Abstract
The NS3 protein of hepatitis C virus (HCV) is a bifunctional protein containing a serine protease in the N-terminal one-third, which is stimulated upon binding of the NS4A cofactor, and an RNA helicase in the C-terminal two-thirds. In this study, a C-terminal hexahistidine-tagged helicase domain of the HCV NS3 protein was expressed in Escherichia coli and purified to homogeneity by conventional chromatography. The purified HCV helicase domain has a basal ATPase activity, a polynucleotide-stimulated ATPase activity, and a nucleic acid unwinding activity and binds efficiently to single-stranded polynucleotide. Detailed characterization of the purified HCV helicase domain with regard to all four activities is presented. Recently, we published an X-ray crystallographic structure of a binary complex of the HCV helicase with a (dU)8 oligonucleotide, in which several conserved residues of the HCV helicase were shown to be involved in interactions between the HCV helicase and oligonucleotide. Here, site-directed mutagenesis was used to elucidate the roles of these residues in helicase function. Four individual mutations, Thr to Ala at position 269, Thr to Ala at position 411, Trp to Leu at position 501, and Trp to Ala at position 501, produced a severe reduction of RNA binding and completely abolished unwinding activity and stimulation of ATPase activity by poly(U), although the basal ATPase activity (activity in the absence of polynucleotide) of these mutants remained intact. Alanine substitution at Ser-231 or Ser-370 resulted in enzymes that were indistinguishable from wild-type HCV helicase with regard to all four activities. A mutant bearing Phe at Trp-501 showed wild-type levels of basal ATPase, unwinding activity, and single-stranded RNA binding activity. Interestingly, ATPase activity of this mutant became less responsive to stimulation by poly(U) but not to stimulation by other polynucleotides, such as poly(C). Given the conservation of some of these residues in other DNA and RNA helicases, their role in the mechanism of unwinding of double-stranded nucleic acid is discussed.
Hepatitis C virus (HCV) is recognized as the main etiologic agent of parenterally transmitted non-A, non-B hepatitis (4, 24) and is responsible for a large proportion of cases of community-acquired hepatitis. The majority of HCV infections become persistent, leading to chronic hepatitis, liver cirrhosis, or even hepatocellular carcinoma (reviewed in reference 13). Development of an effective vaccine against HCV has been slow and difficult, in part due to the quasi-species nature of the virus. Approved therapies for viral hepatitis type C (hepatitis C) include alpha interferon and the combination of alpha interferon and ribavirin, which induce limited long-term response in hepatitis C patients and have relatively severe side effects. Therefore, it is of great interest to develop effective, HCV-specific therapeutic drugs.
HCV is now classified in the Hepacivirus genus (reviewed in reference 13) of the Flaviviridae family (reviewed in reference 40), which includes two additional genera, Flavivirus and Pestivirus. The recently discovered hepatitis G virus (HGV or GBV-C) is closely related to HCV and has been proposed to be the fourth group in the Flaviviridae family. The majority of the 9.6-kb HCV genome encodes a large open reading frame corresponding to a polyprotein precursor of about 3,000 amino acids, which is flanked by 5′ and 3′ noncoding regions. The polyprotein precursor of the HCV H strain is proteolytically processed by cellular signal peptidase(s) and two HCV-encoded proteases into at least 10 distinct products, with the sequence NH2 - C - E1 - E2 - p7 - NS2 - NS3 - NS4A - NS4B - NS5A - NS5B - COOH (7–9, 27). The putative structural proteins include a core protein (C) and two envelope proteins (E1 and E2), whereas the nonstructural (NS) proteins, including two proteases, a combined helicase and nucleoside triphosphatase (NTPase), and an RNA-dependent RNA polymerase, are believed to be components of a complex responsible for viral RNA replication. The C-terminal 450 amino acids of the NS3 protein manifest a polynucleotide-stimulated NTPase activity (14, 45), a 3′-to-5′ unwinding activity (10, 14, 18, 46), and a single-stranded (ss) polynucleotide binding activity (10, 17, 46). Recently, elegant biochemical characterizations of this helicase have been presented by Porter and colleagues and Preugschat et al. (36–39).
