60 years | NM | NM | No |
| Mungara, [69] | JA | India | LS | RCT | A | Pharma | 45 | 20–60 years | NM | NM | No |
| Suman, [70] | JA | India | LS | RCT | A | Pharma | 30 | 20–60 years | NM | NM | No |
| Subbuthai, [71] | AD | India | LS | Open labelled RCT | U | Pharma | 40 | 20–60 years | NM | Yes | No |
| Rai, [72] | JA | India | LS | RCT | A | Combined | 30 | 40–70 years | NM | NM | No |
| Sharma, [73] | JA | India | LS | RCT | A | Pharma | 30 | 18–50 years | NM | NM | No |
| Evangeline, [74] | AD | India | CS | RCT | S | Pharma | 40 | 20–60 years | No | Yes | No |
| Gupta, [75] | AD | India | LS | RCT | A | Pharma | 108 | 18–60 years | NM | Yes | No |
| Brintha, [76] | AD | India | LS | Prospective RCT | S | Pharma | 40 | 31–60 years | NM | Yes | No |
| Lubana, [77] | AD | India | CS | Prospective RCT | S | Pharma | 40 | 20–60 years | NM | Yes | No |
| Prasad, [78] | AD | India | CS | RCT | A | Combined | 40 | 20–69 years | NM | Yes | No |
| Tekur, Padmini [79] | AD | India | LS | Randomized Crossover controlled study | Y | Non-pharma | 80 | 18–60 years | NM | Yes | No |
| Dunleavy, [80] | JA | USA | CS | Quasi-randomized parallel controlled study | Y | Non-pharma | 88 | 55.6 ± 9.0 | Yes | NM | No |
| Kendre, [81] | AD | India | LS | RCT | A | Pharma | 200 | > 60 years | NM | NM | No |
| Niranjana, [82] | AD | India | CS | Non-RCT | S | Combined | 40 | 25–70 years | NM | Yes | No |
| Kalaivani, [83] | AD | India | CS | CT | S | Pharma | 40 | 20–60 years | NM | Yes | No |
| Rajanandhini, [84] | AD | India | CS | CT | S | Combined | 60 | 20–60 years | NM | Yes | No |
| Prakash, [85] | AD | India | LS | CT | S | Pharma | 40 | 30–60 years | NM | Yes | No |
| Sathyakala, [86] | AD | India | CS | CT | S | Combined | 40 | 21–60 years | NM | Yes | No |
| Prathiba, [87] | AD | India | CS | CT | S | Pharma | 60 | 21–60 years | NM | Yes | No |
| Pasupathy, [88] | AD | India | CS | Non-RCT | S | Combined | 40 | 30–60 years | NM | NM | No |
| Jeyabharathi, [89] | AD | India | CS | CT | S | Combined | 40 | 31–70 years | NM | NM | No |
| Venkatachalam, [90] | AD | India | CS | CT | S | Combined | 40 | 31-above 70 years | NM | NM | No |
| Elakkia, [91] | AD | India | CS | CT | S | Combined | 40 | 21–60 years | NM | Yes | No |
| Devi, [92] | AD | India | CS | CT | S | Combined | 40 | 21–60 years | NM | Yes | No |
| Malarvizhi, [93] | AD | India | LS | CT | S | Combined | 40 | 21–60 years | NM | Yes | No |
| Devi, [94] | AD | India | LS | CT | S | Pharma | 40 | 20–60 years | NM | Yes | No |
| Manju, [95] | AD | India | LS | CT | S | Pharma | 40 | 21– > 60 years | NM | NM | No |
| Shalini, [96] | JA | India | CS | Open label trial | A | Pharma | 10 | 18–70 years | NM | NM | No |
| Pradhan, [97] | JA | India | LS | CT | A | Pharma | 60 | < 20- > 60 years | NM | NM | No |
| Jain, [98] | JA | India | CS | OS | Y | Non-pharma | 44 | 45.6 ± 8.3 | NM | NM | No |
| Devi, [99] | AD | India | CS | Open CT | S | Pharma | 40 | 21–50 years | NM | Yes | No |
| Mallinath, [100] | AD | India | LS | CT | A | Pharma | 100 | 20–60 years | NM | NM | No |
| Deebiga, [101] | JA | India | LS | Case study | S | Pharma | 1 | 44 years | NM | NM | No |
| Mishra, [102] | JA | India | LS | Case controlled CT | A | Pharma | 30 | 20–60 years | NM | NM | No |
| Prakash, [103] | JA | India | LS | Case study | A | Pharma | 1 | 49 years | NM | NM | No |
| Kumari, [104] | JA | India | LS | Case study | A | Combined | 1 | 24 years | NM | NM | No |
| Siddiqui, [105] | JA | India | CS | OCS | U | Non-pharma | 30 | 20–60 years | NM | NM | No |
| Ansari, [31] | JA | India | LS | Case study | U | Combined | 1 | 23 years | No | NM | No |
| Rao, [106] | JA | India | Both | Clinical study | A | Pharma | 40 | 20–70 years | NM | NM | No |
| Sharma, [107] | JA | India | CS | PIS | A | Pharma | 60 | 30–65 years | NM | NM | No |
| Shah, [108] | JA | India | LS | Non-randomized PIS | H | Pharma | 20 | 40–55 years | NM | NM | No |
| Shaikh, [109] | JA | India | LS | CT | U | Non-pharma | 20 | 40–60 years | NM | Yes | No |
| Pandey, [110] | JA | India | CS | Open label trial | A | Pharma | 10 | > 18 to < 70 years | NM | NM | No |
| Ghufran, [111] | JA | India | CS | CT | U | Pharma | 15 | 21–60 years | NM | NM | No |
| Yousuf, [112] | JA | India | LS | Interventional OS | U | Pharma | 60 | > 20– < 50 years | NM | NM | No |
| Rajashri, [113] | AD | India | CS | CT | S | Pharma | 40 | 21–70 years | NM | NM | No |
| Selvi, [114] | AD | India | LS | CT | S | Combined | 40 | 20–60 years | NM | Yes | No |
| Usha, [115] | AD | India | LS | CT (pilot study) | S | Pharma | 40 | 31–60 years | NM | Yes | No |
| Tarique, [116] | JA | India | LS | OCS | U | Non-pharma | 30 | 25–60 | NM | Yes | No |
| Patil, [117] | JA | India | LS | CT | Y | Non-pharma | 12 | 36.8 ± 3.8 | NM | Yes | No |
| Paresh, [118] | JA | India | LS | CT | A | Pharma | 20 | 20–80 years | NM | NM | No |
| Sharma, [119] | JA | India | Both | PCT | A | Pharma | 50 | 40.5 ± 11.3 | NM | NM | No |
| Ileana, [120] | JA | Romania | LS | CT | H | Combined | 20 | 20–86 years | NM | NM | No |
| Ahmad, [121] | JA | India | LS | Case series | U | Combined | 3 | 38, 29, 64 years | NM | Yes | No |
| Kumar, [122] | AD | India | CS | Pilot study | S | Combined | 40 | 21–60 years | NM | Yes | No |
| Bharati, [123] | JA | India | LS | OCT (pilot study) | A | Pharma | 27 | 18–70 years | NM | NM | No |
| Nilopher, [124] | AD | India | CS | OCT | S | Combined | 40 | 20–60 years | NM | Yes | No |
| Urooj, [32] | JA | India | Both | Comparative open CT | U | Non-pharma | 98 | 20-60 years | NM | NM | Yes |
| Sreedhana, [125] | AD | India | LS | OCCT | S | Combined | 60 | 30–60 years | NM | Yes | No |
| Rasakumar, [126] | AD | India | CS | OCT | S | Combined | 60 | 20–60 years | NM | Yes | No |
| Madhavikutty, [127] | JA | India | LS | Comparative CT | A | Pharma | 330 | 12–70 years | NM | NM | No |
| Sharma, [128] | JA | India | CS | ROCS | A | Pharma | 48 | 18–70 years | No | NM | No |
| Khan, [129] | JA | India | CS | Controlled CT | U | Combined | 34 | 15—60 years | NM | NM | No |
| Thakur, [130] | IAR | India | LS | Case study | H | Combined | 10 | 36.2 ± 11.1 | No | NM | No |
| Muthumari, [131] | AD | India | LS | Before-after studies | S | Pharma | 20 | 18–60 years | NM | Yes | No |
| Bhatted, [132] | JA | India | LS | Case study | A | Pharma | 1 | 59 years | NM | NM | No |
| Aarthy, [133] | AD | India | LS | OCT | S | Combined | 40 | 20–60 years | NM | Yes | No |
JA journal article, AD academic dissertation, CS cervical spondylosis, LS lumbar spondylosis, A Ayurveda, Y Yoga and Naturopathy, U Unani, S Siddha, H Homoeopathy, RCT randomized controlled trial, QET quasi-experimental trial, OPRT open prospective randomized trial, CT clinical trial, CCT comparative clinical trial, PRT parallel randomized trial, OS observational study, OCS observational clinical study, PIS prospective interventional study, PCT prospective clinical trial, OCT open clinical trial, OCCT open comparative clinical trial, ROCS retrospective observational cohort study, Pharma pharmacological, Non-Pharma non-pharmacological, NM not mentioned
Table 2.
Basic description of selected articles (n = 113)
| Characteristics | n (%) |
|---|---|
| Type of publication | |
| Journal article | 77 (68.1) |
| Academic dissertation | 35 (31.0) |
| Institutional annual report | 1 (0.9) |
| Country | |
| India | 93 (82.3) |
| USA | 12 (10.6) |
| Germany | 4 (3.5) |
| England | 1 (0.9) |
| Romania | 1 (0.9) |
| Saudi Arabia | 1 (0.9) |
| United Kingdom | 1 (0.9) |
| Ayush system | |
| Ayurveda | 37 (32.7) |
| Yoga | 25 (22.1) |
| Unani | 18 (15.9) |
| Siddha | 28 (24.8) |
| Homoeopathy | 5 (4.4) |
| Study population | |
| Cervical spondylosis | 35 (31.0) |
| Lumbar spondylosis | 74 (65.5) |
| Both | 4 (3.5) |
| Type of intervention | |
| Pharmacological | 48 (42.5%) |
| Non-Pharmacological | 30 (26.5%) |
| Combined | 35 (31.0%) |
| Study design | |
| Interventional study | 107 (94.7) |
| Observational study | 6 (5.3) |
| Sample size (range) | |
| Interventional study | 10–313 |
| Observational study | 1–10 |
| Gender | |
| Male | 4 (3.5) |
| Female | 3 (2.7) |
| Both | 95 (84.1) |
| Not reported | 11 (9.7) |
| Number of interventions | |
| One | 45 (39.8) |
| Two | 27 (23.9) |
| Three | 11 (9.7) |
| Four | 12 (10.6) |
| More than four | 18 (15.9) |
In terms of blinding in the intervention studies, 21 (18.6%) were single blinded and four with double and one study with triple blinding. Other 87 (77%) studies did not involve masking (Supp Table 6).
Of the studies included, 30 (26.5%) had a comparison group (Supp Table 7) and 104 (92%) were hospital-based and couple of studies were community-based. Most of the studies (n = 103; 91.2%) were single-centre based and five were multi-centric studies. Informed consent-related details were available for 82 (72.6%) studies. Three-fifths (n = 71) of the studies did not provide any facility/provision/plan to report AE (Supp Table 6). Of the total studies, 29 (25.6%) were registered with Clinical Trials Registry, 68 (60.2%) were mentioned about the ethics committee approval and 44 (38.9%) studies had declared for no conflict interest, 69 (61.1%) studies did not mention the conflict of interest (Supp Table 9).
Studies with a comparison group
Of the 30 studies with a comparison group, six were conducted for cervical spondylosis (CS) and the remaining for lumbar spondylosis (LS). Of the six CS studies, four used Yoga and one study each with Unani and Homoeopathy interventions. In 16 studies, the LS participants were given Yoga as intervention followed by Ayurveda (4 studies) and Unani (3 studies) and a single study with Homoeopathy (Supp Table 7).
Studies without a comparison group
Of the 83 studies without any comparison group, 29 (34.9%) were conducted for cervical spondylosis, 50 (60.2%) for lumbar spondylosis and the remaining for both the conditions. For CS, Siddha intervention was used in 17 studies (58.6%), followed by Ayurveda (n = 6, 20.7%), Unani (n = 4, 13.8%) and Yoga (n = 2, 6.9%). For LS, the Ayurveda system of medicine was used in 23 studies (46%), followed by Siddha (n = 11, 22%), Unani (n = 9, 18%), Yoga (n = 4, 8%) and Homoeopathy (n = 3, 6%). Only four studies involved both cervical and lumbar spondylosis. Of them, three had Ayurveda medicine and single study with Unani intervention (Supp Table 8).
Risk-of-bias assessment among the included studies
We used RoB2 for RCT [High risk = 28 (47.5%), some concerns = 29 (49.2%) Low risk = 2 (3.4%)], ROBINS-I for non-RCT [serious = 4 (8.3%), moderate risk = 27 (56.3%), low risk = 17 (35.4%)] and JBI for case series (low risk = 5 (100%) and case studies (low risk = 1 (100%) to assess the risk of bias and they are reported (Supp Table 10-Table 14)
Adverse events
Of the 113 studies, 15 (13.3%) reported and described AE and these included report from Yoga (n = 11) and two each from Unani and Homoeopathy systems. Eleven of these reports were from lumbar spondylosis and three from cervical spondylosis. A single report was about both cervical and lumbar spondylosis. Of the AE reports, 12 were from non-pharmacological interventions and two were based on pharmacological intervention and a single study report was from combined intervention (Table 3). The number of study participants who experienced any kind of adverse event from the 15 studies ranges from a minimum of 1 to a maximum of 49. Description of intervention such as type and duration of intervention, method of providing the intervention, presence of self-practice for yoga intervention, description of adverse events including, number of participants reported AE, type and description of AE for the 15 studies are provided (Table 4).
Table 3.
Proportion of studies reported any kind of adverse events (n = 113)
| Characteristics | n (%) |
|---|---|
| Ayush system | |
| Ayurveda | 0 (0) |
| Yoga | 11 (9.7) |
| Unani | 2 (1.8) |
| Siddha | 0 |
| Homoeopathy | 2 (1.8) |
| Study population | |
| Cervical spondylosis | 3 (2.7) |
| Lumbar spondylosis | 11 (9.7) |
| Both | 1 (0.9) |
| Intervention type | |
| Pharmacological | 2 (1.8) |
| Non-pharmacological | 12 (10.6) |
| Combined | 1 (0.9) |
Table 4.
Description of adverse events among the studies reported with any type of adverse events (15 studies, 164 participants)
| Author, | Population | System | Age in years (range; mean ± SD) | Intervention | Study group and size | Adverse events | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year | ||||||||||||
| Type | Description | Duration | Yoga sessions handled by | Self-practice | # of participants reported AE | Type of AE | Description of AE | |||||
| Michalsen et al. [12] | CS | Yoga | 18–60 | NP | Iyengar yoga | 9 weeks, 90 min/week | Certified instructor; physician assistant | Yes | Yoga–38 | 1 | Not categorized | Low back pain |
| Some patients | Muscle soreness | |||||||||||
| Exercise–39 | 0 | – | – | |||||||||
| Cramer, [21] | CS | Yoga | 19–60; 47.8 ± 10.4 | NP | Iyengar yoga | 9 weeks, 90 min/week | Certified instructor, physiotherapist | Yes | Yoga–25 | 9 | Not categorized | Transient worsening of neck pain or muscle soreness |
| 3 | Minor adverse effect | Transient limb pain, Migraine and vertigo | ||||||||||
| Exercise–26 | 8 | Not categorized | Transient worsening of neck pain or muscle soreness | |||||||||
| 2 | Minor adverse effect | Transient limb pain, Migraine and vertigo | ||||||||||
| Williams, [22] | LS | Yoga | 18–70; 48.0 ± 1.2 | NP | Iyengar yoga | 24 weeks, 90 min/twice a week | Certified instructor assistants | Yes | Yoga–43 | 1 | Adverse event | Yoga exacerbated LBP |
| SMC–47 | ||||||||||||
| Hepburn [61] | CS | Homoeopathy | 18–65; 36.1 | P | Homoeopathic medicine | 7 days, 3 times/day | NA | NA | Traumeel S and placebo Piroxicam- 25 | 4 | Not categorized | Headache |
| 2 | Aggravation of neck pain | |||||||||||
| 2 | Lethargy | |||||||||||
| Piroxicam and placebo Traumeel S–25 | 2 | Headache | ||||||||||
| 1 | Aggravation of neck pain | |||||||||||
| Groessl et al. [23] | LS | Yoga | 53.4 ± 13.3 | NP | Hatha yoga | 12 weeks, 60 min/twice a week | Certified instructor | Yes | Yoga–76 | 1 | Adverse events | Increased level of back pain |
| 1 | Back went out | |||||||||||
| Delayed Treatment–76 | ||||||||||||
| Saper et al. [28] | LS | Yoga | 44.0 ± 12.0 | NP | Hatha yoga | 12 weeks, four 3 week segments each 75 min | Yoga instructors | Yes | Yoga–30 | 1 | Not categorized | Transient worsening of low back pain |
| Usual care–30 | ||||||||||||
| Williams et al. [25] | LS | Yoga | 23–67; Yoga group: 48.7 ± 10.6 Educational control group: 48.0 ± 1.96 | NP | Iyengar yoga | 16 weeks, 1.5 h/week | One yoga instructor | Yes | Yoga–30 | 1 | Adverse event | Herniated disc*** |
| Education–30 | ||||||||||||
| Tilbrook et al. [46] | LS | Yoga | 18–65; Yoga group: 46.3 ± 11.5 Usual care group: 46.4 ± 11.3 | NP | Not available | 75 min/week | 20 yoga teachers ( 10 each from British Wheel of Yoga and Iyengar Yoga) | Yes | Yoga–156 | 1 | Serious adverse event | Increased back pain possibly or probably related to Yoga |
| 1 | Nonserious adverse event | Accident/injury* | ||||||||||
| 4 | Increased back pain possibly or probably related to Yoga | |||||||||||
| 3 | Increased back pain unrelated to Yoga | |||||||||||
| 3 | Other pain probably related to Yoga** | |||||||||||
| Usual care–157 | 1 | Serious adverse event | Accident/injury* | |||||||||
| 1 | Death | |||||||||||
| Michalsen et al. [26]˥ | LS | Yoga | 18–75; 55 ± 10 | NP | Jyoti meditation | 8 weeks, 90 min/week | Information not available | Yes | Meditation–32 | 2 | Not categorized | Increase of an already existing tinnitus |
| Exercise–36 | 1 | Slightly increased dizziness and headache | ||||||||||
| Sherman et al. [27] | LS | Yoga | 48.4 ± 9.8 | NP | Viniyoga | 12 weeks, 75 min/week | Yoga instructor physical therapists | Yes | Yoga–87 | 13 | Mild/moderate adverse event | Increased Back pain |
| 1 | Not categorized | Herniated disk | ||||||||||