Helicases are enzymes that are able to separate the strands of duplex DNA or RNA by utilizing energy derived from hydrolysis of nucleoside 5′-triphosphates (NTPs, usually ATP) (reviewed in reference 30). Accordingly, the NTPase activity of helicases can be stimulated by polynucleotide. More than 200 proteins have been identified as putative helicases based on the presence of some or all of seven conserved amino acid motifs (for a recent review, see reference 6). Until now, only a handful of these putative helicases have been purified and shown to have duplex nucleic acid unwinding activity. Helicases have been shown to be involved in DNA metabolism (replication, repair, and genome recombination) (reviewed in references 30 and 32) and RNA metabolism (transcription, splicing or processing, transport, and translation initiation of RNA) (for a recent review, see reference 31). Mutations in several helicase genes have been associated with six human genetic disorders: Werner’s syndrome, Bloom’s syndrome, xeroderma pigmentosum, trichothiodystrophy, Cockayne’s syndrome, and α thalassemia-related mental retardation associated with the X chromosome (for a review, see reference 5). Helicases may also play critical roles in processes involved in the life cycles of many DNA and RNA viruses, including replication and recombination of viral DNA or RNA genome, RNA transcription, and translation (for a review, see references 16 and 21).
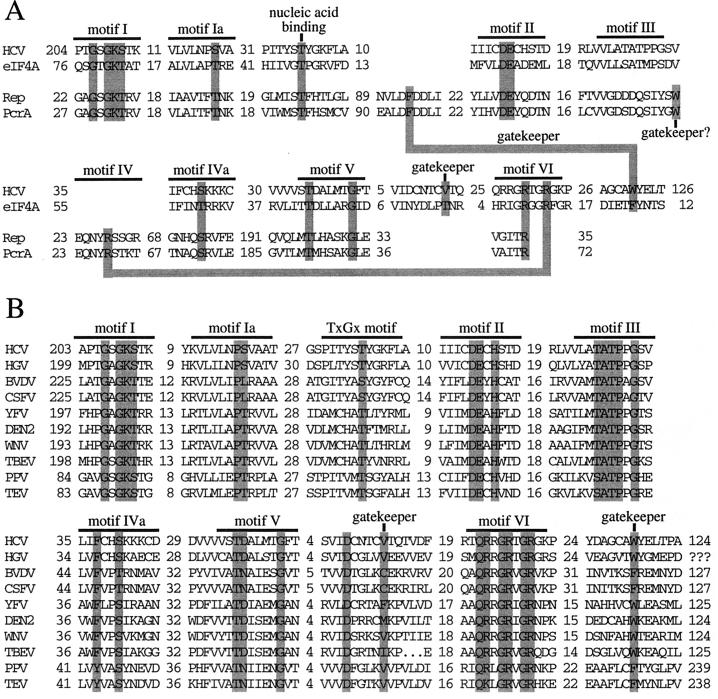
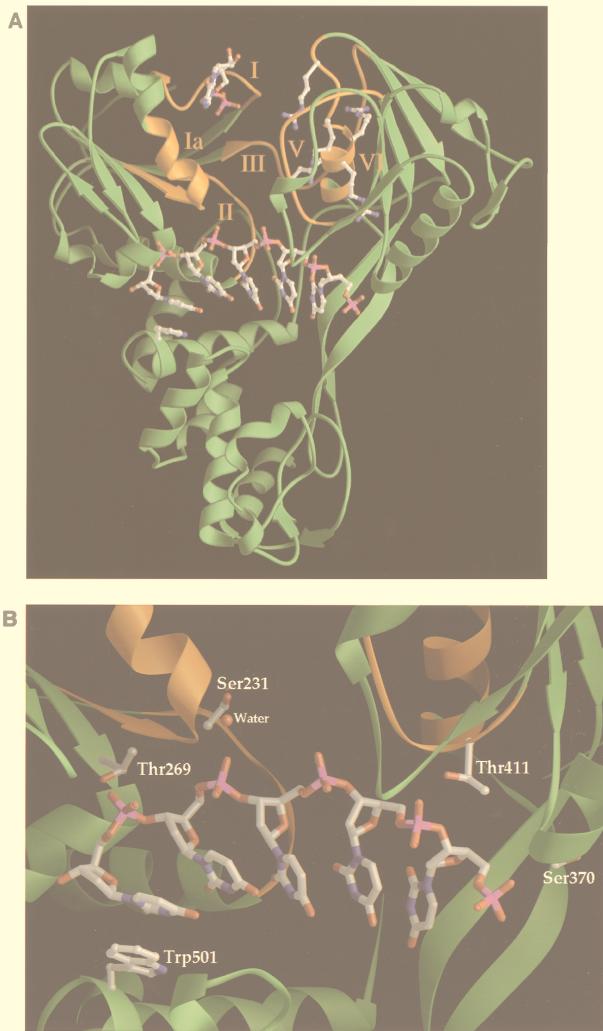
Recently, four groups reported the X-ray crystallographic structures of three different helicases, i.e., the DNA helicases PcrA (44) and Rep (22) and the HCV NS3 RNA helicase (20, 48). These structures demonstrated that helicases have many common features in terms of protein folding and tertiary structure, although there is very limited homology in their primary amino acid sequences. For all three helicases, most of the six or seven conserved motifs (motifs I through VI) (see Fig. 4A) are located at similar positions within a cleft between two domains of the enzyme (see Fig. 3A, domains 1 and 2 of the HCV RNA helicase, and domains 1A and 2A of Rep or PcrA helicases (22, 44). The NTP-binding pocket, composed of residues of motifs I and II, is located on the side of domain 1 in this cleft. Our X-ray structural analysis of the HCV helicase bound to an ss (dU)8 oligonucleotide showed that the oligonucleotide binds in a groove that separates domain 3 from domains 1 and 2 (reference 20, and also see Fig. 3A). This nucleic acid-binding groove is oriented perpendicular to the NTP-binding cleft between domains 1 and 2. In this structure, most of the interactions between the enzyme and bound (dU)8 oligonucleotide involve hydrogen bonds with the phosphate backbone but not the bases of the oligonucleotide (20), which explains the sequence-independent nature of these helicases with regard to the duplex nucleic acid substrate. The most significant enzyme-base interaction involves a hydrophobic stacking interaction between Trp-501 of the HCV helicase and a base near the 3′ end of the bound (dU)8 oligonucleotide (see Fig. 3B). This structural analysis identifies several amino acids in this nucleic acid-binding groove that interact with the oligonucleotide (see Fig. 3B). Although some of these residues are conserved within various HCV strains, they are not a part of the previously reported conserved helicase motifs (motifs I through VI) (see Fig. 4A). In the present study, site-directed mutagenesis was used to investigate the role of these residues in the mechanism of action of the HCV NS3 helicase.
FIG. 4.
Alignment of functional conserved motifs or residues of selected helicases from SF1 and SF2. (A) Alignment of several conserved motifs and other functional conserved residues of two SF2 RNA helicases (HCV NS3 helicase and the eukaryotic translation factor eIF4A) or two SF1 DNA helicases (Rep and PcrA helicases) is shown. It should be noted that the previously predicted motif IV exists only in SF1 helicases and not in SF2 helicases. Homologous residues with similar function in the two superfamilies are highlighted by shadowed boxes. The number of amino acids between any two motifs is indicated. (B) Sequence alignment of several viral SF2 helicases, which are homologous to the HCV NS3 helicase. These proteins include the homologous NS3 proteins of HGV, two pestiviruses (bovine viral diarrhea virus [BVDV] and classical swine fever virus [CSFV]), and four flaviviruses (yellow fever virus [YFV], dengue virus 2 [DEN2], West Nile virus [WNV], and tick-borne encephalitis virus [TBEV]). Also shown is CI helicase of two plant potyviruses, plum pox virus (PPV) and tobacco etch virus (TEV). Again, homologous residues with functions presumably similar to those of their counterparts of the HCV NS3 helicase are highlighted by shadowed boxes.