| CSE–75 | 13 | Mild/moderate adverse event | Increased Back pain | |||||||||
| Self-care book–45 | 1 | Not categorized | Increased pain | |||||||||
| Saper et al. [28] | LS | Yoga | 18–64; Yoga group: 46.4 ± 10.4; physical therapy: 46.4 ± 11.1; education: 44.2 ± 10.8 | NP | Yoga | 12 weeks, 75 min/week | Yoga instructors | Yes | Yoga–127 | 9 | Adverse events | Mild self-limited joint and back pain |
| Physical therapy–129 | 14 | |||||||||||
| Education–64 | 1 | |||||||||||
| Stam et al. [30]# | LS | Homoeopathy | 18–65; SRL group: 40.6 ± 13.6; CCC group: 41.0 ± 12.8 | P | Homoeopathic gel | 1 week; 3 gm/day; 3 times a day | NA | NA | SRL–83 | 9 | Adverse events | Not reported |
| 3 | Adverse drug reactions | |||||||||||
| CCC–78 | 19 | Adverse events$ | ||||||||||
| 18 | Adverse drug reactions | |||||||||||
| Ansari et al. [48] | LS | Unani | 25–50 | C | Unani formulation and cupping therapy | 30 days, cupping-3 times/day; internal medicine 2 times/day | NA | NA | Habb-e-Asgandh & Habb-e-Suranjan–30 | |||
| Wet cupping therapy–30 | 2 | Side effect | Fainting (vaso-vagal shock) | |||||||||
| Neyaz et al. [29] | LS | Yoga | 18–55; 35.8 ± 10.6 | NP | Hatha yoga | 12 weeks, 35 min/week | One trained yoga therapist | Yes | Yoga–35 | 3 | Nonserious side effects | Slight increased pain |
| CTE–35 | ||||||||||||
| Urooj et al. [32]˥ | Cervical OA | Unani | 20–60 | NP | Cupping therapy | 4 consecutive days, 20 min/sitting | NA | NA | Group A–33 | 2 | Not categorized | Ecchymosis |
| Lumbar OA | Group B–33 | |||||||||||
| Knee OA | Group C–32 | |||||||||||
P pharmacological, NP non-pharmacological, C combined, AE adverse events, CS cervical spondylosis, LS lumbar spondylosis, OA osteoarthritis, NA not applicable, SMC self-directed standard medical care, CSE conventional stretching exercises, SRL Sprioflor gel, CCC Cremor Capsici Compositus, CTE conventional therapeutic exercises
#4 ADRs in the CCC were rated ‘severe’ compared to none of the ADRs in the SRL group
*Unrelated to intervention
**All patients had a history of other pain
***Participant with symptomatic osteoarthritis
$Out of 19 subjects in CCC group, one subject who experienced two AEs
˥Adverse event was not reported in which group
Low back pain, muscle soreness, transient worsening of neck pain or muscle soreness, transient limb pain, migraine and vertigo were reported by the CS participants with yoga intervention [20, 21]. Exacerbated/increased level of back pain, transient worsening of low back pain, herniated disc, increased back pain possibly or probably related to yoga, increased back pain unrelated to yoga, other pain probably related to yoga, accident/injury, increase of an already existing tinnitus, slightly increased dizziness and headache, increased back pain, herniated disk, increased back pain, increased pain, mild self-limited joint and back pain and slight increased pain reported by the LS participants with yoga interventions [22–29]. One death due to accident was reported during the intervention in the study by Tilbrook [46]. Headache, aggravation of neck pain and lethargy was reported by CS participants who were taking homoeo intervention [61]. Another homoeo study for LS mentioned that it had adverse events, adverse drug reactions, adverse events, adverse drug reactions, however they were not described in detail [30]. Fainting (vaso-vagal shock) was reported in an Unani intervention for LS [31] and ecchymosis was mentioned in an Unani for both cervical and lumbar spondylosis [32].
Withdrawal of study participants
Of the 113 studies, 32 (28.3%) had reported withdrawal of participants (n = 400). Studies reporting withdrawal included nine of 37 from Ayurveda, 15 of 25 from Yoga, seven of 18 studies from Unani, one from 5 homoeopathy studies and none from 28 Siddha based studies. Among the total, 80 studies had no information on withdrawal of study participants, but one reported that there was no withdrawal during the study. Nine of 48 studies which had pharmacological interventions (n = 122; 30.5%) and 18 of 30 non-pharmacological (n = 238; 59.5), five of 35 studies combined interventions (n = 40; 10%) had withdrawals. The 15 studies reported AE had withdrawals and drop outs due to various reasons. Description of intervention such as the number of participants in study groups, detailed description of intervention with name of pharmacological and non-pharmacological intervention, number of follow-ups and the timeline, number of withdrawal and dropouts and reasons for the same for the 15 studies are provided (Table 5).
Table 5.
Description of intervention and withdrawals for the studies reported with AE
| Author, year, country | Intervention description | Follow-up | # Withdrawals and reason |
|---|---|---|---|
| Cramer et al. [21] Germany | Yoga group (n = 25): Iyengar yoga [Bharadvaja’s twist, Bridge pose, Corpse pose, Downward facing dog, Downward facing hero, Extended side angle, Extended triangle, Mountain pose, Prosperous pose, Reclining big toe, Standing half forward bend (at wall), Thunderbolt pose, Upward hand pose, Warrior pose II]; | Baseline; at 12 weeks; at 24 weeks | Yoga group (n = 3): 1-Symptom worsening, 1-Acute illness, 1-Scheduling problems |
| Exercise group (n = 26) Self-care manual designed by a large Statutory German Health insurance company to relieve neck pain and stiffness | Baseline; at 12 weeks; at 24 weeks | Exercise group (n = 0) | |
| Williams et al. [22] USA | Yoga group (n = 43): Iyengar yogaSavasana II Supine; Savasana prone; Supta padangusthasana II prone; Supta tadasana; Supta urdhva hastasana; Supta padangusthasana I and II; Supta pavanmuktasana; Urdhva prasarita padasana at the wall; Pavanmuktasana-over bolster on 2 chairs; Parsava pavanamuktasana: Utthita hasta padangusthasana I and II; Ardha uttanasana; Uttanasana; Adho mukha svanasana; Tadasana; Urdhva hastasana; Utthita padmasana; Utthita hasta padasana*; Parsva hasta padasana*; Utthita parsvakonasana*; Utthita trikonasana*; Virabhadrasana II*; Ardha chandrasana*; Prasarita padottanasana; Parsvottanasana; Parsvottanasana; Parivrtta trikonasana*; Bharadvajasana-seated on chair; Utthita Marichyasana; Dandasana; Marichyasana III; Adho mukha virasana-with bolster under abdomen. ( *with support of upper wall rope) | ||
| Standard medical care group (n = 47): Continued self-directed standard medical care, no attempt was made to regulate treatment received. Participants were waitlisted and offered the yoga classes 6 months after the conclusion of the study | |||
| Hepburn, [61], Canada |
Group 1 (n = 25): Traumeel S sachets and placebo Piroxicam (corn starch); Placebo: 40 mg (2 × 20 mg capsules) orally for the first two days, followed by 20 mg (1 × 20 mg capsule) daily orally for the following 5 days Traumeel S: One tablet to be sucked 3 times a day for 7 days |
At day 1; at day 3; at day 7 | Drop outs (n = 10) [3-Took medication during the trial which would affect results, 1-Missed appointment on day three, 6-Missed appointment on day seven] |
| Group 2 (n = 25): Piroxicam and placebo Traumeel S (Lactose powder); Piroxicam: 40 mg (2 × 20 mg capsules) orally for the first two days, followed by 20 mg (1 × 20 mg capsule) daily orally for the following 5 days. Placebo: One tablet to be sucked 3 times a day for 7 days | |||
| Groessl et al. [23] , USA | Yoga group (n = 76): Hatha yoga; Physical yoga postures, movement sequences, and regulated breathing. Directed attention and brief meditation | Baseline; at 6 weeks; at 12 weeks; at 6 months | Yoga group (n = 28) [1-withdrew from study, 7-work conflict, 8-transportation, 1-no reason given, 4-non yoga injury, 3-other medical, 2-homeless, 1-back pain, 1-type of yoga] |
| Delayed treatment group (n = 76): Attend the yoga intervention after 6 months | Delayed treatment group (n = 4) [1-withdrew from study, 3-did not wait 6 months to use yoga] | ||
| Williams et al. [25], USA | Yoga group (n = 30): Hatha yoga Svasana relaxation and breathing exercises*, Knee to chest*, Knee to chest with twist*, Pelvic clocks*, Cat and dog pose (and modifications)*, Chair pose (and modified)*, Mountain pose*, Shoulder opener*, Half moon*, Child's pose*, Reclining cobbler*, Downward-facing dog (and modified at wall)*, Triangle pose at wall, Locust pose, Reclining big toe pose, Warrior I pose, Downward-facing dog, Lunge with wall assist, Standing squat with half forward bend, Baby dancer pose, Deep lunge, Spinal rolls, Svasana relaxation and breathing exercises*. Each class began and ended with Svasana, a relaxation exercise. (* Exercises included on the audio CD provided to participants for home practice) | Pre-intervention, post-intervention, 3-month follow-up | Yoga intervention group: At 16 weeks (n = 10) [3-no shows after baseline, 3-quit, 1-adverse event, 2-medically ineligible, 1-unwilling to perform active postures]; After 3 months follow-up (n = 0) |