FIG. 3.
(A) Locations of conserved motifs in the ternary complex of HCV NS3 helicase, (dU)8 oligonucleotide, and ADP. The overall fold of the HCV NS3 helicase is illustrated by a green ribbon diagram. The HCV NS3 helicase consists of three domains: domain 1 (top left), domain 2 (top right), and domain 3 (bottom). The conserved amino acid motifs I through VI, which are located at the interface between domains 1 and 2, are colored orange and labeled with the corresponding Roman numerals. The bound (dU)8 oligonucleotide, shown in stick form, binds in the groove that separates domain 3 from the other two domains. ADP, shown in stick form, binds to the phosphate binding loop (motif I) of domain 1. Four arginine residues (Arg-461, Arg-462, Arg-464, and Arg-467) of motif VI (QRRGRTGR) in domain 2 are shown in stick form. The side chains of Arg-464 and Arg-467 point into the interdomain cleft between domains 1 and 2, while that of Arg-461 points toward domain 3. Trp-501 of domain 3, shown in stick form, stacks against the 3′ uridine base of the bound oligonucleotide. (B) Close-up view of the RNA-binding groove of the HCV RNA helicase. Five nucleotides of the bound (dU)8 oligonucleotide are shown in stick form. Trp-501 stacks on a base at the 3′ end of the oligonucleotide, while the γ-OH groups of four HCV helicase residues (Ser-231, Thr-269, Ser-370, and Thr-411) form either direct or water-mediated hydrogen bonds with the phosphate backbone of the bound (dU)8 oligonucleotide.
MATERIALS AND METHODS
Construction of plasmids and site-directed mutagenesis.
The HCV NS3 helicase domain (the C-terminal 465 amino acids of the 631-residue NS3 protein) was subcloned from a cDNA of the HCV strain H (9, 28) into a pET expression vector as described previously (20). The resulting plasmid, pET-BS(+)/HCV/NS3-C465-His, was used as a template for ss phagemid DNA-based, site-directed mutagenesis performed as described previously (23, 26) with some minor modifications described below. The phagemid ssDNA packaged in the presence of M13 helper phage corresponds to the HCV positive strand. A single colony of the Escherichia coli strain CJ 326, transformed with pET-BS(+)/HCV/NS3-C465-His, was grown in YT medium containing 0.25 μg of uridine/ml and 50 μg of carbenicillin/ml. After three serial passages, the M13 helper phage (Bio-Rad) was used to rescue uridylated phagemid ssDNA, which was then used as a template for oligonucleotide-directed mutagenesis (23). ABI automatic sequencing (PE Applied Biosystems, Foster City, Calif.) was used to confirm mutations and ensure that there were no other, unintended mutations within the HCV NS3 helicase domain sequences. Each construct containing a mutation was named according to the position of the substituted residue in the full-length HCV NS3 protein.
Protein expression and purification.
The wild-type (wt) or mutated HCV NS3 helicase proteins were expressed in the E. coli strain BL21(DE3) after IPTG (isopropyl-β-d-thiogalactopyranoside) induction essentially as described before (20). All the protein purification procedures were performed at 4°C. Typically, 10 g of cell paste was resuspended in 50 ml of buffer A (50 mM HEPES [pH 8.0], 300 mM NaCl, 10% glycerol, and 2.5 mM β-mercaptoethanol) containing 0.2 mM phenylmethylsulfonyl fluoride and lysed using a microfluidizer. The lysate was clarified by centrifugation at 100,000 × g for 35 min. Five millimolar imidazole (pH 8.0) was added to the supernatant, and the resulting solution was batch absorbed with 2 ml of Ni-nitrilotriacetic acid (NTA)-agarose (Qiagen) for 2 h at 4°C. The resin was packed into a column, washed with 10 bed volumes of buffer A containing 5 mM imidazole followed by 10 bed volumes of buffer A containing 15 mM imidazole, and eluted with buffer A containing 100 mM imidazole. The eluate was desalted with buffer B (50 mM HEPES [pH 8.0], 10% glycerol, and 2.5 mM β-mercaptoethanol) containing 50 mM NaCl on a PD-10 column (Pharmacia). The desalted solution was loaded onto a heparin-Sepharose column (Pharmacia). The flowthrough was then applied onto a Q-Sepharose column (Pharmacia) and washed with 10 bed volumes of buffer B containing 50 mM NaCl. The column was then eluted with a gradient of NaCl ranging from 50 mM to 2 M in buffer B. The peak fraction containing the HCV NS3 helicase domain protein was shown by gel filtration chromatography to be monomeric. The purified protein was confirmed to be greater than 90% pure by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Coomassie brilliant blue R-250 staining. Protein concentration was estimated by UV absorption spectroscopy at 280 nm by using a molar extinction coefficient of 64,000 M−1 cm−1 (39).
Helicase assay.
The standard 3′-tailed double-stranded RNA-DNA hybrid was prepared as described before (20). The 98-nucleotide RNA template was transcribed from a BsrBI-digested plasmid, pSP65 (Promega), in the presence of [α-32P]GTP (New England Nuclear). The 34-nucleotide DNA release strand corresponds to an SP6 RNA transcript from a BamHI-digested pSP64 (Promega). Standard helicase unwinding reactions (reaction volume, 20 μl) were carried out as follows, unless noted otherwise in the text. HCV NS3 helicase domain protein (0.3 nM) was added to a mixture of 25 mM MOPS (morpholinepropanesulfonic acid)–NaOH (pH 6.5), 1 mM ATP, 0.5 mM MnCl2, 2 mM dithiothreitol (DTT), 0.1 mg of bovine serum albumin (BSA) per ml, 4 U of RNasin (Promega), and 5 nM 3′-tailed double-stranded RNA-DNA hybrid substrate. Mixtures were incubated for 20 min at 37°C, and the reaction was stopped by the addition of 5 μl of 5× loading buffer (100 mM Tris-Cl [pH 7.5], 20 mM EDTA, 50% glycerol, 0.5% SDS, 0.1% NP-40, 0.1% bromophenol blue, and 0.1% xylene cyanol). The reaction products were then analyzed by 10% PAGE with 0.5× Tris-borate-EDTA and 0.1% SDS. The gels were dried and exposed by using a Fuji 1500 phosphorimager. Helicase activity was determined by measuring the radioactivity of the double-stranded substrate and the ss template.
ssRNA binding assay.
The binding of ssRNA to the HCV NS3 helicase was measured by a nitrocellulose filter-binding assay. A 34-nucleotide RNA transcript was generated from a BamHI-digested pSP64 plasmid by using SP6 RNA polymerase in the presence of [α-32P]GTP. Standard ssRNA-binding reactions (reaction volume, 40 μl) were carried out as follows, unless noted otherwise in the text. The HCV NS3 helicase domain protein (6.25 nM) was added to a mixture containing 25 mM MOPS–NaOH (pH 7.0), 2 mM DTT, 0.1 mg of BSA per ml, 4 U of RNasin (Promega), and 5 nM 32P-labeled ssRNA substrate. Mixtures were incubated for 15 min at 30°C and filtered through a prewet nitrocellulose membrane (Millipore). Filters were washed twice with washing buffer (50 mM MOPS-NaOH [pH 7.0] and 1 mM EDTA), dried, and quantified in a scintillation counter.
ATPase assay.
The hydrolysis of ATP to ADP was measured by a thin-layer chromatography method. Standard ATPase reactions (reaction volume, 10 μl) were carried out as follows, unless noted otherwise in the text. The HCV NS3 helicase domain protein (2 nM) was added to a mixture containing 50 mM MOPS–NaOH (pH 7.0), 0.1 mM ATP, 2.5 μCi of [α-32P]ATP (New England Nuclear), 0.5 mM MgCl2, 1 mM DTT, and 0.1 mg of BSA per ml. Reaction mixtures were incubated in the absence or presence of 5 μM poly(U) (5 μM refers to the uridine concentration) (Pharmacia) for 30 min at 37°C, and the reaction was terminated by the addition of EDTA to a final concentration of 20 mM. A 5-μl sample of the reaction product was spotted on a polyethyleneimine-cellulose plate (E. Merck), and 32P-labeled ATP and ADP were separated by ascending chromatography in 0.375 M potassium phosphate (pH 3.5) and quantified by a Fuji 1500 phosphorimager.