| Usual care group (n = 30): Continued to receive their routine medical care and medications. Offered yoga interventions after 26 weeks | Educational control group: At 16 weeks (n = 6) [3-lost to follow-up, 2-ineligible due to other CAM use for CLBP, 1-no show at baseline]; After 3 months follow-up (n = 2) [1- lost to follow-up, 1-died] | ||
| Tilbrook et al. [46], UK | Yoga group (n = 156): Asana, pranayama, relaxation techniques, mental focus, and philosophy. Classes consisted of an introduction to the weekly theme; pain-relieving or settling-in relaxing poses; a program of seated, standing, prone, and supine poses; educative postural advice; and 5 to 15 min of relaxation | Baseline; at 3 months; at 6 months; at 12 months | Yoga group: At baseline (n = 4) [1-lost to follow-up at baseline, 2-did not want to continue, 1-questionnaire not returned]; At 3 months (n = 21) [5-lost to follow-up,3-did not want to continue, 3- ineligible after randomization, 1-unable to attend classes, 8-questionnaire not returned, 1-change from baseline scores could not calculated); At 6 months (n = 19) [6-lost to follow-up, 4-did not want to continue, 3- deemed ineligible after randomization, 1-unable to attend classes, 4- questionnaire not returned, 1-change from baseline scores could not calculated]; At 12 months (n = 21) [13-lost to follow-up, 4-did not want to continue, 3- deemed ineligible after randomization, 1-unable to attend classes] |
| Usual care group (n = 157): Offered a 1-time session of yoga after final follow-up | |||
| Michalsen et al. [26], Germany | Focused meditation technique (Jyoti meditation) (n = 32): Jyoti meditation for controlling and directing attention away from the physical body and sensations, from the emotions and thoughts to a place of relaxation or peace within the organism | Baseline; at 4 weeks; at 8 weeks | Meditation group (n = 12) [1-health problem, 8-non-compliance, 3-did not participate at all] |
| Exercise group (n = 36): A total of 15 exercises were described focusing on muscle stretching, strengthening and joint mobility with proper posture depiction | Exercise group (n = 4) [1-health problem, 3- non-compliance] | ||
| Sherman et al. [27], USA | Yoga group (n = 87): Viniyoga, and included 17 relatively simple postures, with variations and adaptations. Each class included breathing exercises, 5 to 11 postures (lasting approximately 45–50 min), and guided deep relaxation. Six distinct and progressive classes were taught in pairs | Baseline; at 6 weeks; at 12 weeks; at 26 weeks | Yoga group (n = 5 [sickness, family emergency, time conflict] |
| Stretching group (n = 75): Aerobic exercises, 10 strengthening exercises, and 12 stretches, held for 30 s each (a total of 10.5 min of stretching). Classes consisted of 15 exercises designed to stretch the major muscle groups but emphasizing the trunk and legs (a total of 52 min of stretching), and 4 strengthening exercises | Exercise group (n = 5) [sickness, family emergency, time conflict] | ||
| Self-care group (n = 45): Self-care book; Self-care participants received the Back Pain Helpbook, which provides information on the causes of back pain and advice on exercising, making appropriate lifestyle modifications and managing flare-ups | self-care group (n = 0) | ||
| Saper et al. [28], USA | Yoga group (n = 127): Hatha yoga group: Svasana Relaxation/ Breathing Exercise; Knee to Chest*; Knee Together Twist*; Shoulder Opener*; Mountain*; Chair twists, standing and seated; Cobra*; Bridge; Downward Facing dog (and at wall)*; Pelvic Tilt*; Cat and Cow*; Chair Pose*; Crescent Moon*; Reclining Cobbler*; Locust*; Child Pose*; Triangle (with and without the wall); Sphinx*; Standing forward bend at wall*; Extended Leg*; Warrior*; Sun salutations; Baby Dancer*; Spinal Rolls; Svasana Integrative Relaxation (* These exercises were included in the home practice videos provided to participants) | Baseline; at 26 weeks; at 40 weeks; at 52 weeks | Yoga group: Treatment phase (n = 2) [2-did not complete any follow-up surveys]; Maintenance phase (n = 8) [8-did not attend any treatment phase classes and were discontinued after 12 weeks] |
| Physical therapy group (n = 129): Abdominal bracing; Bracing with heel slides; Bracing with leg lifts; Bracing with bridging; Bracing with standing; Bracing with standing; Bracing with standing row exercise; Bracing with walking; Quadruped arm lifts with bracing; Quadruped leg lifts with bracing; Quadruped alternative arm & leg lifts w/bracing; Side support with knees flexed; Side support with knees extended; Side support with knees flexed; Side support with knees extended | Physical therapy: Treatment phase (n = 16) [15- did not complete any follow-up surveys, 1- was found to meet exclusion criterion and was discontinued]; Maintenance phase (n = 7) [5-did not attend any treatment phase classes and were discontinued after 12 weeks, 1- was found to meet exclusion criterion and was discontinued, 1-withdrew due to an unrelated illness] | ||
| Education (n = 64): Participants received the Back Pain Help Book which includes information on CLBP, self-management, stretching, strengthening, and the role of emotions an fear avoidance | Education group: Treatment phase (n = 3) [3-did not complete any follow-up surveys]; Maintenance phase (n = 0) | ||
| Ansari et al. [48] India | GROUP A (n = 30): Habb-e-Asgandh & Habb-e-Suranjan- Unani formulation and cupping therapy. Unani formulation, i.e. Habb-e-Asgandh14 (2 T.I.D.) and Habb-e-Suranjan15 (2 T.I.D.). The medicines were given orally and the patients were advised to take them after meals | Baseline; at day15; at day 30; at day 60 | No information |
| GROUP B (n = 30): Wet cupping therapy—30; Cupping procedure on 0, 15th and 30th days on the lower part of back 3–5 cm lateral to midline at the level of L2, 3, 4 vertebrae on both sides | |||
| Neyaz et al. [29] India | Yoga group (n = 35): Hatha yoga: Hatha yoga: Introduction of yoga philosophy; Sithilikaran Vyayama (Flexibility practice): Supta Udarakarshanasana (folded leg lumbar stretch), Shavaudarakarshanasana (Crossed leg lumbar stretch), Supta Pawanmuktasana (leg lock pose); Yogasanas: Ustrasana (Camel pose), Bhujangasana (Cobra pose), Salabhasana (Grasshopper pose), Settubandasana (Bridge pose); Quick relaxation technique: Savasana (Corpse pose) with pranayama; Pranayama (breath control): Nadi suddhi (Alternate nostril breathing), Bhramari (Humming breathing); Medication (deep relaxation technique): Savasana (Chanting AUM or OM) | Baseline; at 6-week; at 12-week | Yoga group: At 6 weeks (n = 15) Lost to follow-up [13- Discontinued allocated intervention, 2-Reason-not specified); At 12 weeks (n = 3) [2-Shifted elsewhere,1- Pain recurred] |
| Conventional therapeutic exercise group (CTE) (n = 35): Short introduction regarding benefits of exercises; Warm-up exercises; Hip extensors strengthening both sides, Hamstring stretching both sides, Rectus abdominis strengthening, Erector spinae stretching, Erector spinae strengthening, Pyriformis stretching both sides, Oblique abdominal muscle strengthening both sides, Hip abductor strengthening both sides; Relaxation | Exercise group: At 6 weeks (n = 12) Lost to follow-up [11- Discontinued allocated intervention, 1-Reason not specified); At 12 weeks (n = 5) [2-Shifted elsewhere, 1- ill health, 2-Too busy] | ||
| Urooj et al. [32] India | Group A (cervical osteoarthritis) (n = 33): dry cupping | Baseline, at day 1, day 2, day 3, day 4 | Drop outs (n = 8) [2-Ecchymosis, 3-registered no response, 3-missed the cupping session as per the study protocol] |
| Group B (lumbar osteoarthritis) (n = 33): dry cupping | |||
| Group C (knee osteoarthritis) (n = 32): dry cupping | |||
| Michalsen et al. [20] Germany | Yoga group (n = 38): Iyengar yoga | Baseline; at 4 weeks; at 10 weeks | Yoga group (n = 13) [1-didn’t receive allocated intervention, 5- adverse events (1-related to intervention), 5-study non-compliance (bronchitis, sinusitis, migraine, low back pain), 2-other reasons (death of relative, change of workplace)] |
| Exercise group (n = 39): Participants received standard self-care manual (developed by German Health Insurance Company) that specifically address exercise and education for chronic neck pain | Exercise group (n = 11) [1-adverse event (had surgery of hip joint earlier than expected, none related to intervention), 10-study non-compliance (wish to immediately start additional yoga or similar treatment)] |
Discussion
In the current review, we synthesized the adverse events following Ayush interventions (pharmacological and/or non-pharmacological) for cervical and lumbar spondylosis. In this review of predominantly interventional studies from published literature mostly conducted in India, one in eight of the studies had reported adverse events.