RESULTS
Expression and purification of HCV NS3 helicase domain.
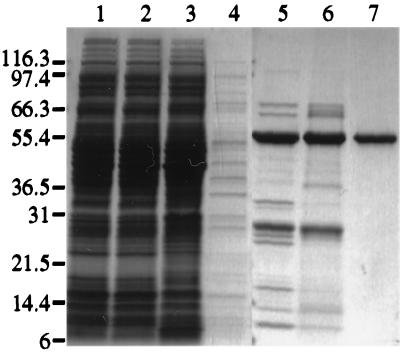
A cDNA encoding the HCV NS3 helicase domain (the C-terminal 465 amino acids of the 631-residue NS3 protein) was subcloned into a pET-based vector for high-level expression in the E. coli strain BL21(DE3). A hexahistidine tag was fused to the C terminus of the HCV NS3 helicase domain to facilitate protein purification. Expression of the recombinant HCV NS3 helicase protein was induced by the addition of IPTG. Cell paste was lysed by using a microfluidizer, and cell lysate (Fig. 1, lane 1) was clarified by centrifugation at 100,000 × g. The supernatant (lane 2) was batch absorbed to Ni-NTA-agarose. The Ni-agarose beads were then packed into a column, washed, and eluted with the buffer A containing 100 mM imidazole. The eluate (lane 5) was desalted and applied onto a heparin-Sepharose column. Most proteins, including the HCV helicase domain, did not bind to the heparin-Sepharose column. The flowthrough (lane 6) was then loaded onto a Q-Sepharose column, and the HCV NS3 helicase domain protein was eluted with an NaCl gradient in buffer B (Fig. 1, lane 7). As shown in lane 7, the peak fraction of HCV helicase protein had a purity of more than 90%. The identity of the purified HCV helicase domain protein was confirmed by internal amino acid sequencing analysis (data not shown).
FIG. 1.
Purification of recombinant HCV NS3 helicase domain protein from E. coli. The wt or mutated HCV helicase domain proteins were expressed in E. coli BL21(DE3) and purified by affinity and ion-exchange chromatography as described in the Materials and Methods section. The main fractions collected at each purification step were analyzed by SDS-PAGE followed by Coomassie brilliant blue R-250 staining. Lane 1, crude lysate of E. coli cells obtained after lysis by microfluidization; lane 2, supernatant collected after 100,000 × g centrifugation; lanes 3 and 4, flowthrough fraction and 15 mM imidazole washing fraction, respectively, from the Ni-NTA agarose column; lane 5, peak fraction of 100 mM imidazole eluate from the Ni-NTA agarose column; lane 6, flowthrough fraction from the heparin-Sepharose; lane 7, peak fraction containing the HCV NS3 helicase domain protein eluted from Q-Sepharose. The sizes (expressed in kilodaltons) of molecular mass markers are indicated at the left. The purified HCV NS3 helicase domain protein was shown by gel-filtration chromatography to be monomeric and judged to be greater than 90% pure based on the SDS-PAGE with Coomassie brilliant blue R-250 staining.
Helicase unwinding activity of the HCV NS3 helicase domain protein.