Studies reporting AEs mainly used Yoga as an intervention, and a couple of studies used Homoeopathy or Unani interventions. Among the Ayush systems, none of the Siddha or Ayurveda studies reported any adverse events for cervical and lumbar spondylosis treatment. When compared to cervical spondylosis, more AEs were reported by lumbar spondylosis participants. Although all the reviewed studies mentioned in the objective statement or in the methods section that they would observe and record the AEs, only one-sixth of them described the adverse events. This could lead to considerable underreporting of AE among the Ayush studies for cervical and lumbar spondylosis.
Besides, our review of abstract reports and published documents from the Country’s pharmacovigilance centres at different levels (National; Intermediary and Peripheral) for Ayush systems did not yield any AE documentation for Ayush interventions [33]. The practitioners, researchers did not use these centres for reporting. Another reason for underreporting may be that more academic dissertations were included in this study. There may be a lack of vigilant reporting of AE by scholars.
The strength of our systematic review is that, according to the best of our knowledge, this may be a maiden attempt to document and synthesize evidence for adverse events of Ayush interventions for lumbar and cervical spondylosis. There are some limitations in this systematic review. There was a difficulty to search the articles for Ayush systems of medicine as majority of the articles are published in non-indexed journals, and moreover the Ayush databases (Ayush research portal, DHARA, and Shodhganga) are not compatible to use structured search strategy. This estimation of AE may result from the bias and limitations of the original studies included in the qualitative synthesis. For instance, majority of the included RCTs suffered from high or moderate risk of bias. Case reports and case series had a low risk of bias. Hence, there is a need for more high-quality original studies. In conclusion, the current systematic review documented considerably low frequency of adverse events following Ayush interventions for cervical or lumbar spondylosis. There is an urgent need to address, capture and reporting of adverse events in the Ayush studies for cervical and lumbar spondylosis through practitioners, researchers and health care system professionals.
Supplementary Information
Supplementary Material 1: Table 1: PRISMA 2020 for Abstract checklist. Table 2: PRISMA 2020 checklist. Table 3: Inclusion and Exclusion criteria. Table 4: Diagnostic classification and codes for cervical and lumbar spondylosis in Allopathy and Ayush systems of medicine. Table 5: Database specific search terms and the number of articles retrieved. Figure 1: Description of selected articles based on Population, Intervention and study design. Figure 2: Publication of Ayush studies on Cervical and lumbar spondylosis during 1976-2021. Table 6: General Characteristics of selected studies. Table 7: Description of selected articles with comparison group. Table 8: Description of selected articles without comparison group. Table 9: Compliance of research mandates reported in selected articles. Table 10: Risk of bias assessment of selected articles. Table 11: Risk of bias assessment for Randomized Controlled Trials (RoB2). Table 12: Risk of bias assessment for Non- Randomized Controlled Trials (ROBINS-I tool). Table 13: Risk of bias assessment for Case reports (JBI tool). Table 14: Risk of bias assessment for Case series (JBI tool).
Acknowledgements
We acknowledge S. Arul Priya and A. Senthamilselvi and G. Keerthika for retrieving the articles, M Prakash and V Saravanakumar for providing suggestions for data analysis.
Author contributions
Manickam Ponnaiah*: conseptualization, methodology, investigation, resources, writing—review and draft, supervision, project administration, funding acquisition. Rajalakshmi Elumalai*: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing original draft, writing—review and draft, visualization, supervision. Sendhilkumar Muthappan: conceptualization, methodology, investigation, supervision, project administration. Saranya J: validation, formal analysis, investigation, data curation, writing original draft, visualszation. Bhavani Shankara Bagepally: methodology, validation, investigation, resources, writing—review and draft, supervision. Satish Sivaprakasam: investigation, resources. Ganeshkumar Parasuraman: methodology.
Funding
Indian Council of Medical Research, Sanction order. No. 5/7/1705/CH/Adhoc/RBMCH/2020—dated 27 November 2020. The funders played no role in the study’s conception, execution, or manuscript preparation.
Availability of data and materials
The datasets used and analysed during the present study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethics committee review is exempted for conducting systematic reviews as per the guidelines of Indian Council of Medical Research—National ethical guidelines for biomedical and health research involving human participants, 2017.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manickam Ponnaiah and Rajalakshmi Elumalai have contributed equally.
References
- 1.Government of India. Ministry of Ayush. https://www.ayush.gov.in/
- 2.Rudra S, Kalra A, Kumar A, Joe W. Utilization of alternative systems of medicine as health care services in India: evidence on AYUSH care from NSS 2014. PLoS ONE. 2017;12(5):e0176916. 10.1371/journal.pone.0176916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.University of Washington, Public Health Foundation of India, Indian Council of Medical Research. India: health of the nation's states: the India state-level disease burden initiative: disease burden trends in the states of India, 1990 to 2016. New Delhi, India: Indian Council of Medical Research; 2017 2017. 214 pages p.
- 4.Chopra A, Saluja M, Patil J, Tandale HS. WHO-international league of associations for rheumatology. Community oriented program for control of rheumatic diseases. J Rheumatol. 2002;29(3):614–21. [PubMed] [Google Scholar]
- 5.Corp N, Mansell G, Stynes S, Wynne-Jones G, Morsø L, Hill JC, et al. Evidence-based treatment recommendations for neck and low back pain across Europe: a systematic review of guidelines. Eur J Pain. 2021;25(2):275–95. 10.1002/ejp.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrotra RP, Saxena SA. Status and role of Ayush and local health traditions under the National Rural Health Mission: report of a study. New Delhi 2010 2010. 308 p.
- 7.Jose J, Rao PGM, Kamath Ms, Jimmy B. Drug safety reports on complementary and alternative medicines (Ayurvedic and homeopathic medicines) by a spontaneous reporting program in a tertiary care hospital. J Altern Complement Med. 2009;15(7):793–7. 10.1089/acm.2008.0128 [DOI] [PubMed] [Google Scholar]
- 8.Paudyal B, Thapa A, Sigdel KR, Adhikari S, Basnyat B. Adverse events with Ayurvedic medicines—possible adulteration and some inherent toxicities. Wellcome Open Res. 2019;4:23. 10.12688/wellcomeopenres.15096.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dora BB, Gupta S, Sital S, Singh M. Importance of AYUSH in present health care perspective. Res Rev: J Med Sci Technol. 2019;4(3):5–8. [Google Scholar]
- 10.World Health Organization. WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. Geneva: World Health Organization; 2004. [Google Scholar]
- 11.Xu Y, Patel DN, Ng S-LP, Tan S-H, Toh D, Poh J, et al. Retrospective study of reported adverse events due to complementary health products in Singapore from 2010 to 2016. Front Med. 2018. 10.3389/fmed.2018.00167. 10.3389/fmed.2018.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140(10):795–801. 10.7326/0003-4819-140-10-200405180-00009 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation. The Importance of Pharmacovigilance. https://apps.who.int/iris/bitstream/handle/10665/43034/9241592214_eng.pdf
- 14.Xu JC, Goel C, Shriver MF, Tanenbaum JE, Steinmetz MP, Benzel EC, et al. Adverse events following cervical disc arthroplasty: a systematic review. Glob Spine J. 2018;8(2):178–89. 10.1177/2192568217720681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponnaiah M, Muthappan S, Elumalai R, Muthuperumal P, Parasuraman G, Bagepally BS, et al. Adverse drug events in AYUSH interventions for cervical and lumbar spondylosis: a protocol for systematic review. Med: Case Rep Study Protoc. 2021;2(12):e0192. [Google Scholar]
- 17.Vanhecke TE. Zotero. J Med Lib Assoc: JMLA. 2008;96(3):275. 10.3163/1536-5050.96.3.022 [DOI] [Google Scholar]
- 18.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathur R, Swaminathan S. National ethical guidelines for biomedical & health research involving human participants, 2017: a commentary. Indian J Med Res. 2018;148(3):279. 10.4103/0971-5916.245303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michalsen A, Traitteur H, Ludtke R, Brunnhuber S, Meier L, Jeitler M, et al. Yoga for chronic neck pain: a pilot randomized controlled clinical trial. J Pain. 2012;13(11):1122–30. 10.1016/j.jpain.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Cramer H, Lauche R, Hohmann C, Lüdtke R, Haller H, Michalsen A, et al. Randomized-controlled trial comparing yoga and home-based exercise for chronic neck pain. Clin J Pain. 2013;29(3):216–23. 10.1097/AJP.0b013e318251026c [DOI] [PubMed] [Google Scholar]
- 22.Williams K, Abildso C, Steinberg L, Doyle E, Epstein B, Smith D, et al. Evaluation of the effectiveness and efficacy of Iyengar yoga therapy on chronic low back pain. Spine. 2009;34(19):2066–76. 10.1097/BRS.0b013e3181b315cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groessl EJ, Liu L, Chang DG, Wetherell JL, Bormann JE, Atkinson JH, et al. Yoga for military veterans with chronic low back pain: a randomized clinical trial. Am J Prev Med. 2017;53(5):599–608. 10.1016/j.amepre.2017.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saper RB, Sherman KJ, Cullum-Dugan D, Davis RB, Phillips RS, Culpepper L. Yoga for chronic low back pain in a predominantly minority population: a pilot randomized controlled trial. Altern Ther Health Med. 2009;15(6):18–27. [PMC free article] [PubMed] [Google Scholar]
- 25.Williams KA, Petronis J, Smith D, Goodrich D, Wu J, Ravi N, et al. Effect of iyengar yoga therapy for chronic low back pain. Pain. 2005;115(1–2):107–17. 10.1016/j.pain.2005.02.016 [DOI] [PubMed] [Google Scholar]
- 26.Michalsen A, Kunz N, Jeitler M, Brunnhuber S, Meier L, Ludtke R, et al. Effectiveness of focused meditation for patients with chronic low back pain-A randomized controlled clinical trial. Complement Ther Med. 2016;26:79–84. 10.1016/j.ctim.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 27.Sherman KJ, Cherkin DC, Wellman RD, Cook AJ, Hawkes RJ, Delaney K, et al. A randomized trial comparing yoga, stretching, and a self-care book for chronic low back pain. Arch Intern Med. 2011;171(22):2019–26. 10.1001/archinternmed.2011.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saper RB, Lemaster C, Delitto A, Sherman KJ, Herman PM, Sadikova E, et al. Yoga, physical therapy, or education for chronic low back pain: a randomized noninferiority trial. Ann Intern Med. 2017;167(2):85–94. 10.7326/M16-2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neyaz O, Sumila L, Nanda S, Wadhwa S. Effectiveness of hatha yoga versus conventional therapeutic exercises for chronic nonspecific low-back pain. J Altern Complement Med. 2019;25(9):938–45. 10.1089/acm.2019.0140 [DOI] [PubMed] [Google Scholar]
- 30.Stam C, Bonnet MS, van Haselen RA. The efficacy and safety of a homeopathic gel in the treatment of acute low back pain: a multi-centre, randomised, double-blind comparative clinical trial. Br Homeopath J. 2001;90(1):21–8. 10.1054/homp.1999.0460 [DOI] [PubMed] [Google Scholar]
- 31.Ansari AP, Dar PA, Kalam MA, Rather SA, Arif M, Nasir A. Therapeutic effect of inkibab (steam application) and hijama muzliqa (massage cupping) in case of waj al-zahr (non-specific low back pain): a case report. J Ayurvedic Herb Med. 2018;4:150–3. 10.31254/jahm.2018.4402 [DOI] [Google Scholar]
- 32.Urooj S, Jahangir U, Khan AA, Zaman F. Analgesic effect of cupping therapy in osteoarthritis on timeline: an open comparative clinical study. Group. 2016;100(32):30. [Google Scholar]
- 33.AyushSuraksha – AyushSuraksha. https://www.ayushsuraksha.com/
- 34.Zarekar DL, Shinde ST. Role of raktamokshana in katigatvata-randomized controlled trial. International Journal of Research in Ayurveda and Medical Sciences. 2:164–70.
- 35.P.Y.Yadaiah. Management of grudhrasi with katibasti. Aryavaidyan. 1989; 55–8.
- 36.Colgrove YM, Gravino-Dunn NS, Dinyer SC, Sis EA, Heier AC, Sharma NK. Physical and physiological effects of yoga for an underserved population with chronic low back pain. Int J Yoga. 2019;12(3):252. 10.4103/ijoy.IJOY_78_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haldavnekar RV, Tekur P, Nagarathna R, Nagendra HR. Effect of yogic colon cleansing (laghu sankhaprakshalana kriya) on pain, spinal flexibility, disability and state anxiety in chronic low back pain. Int J Yoga. 2014;7(2):111–9. 10.4103/0973-6131.133884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi F, Mahanta V, Dudhamal TS, Gupta SK. Effect of agnikarma (therapeutic heat burns) and raktamokshana (therapeutic bloodletting) in the management of kati sandhigata vata (lumbar spondylosis). Ayu. 2019;40(2):79–88. 10.4103/ayu.AYU_142_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Rampp T, Kessler C, Jeitler M, Dobos GJ, Ludtke R, et al. Effectiveness of Ayurvedic massage (sahacharadi taila) in patients with chronic low back pain: a randomized controlled trial. J Altern Complement Med. 2017;23(2):109–15. 10.1089/acm.2015.0272 [DOI] [PubMed] [Google Scholar]
- 40.Hanan SA, Eman SE. Cupping therapy (Al-Hijama): it’s impact on persistent non-specific lower back pain and client disability. Life Sci J. 2013;10:631–42. [Google Scholar]
- 41.Nair R. Comparative clinical study on gridrasi with sahacaradi taila vis a vis bhadradarvabi taila. J Res Ayur and Siddha. 1985;6(2):121. [Google Scholar]
- 42.N.P. Vijayam PRN, Madhavikutty. Clinical evaluation of prabhanjana vimardanam taila and sodhana therapy in the treatment of gridhrasi. Journal of Research in Ayurveda and Siddha. 1991; 19–32.
- 43.Singh B, Ahmad N. Clinical evaluation of Hijamat Bila Shurt (dry cupping) in the management of Waja ul zahar (low back pain)-A randomized, single blind, standard controlled study. J Med Plants. 2016;4(5):189–92. [Google Scholar]
- 44.Mahanta V. A comparative clinico pathological study of Sandhigata Vata W S R To Lumber spondylosis and its management by Agnikarma and Lakshya Guggulu Chikitsa [Thesis]: Sambalpur University; 2013.
- 45.Rae L, Dougherty P, Evertz N. yoga vs stretching in veterans with chronic lower back pain and the role of mindfulness: a pilot randomised controlled trial. J Chiropr Med. 2020;19(2):101–10. 10.1016/j.jcm.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilbrook HE, Cox H, Hewitt CE, Kang’ombe AR, Chuang LH, Jayakody S, et al. Yoga for chronic low back pain: a randomized trial. Ann Intern Med. 2011;155(9):569–78. 10.7326/0003-4819-155-9-201111010-00003 [DOI] [PubMed] [Google Scholar]
- 47.Jadhav NS, Jadhav SN. Efficacy of comprehensive ayurveda management of vertebral disc lesions by panchakarma therapies and herbomineral formulations. Anc Sci Life. 2012;32:S17. 10.4103/0257-7941.111974 [DOI] [Google Scholar]
- 48.Ansari MS, Yasir M. Evaluation of efficacy of wet cupping (hejamat-bil-shurt) in cases of back pain (Waja-uz-Zahr). Hamdard Medicus. 2013;56(1):52–60. [Google Scholar]
- 49.Aafreen S, Siddiqui MA, Quamri MA, Tarique BM, Saba K. Efficacy of Unani Formulations in Waja-ul-Khasirah (Low Back Pain)-A Randomised Double Dummy Clinical Trial.
- 50.Lari A, Nayab M, Tausif M, Lari JAH. Efficacy of Hijamat-Bila-Shart (dry cupping) in Waja-uz-Zahr (Low back pain): An open randomized controlled clinical trial.
- 51.Tekur P, Chametcha S, Hongasandra RN, Raghuram N. Effect of yoga on quality of life of CLBP patients: a randomized control study. Int J Yoga. 2010;3(1):10–7. 10.4103/0973-6131.66773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smeeta BK, Rukmani V. Effect of isolated and combined practice of Yoga and Ayurveda therapy on pain among cervical osteoarthritis patients. Int Peer Rev Index Bimonthly J Res AYUSH. 2016;1(1):12–5. [Google Scholar]
- 53.Tekur P, Singphow C, Nagendra HR, Raghuram N. Effect of short-term intensive yoga program on pain, functional disability and spinal flexibility in chronic low back pain: a randomized control study. J Altern Complement Med. 2008;14(6):637–44. 10.1089/acm.2007.0815 [DOI] [PubMed] [Google Scholar]
- 54.Monro R, Bhardwaj AK, Gupta RK, Telles S, Allen B, Little P. Disc extrusions and bulges in nonspecific low back pain and sciatica: exploratory randomised controlled trial comparing yoga therapy and normal medical treatment. J Back Musculoskelet Rehabil. 2015;28(2):383–92. 10.3233/BMR-140531 [DOI] [PubMed] [Google Scholar]
- 55.Sharma P, Wetal V, Gupta A. comparative study of bastikarma and siravedha in the management of gridhrasi (SCIATICA). Int J Res Ayurveda Pharm. 2020;11:48–55. 10.7897/2277-4343.110229 [DOI] [Google Scholar]
- 56.Tausif M, Ali H, Lari A. Comparative evaluation of effects of Hijama bila Shart and tens in Wajaur raqaba (Cervical spondylosis).
- 57.Deepak SP, Chandaliya Sachin S. Comparative clinical trial b rahita erandamoola (Ri “KATIGA.
- 58.Morone NE, Rollman BL, Moore CG, Li Q, Weiner DK. A mind-body program for older adults with chronic low back pain: results of a pilot study. Pain Med. 2009;10(8):1395–407. 10.1111/j.1526-4637.2009.00746.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ajay Kumar Sharma KR. clinical evaluation of the efficacy of trayodashanga guggulu and kati vasti in the management of gridhrasi roga (SCIATICA). Journal of Research in Ayurveda and Siddha. 2010; 65–80.
- 60.Highland KB, Schoomaker A, Rojas W, Suen J, Ahmed A, Zhang Z, et al. Benefits of the restorative exercise and strength training for operational resilience and excellence yoga program for chronic low back pain in service members: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2018;99(1):91–8. 10.1016/j.apmr.2017.08.473 [DOI] [PubMed] [Google Scholar]
- 61.Hepburn SE. The relative effectiveness of non-steroidal anti-inflammatory medication as compared to a homoeopathic complex in the treatment of cervical facet syndrome [Thesis] 2000.
- 62.Vaneet Kumar J, Dudhamal TS, Gupta SK, Mahanta V. A comparative clinical study of Siravedha and Agnikarma in management of Gridhrasi (sciatica). Ayu. 2014;35(3):270–6. 10.4103/0974-8520.153743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheeraz M, Quamri MA, Ahmed Z. A comparative clinical study on the effects of mehjama nariya (fire cupping) and hijamat bila shurt (dry cupping) in irqunnasa (sciatica). Spatula DD. 2013;3(4):161–6. 10.5455/spatula.20131127023056 [DOI] [Google Scholar]
- 64.Sawarkar P, Deshmukh M, Sawarkar G, Bhojraj N. A comparative efficacy study of the Panchtikta Ghrita Matra Vasti and Panchtikta Ghrita Marsha Nasya in cervical spondylosis. Int J Ayurvedic Med. 2020;11(2):218–27. 10.47552/ijam.v11i2.1432 [DOI] [Google Scholar]
- 65.Kumari A, Mahto R, Dave A, Shukla V. A comparative study on the effect of an indegenous compound drug & matra-basti in the management of Gridhrasi. AYU Int Quarter J Res Ayurveda. 2009;30(4):495–302. [Google Scholar]
- 66.Nandini B, Mooventhan A, Manjunath NK. Add-on effect of hot sand fomentation to yoga on pain, disability, and quality of life in chronic neck pain patients. Explore. 2018;14(5):373–8. 10.1016/j.explore.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 67.Telles S, Bhardwaj AK, Gupta RK, Sharma SK, Monro R, Balkrishna A. A randomized controlled trial to assess pain and magnetic resonance imaging-based (MRI-Based) structural spine changes in low back pain patients after yoga practice. Med Sci Monit. 2016;22:3228–47. 10.12659/MSM.896599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baig MG, Quamri MA. A randomized open labeled comparative clinical study on the efficacies of Hijamat Bila Shurt and Habbe Gule Aakh in cervical spondylosis. Int J Cur Res Rev. 2015;7(2):41. [Google Scholar]
- 69.Mungara B, Shukla V, Dave A, Bhatt N. A study on the role of Parijata Vati in the management of Gridhrasi w.s.r. to Sciatica. AYU Int Quarter J Res Ayurveda. 2009;30:342. [Google Scholar]
- 70.Suman Ahuja CBS, Ajay Kr S. Clinical evaluation of efficacy of kati basti and rasnadi guggulu in the management of katishoola (LUMBAGO). Journal of Ayurveda. 2010; 5–14.
- 71.Subbuthai M. A Clinical Study on Thandaga Vatham with Sagala Vatha Chooranam [Thesis]: Government Siddha Medical College, Palayamkottai; 2016.
- 72.Rai M, Dudhamal TS. A clinical evaluation of raktamokshana and trayodashanga guggulu in management of katigata vata wsr to lumbar spondylosis. Healer. 2020;1(1):7–18. 10.51649/healer.2 [DOI] [Google Scholar]
- 73.Dr. Gyan Prakash Sharma DOPS. A clinical study of Matra Basti & Kati Basti in the manegement of Gridhrasi w.s.r.to sciatica. Journal of Ayurveda. 2008; 55–60.
- 74.T Evangeline P. An open clinical Evaluation on “Thandaga Vatham (Lumbar Spondylosis)” with the siddha trial drugs “Jeevasakthi Thiravagam” (Internally)“Chennakara Pattai Thailam”(Externally) and “Kaduppu Vadhathirkku Poochu Therapy” [Thesis]: Government Siddha Medical College, Chennai; 2019.
- 75.Gupta S. A Comparative Clinical study of Kati Basti Patrapinda Sveda and Matra Basti in Kati Shoola [Thesis]: Dr. Sarvepalli Radhakrishnan Rajasthan Ayurved University; 2017.
- 76.Jensin Brintha L. A Prospective open labeled randomised clinical trial on Thandaga Vatham (Lumbar Spondylosis) with Vaeppam Pattai Kudineer [Thesis]: Government Siddha Medical College, Palayamkottai; 2018.
- 77.Nahitha Lubana A. A Prospective open labeled randomized clinical study on Cegana Vatham with Ingi Chooranam [Thesis]: Government Siddha Medical College, Palayamkottai; 2018.
- 78.Prasad JA. Efficacy of Agnikarma in the management of manyastambha-a clinical study [Thesis]: RGUHS; 2011.
- 79.Tekur P. Effect of yoga therapy on chronic low back pain a randomized control study [Thesis]: Swami Vivekananda Yoga Anusandhana Sansthana; 2012.
- 80.Dunleavy K, Kava K, Goldberg A, Malek MH, Talley SA, Tutag-Lehr V, et al. Comparative effectiveness of Pilates and yoga group exercise interventions for chronic mechanical neck pain: quasi-randomised parallel controlled study. Physiotherapy. 2016;102(3):236–42. 10.1016/j.physio.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 81.Kendre. Comparative Study of Efficacy of Katibasti Kalabasti and Anchan Lumber Traction in the Management of Gridhrasi [Thesis]: Sant Gadge Baba Amravati University; 2008.
- 82.Niranjana N. A study of Saganavatham [Thesis]: Government Siddha Medical College, Tirunelveli; 2013.
- 83.Kalaivani R. An Open Clinical evaluation on “saganavatham”(Cervical spondylosis) with siddha trial drug “Pooranathi Chooranam”(Internal),“Vali Kuthaluku Ulli Ennai”(External) and “Veppam Pinnakku Ottradam”(External Therapy) [Thesis]: Government Siddha Medical College, Chennai; 2019
- 84.Rajanandhini M. An open comparative clinical evaluation on “Sagana Vadham”(cervical spondylosis) with siddha trial drugs “Pancha Pashana Chendhuram”(internal),“Kurunthotti Thailam”(external) and “Varmam Therapy” [Thesis]: Government Siddha Medical College, Chennai; 2017.
- 85.Prakash N. A Prospective Open Labelled Non Randomised Phase-II Clinical Trial on Thandagavatham (Lumbar Spondylosis) with Munnai Ilai Kudineer [Thesis]: Government Siddha Medical College, Palayamkottai; 2019.
- 86.Sathyakala S. A study on cegana vatham. Chennai: National Institute of Siddha; 2012. [Google Scholar]
- 87.Prathiba M. A Study on Cegana Vaatham [Thesis]: National Institute of Siddha, Chennai; 2007.
- 88.Pasupathy T. A Prospective Open Labelled Non Randomized Phase-II Clinical Trial of “Appalakara Chooranam” for Cegana Vatham (Cervical Spondylosis) [Thesis]: Government Siddha Medical College, Palayamkottai; 2019.
- 89.Jeyabharathi S. A study on cegana vatham [master’s thesis on the intenet]. government siddha medical college. Palayamkottai: The Dr MGR Medical University; 2009. [Google Scholar]
- 90.Venkatachalam D. A Study on Sagana Vatham [Thesis]: Government Siddha Medical College, Palayamkottai; 2008.
- 91.Elakkia K. A Study on Cegana Vatham (Cervical Spondylosis): Government Siddha Medical College, Palayamkottai; 2018.
- 92.Nimeshika Devi SVL. A Study on Cegana Vatham (Cervical Spondylosis) [Thesis]: Government Siddha Medical College, Palayamkottai; 2019.
- 93.Malarvizhi M. A Study on Thandagavatham (Lumbar spondylosis) [Thesis]: Government Siddha Medical College, Palayamkottai; 2018.
- 94.Saranya Devi A. A Study on Thandaga Vatham (Lumbar Spondylosis) [Thesis]: Government Siddha Medical College, Palayamkottai; 2019.
- 95.Manju N. A Study on Thandaga Vatham (Lumbar spondylosis) [Thesis]: Government Siddha Medical College, Palayamkottai; 2013.
- 96.Shalini PYK. Clinical evaluation of shallaki niryas (boswellia serrata) in the management of Grivaasthi sandhi gata vata (cervical spondylosis). International Ayurvedic Medical Journal. 2013; 1–7.
- 97.Sangram Keshari Pradhan SRCM. A study on vaitaran basti with special reference to gridhrasi (Sciatica). Journal of Research in Ayurveda and Siddha. 2015; 72–88.
- 98.Jain M, Sahoo DP, Sahoo J, Kumar DS, Manik R. Effect of selected group of asana when used as an adjunct in management of cervical spondylosis of mild to moderate severity: an observational study. J Ayurveda Integr Med. 2021. 10.1016/j.jaim.2021.01.011. 10.1016/j.jaim.2021.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Devi S. Pre Clinical and Clinical study on Cegana Vatham and the drug of choice is Kariuppu Chenduram [Thesis]: National Institute of Siddha, Chennai; 2013.
- 100.Mallinath ZS. Study the efficacy of tryodashang guggulu in the management of grudhrasi [Thesis]: Bharati Vidyapeeth Deemed University; 2016.
- 101.Deebiga DR. Markable pain reduction in external therapy amukkara kilangu pattru. Int J Res Writ. 2020;2(6):86–91. [Google Scholar]
- 102.Mishra SS, Dash NC, Das BK. A clinical study on Gridhrasi (sciatica) and its management with nirgundi.9.
- 103.Prakash S, Singh SK. Ayurvedic Management for Gridhrasi with Special Reference to Sciatica-A Case Report. 2015.
- 104.Varsakiya J, Kumari R, Singh N. An Ayurvedic approach to prolapsed intervertebral disc (gridhrasi): a case report. J Res Ayurvedic Sci. 2020;4:38–43. 10.5005/jras-10064-0101 [DOI] [Google Scholar]
- 105.Siddiqui MMH, Anwar M, Shoaib M, Khan MSA. Effect of Hijdma bi6 Shart (Dry cupping therapy) in the management of Waja'al-'Unug (cervical Spondylosis).6.
- 106.Tejeswar Rao P, Gupta ML. Rumalaya in cervical and lumbar spondylosis. Ind Med Gaz. 1976;6:227. [Google Scholar]
- 107.Radhakrishnan P, Dhiman KS, Makhija R, Dua M, Deep VC, Sharma S, et al. Evaluation of the effect of jambira pinda sweda in cervical spondylosis w.r.t. Clinical symptomatology. J Res Ayurvedic Sci. 2020;4(1):18–25. 10.5005/jras-10064-0103 [DOI] [Google Scholar]
- 108.Shah IP. Assessment of the effectiveness of homoeopathic remedies in improving quality of life of chronic low back pain: a prospective study.
- 109.Shaikh N, Alam H. Effect of Hijama (Wet Cupping Therapy) In Sciatica Pain Management.
- 110.Pandey YK, Shalini SAK. Effect of griva vasti in management of griva asthi sandhi gata vata (cervical spondylosis). Anc Sci Life. 2013;33(1):71–5. 10.4103/0257-7941.134618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghufran BM, Quamri MA. Effect of dalk layyen with roghane gule aakh in cervical spondylosis–a pre and post analysis clinical study. Int Res J Med Sci. 2015;3(1):p5-8. [Google Scholar]
- 112.Yousuf KT, Wani AI. Effect of dalk layyain kaseer (soft massage) with roghan sosan (medicated oil) in slowing the progress of Waja-ul-Zahar (low back pain). Int J Adv Ayurveda, Yoga, Unani, Siddha Homeopat. 2018. 10.2395/cloud.ijaayush.357. 10.2395/cloud.ijaayush.357 [DOI] [Google Scholar]
- 113.Rajashri R. A Study on Sagana Vatham [Thesis]: Government Siddha Medical College, Palayamkottai; 2013
- 114.Selvi R. A Study on Thandaga Vatham [Thesis]: National Institute of Siddha, Chennai; 2013.
- 115.Usha A. A Study on Thandaga Vatham [Thesis]: National Institute of Siddha, Chennai; 2012.
- 116.Tarique M, Ansari AH, Zulkifle M. Effects of Hijamat bish Shart in Wajauz Zahr (Low back pain) and associated disability. 2016.
- 117.Patil NJ, Nagarathna R, Tekur P, Patil DN, Nagendra HR, Subramanya P. Designing, validation, and feasibility of integrated yoga therapy module for chronic low back pain. Int J Yoga. 2015;8(2):103–8. 10.4103/0973-6131.158470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deshmukh VPR, Pargaon S, Fadnavis VKK. Clinical Trial of Tikta Ksheera Basti in the Management of Lumbar Spondylosis.
- 119.Sharma K, Sahoo J, Sahu D, Chattopadhyay A, Kumar S, Mishra SS. Therapeutic evaluation of “Ayush Tulsi Jiwan Plus” oil for chronic musculoskeletal pain relief. Ayu. 2015;36(4):387–96. 10.4103/0974-8520.190687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leana R, Mihai B. Effect of Traumeel S associated with conventional treatment in patients with low-back pain. Med Sport: J Roman Sports Med Soc. 2017;13(1):2845. [Google Scholar]
- 121.Ahmad I, Nayab M, Ahmad T. Effect of gliding cupping with Roghan-e-Surkh in low back ache (Waja-uz-Zahr): a case series study. Drug Metab Pers Ther. 2021;36(3):247–50. 10.1515/dmpt-2020-0177 [DOI] [PubMed] [Google Scholar]
- 122.Vikram Kumar V. Preclinical and Clinical Study on Ceganavatham [Thesis]: National Institute of Siddha, Chennai; 2013.
- 123.Kumar A, Bhikshapati T. Kati Basti-a clinical study. AYU Int Quarter J Res Ayurveda. 2008;29(1):42. [Google Scholar]
- 124.Nilopher L. Preclinical and comparative clinical trial of Siddha drugs “Sigamani Chooranam” (Internal) and “Arkkasheerathy Thylam”(External) in the treatment of “Cagana Vaatham”(Cervical Spondylosis) with and without Varmam therapy [Thesis]: National Institute of Siddha, Chennai; 2019.
- 125.Sreedhana CR. An open comparative clinical study on “Thandaga Vatham” (Lumbar Spondylosis) with the evaluation of trial drugs “Naga Chendhuram”(Int)“Moolayoga Nirkundi Thailam”(Ext) and “Varmam Therapy” [Thesis]: Government Siddha Medical College, Chennai; 2017.
- 126.Rasakumar R. An Open Comparative Clinical Evaluation on “Sagana Vatham”(Cervical Spondylosis) with siddha herbal formulation drug “Kurunthotti Kashayam”(Internal),“Azhinjil Thylam”(External) and “Varmam Therapy [Thesis]: Government Siddha Medical College, Chennai; 2018.
- 127.Madhavikutty P, Deep VC, Radhakrishnan P, Jaya N, Channabasavanna BM, et al. A study on the comparative efficacy of samana and sodhana therapy in the treatment of Gridhrasi (sciatica) with dasamoola bala taila and Kwatha. J Res Ayurveda Siddha. 2016;37:78–86. [Google Scholar]
- 128.Goel S, Sharma O, Arya D, Lavaniya V. Clinical observation of panchamrit lauha guggulu and panchguna taila in the management of greevastambha (cervical spondylosis): retrospective analysis. J Res Ayurvedic Sci. 2019;2(3):176–9. [Google Scholar]
- 129.M. Saad Ahmad Khan AR. Clinical Study of Wajul- Fiqaria Unqi (Cervical Spondylosis) and Efficacy of Safoof-e- Suranjan and Habb-e-Gul-e- Aakh along with Exercise and Massage with Roghan-e- Baboona. Hippocratic Journal of Unani Medicine. 2012; 25–35.
- 130.Thakur DT, Rao DBV. Chronic lower back pain treated with combination of individualised homoeopathy and yoga- a novel case study.5.
- 131.Muthumari KR. A clinical study on Vathasthambam (Sciatica) with the evaluation of siddha drug Ayakaantha Chendhooram [Thesis]: Government Siddha Medical College, Chennai; 2017.
- 132.Bhatted S, Gauttam J, Yadav U. Management of spondylosis induced sciatica through panchakarma wsr to vata kaphaja gridhrasi-a case study. J Ayurveda Integr Med Sci. 2019;4(4):366. [Google Scholar]
- 133.Aarthy K. Pre clinical and comparative clinical trial of Siddha drugs Raajamaarthandha Ilagam (Internally) and Vaathakajakesari Thylam (Externally) in the treatment of Vathasthambam (Sciatica) with and without Varmam therapy [Thesis]: National Institute of Siddha, Chennai; 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table 1: PRISMA 2020 for Abstract checklist. Table 2: PRISMA 2020 checklist. Table 3: Inclusion and Exclusion criteria. Table 4: Diagnostic classification and codes for cervical and lumbar spondylosis in Allopathy and Ayush systems of medicine. Table 5: Database specific search terms and the number of articles retrieved. Figure 1: Description of selected articles based on Population, Intervention and study design. Figure 2: Publication of Ayush studies on Cervical and lumbar spondylosis during 1976-2021. Table 6: General Characteristics of selected studies. Table 7: Description of selected articles with comparison group. Table 8: Description of selected articles without comparison group. Table 9: Compliance of research mandates reported in selected articles. Table 10: Risk of bias assessment of selected articles. Table 11: Risk of bias assessment for Randomized Controlled Trials (RoB2). Table 12: Risk of bias assessment for Non- Randomized Controlled Trials (ROBINS-I tool). Table 13: Risk of bias assessment for Case reports (JBI tool). Table 14: Risk of bias assessment for Case series (JBI tool).
Data Availability Statement
The datasets used and analysed during the present study are available from the corresponding author upon reasonable request.