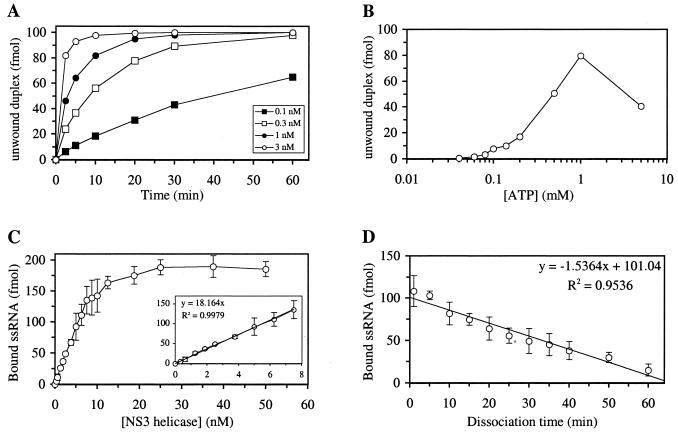
Unwinding activity of the purified HCV helicase domain was characterized with regard to the following parameters: protein concentration, incubation time, incubation temperature, ATP concentration, pH, and concentration of monovalent cation (Na+) or divalent cation (Mn2+ or Mg2+). The amounts of unwound products increased as protein concentration or incubation time increased (Fig. 2A). At a concentration of 0.1 nM for the HCV NS3 helicase, the reaction rate was nearly linear at incubation times up to 30 min (Fig. 2A). Several HCV helicase mutants purified by the same chromatographic method did not show any unwinding activity (Table 1), indicating that the unwinding activity shown here is due to the purified HCV NS3 helicase and not to contaminant proteins from E. coli. Higher incubation temperature also led to more rapid unwinding of the substrate (data not shown), presumably due to a lower energy requirement for breaking the hydrogen bonds between the two nucleic acid strands at a higher temperature. The unwinding activity of the HCV NS3 helicase domain was optimal at pH 6.5 and displayed a very narrow pH window (data not shown). In addition, the unwinding reaction was very sensitive to the presence of monovalent cation, such as Na+ (data not shown). Addition of 25 mM NaCl decreased the unwinding activity by 85% compared to that in the absence of NaCl. The helicase activity was absolutely dependent on the presence of ATP (Fig. 2B) or other NTPs (data not shown). The unwinding activity increased almost linearly with ATP concentration up to 1 mM (Fig. 2B). However, the unwinding activity was lower at 5 mM ATP than at 1 mM ATP, probably due to inhibition by Na+ introduced together with the ATP. The helicase activity was also dependent on the presence of divalent cation, such as Mn2+ or Mg2+ (data not shown). However, if the concentration of divalent cation was higher than that of ATP, inhibition of the helicase activity was observed. At equal concentrations of ATP and divalent cation (1 mM or 5 mM), the HCV NS3 protein showed higher unwinding activity in the presence of Mn2+ than that in the presence of Mg2+ (data not shown).
FIG. 2.
Characterization of duplex nucleic acid unwinding and ssRNA binding activities of the wt HCV NS3 helicase. Standard helicase unwinding reactions (reaction volume, 20 μl) with the 3′-tailed double-stranded RNA-DNA hybrid substrate were carried out as described in the Materials and Methods section unless noted otherwise below. Effects of various parameters were examined as follows. (A) For characterization of the effect of enzyme concentration, 0.1, 0.3, 1, or 3 nM enzyme was incubated with 5 nM duplex substrate at 37°C for 0, 2.5, 5, 10, 20, 30, or 60 min. (B) For ATP 0.3 nM enzyme was incubated with 5 nM duplex substrate for 20 min at 37°C in the presence of 0.04, 0.06, 0.08, 0.1, 0.14, 0.2, 0.5, 1, or 5 mM ATP (the sodium salt form). Standard ssRNA binding reactions (reaction volume, 40 μl) with the 32P-labeled 34-nucleotide ssRNA were carried out as described in the Materials and Methods unless noted otherwise below. Effects of various parameters were examined as follows. (C) For examination of the effect of enzyme concentration, 0.3125, 0.625, 1.25, 1.875, 2.5, 3.75, 5, 6.25, 7.5, 8.75, 10, 12.5, 18.75, 25, 37.5, or 50 nM enzyme was incubated with 5 nM 32P-labeled ssRNA substrate at 30°C for 15 min. (D) For examination of the effect of the dissociation of ssRNA from the HCV helicase, 6.25 nM enzyme was incubated with 5 nM 32P-labeled ssRNA for 15 min at 30°C, and then 250 nM 3H-labeled ssRNA was added to the reaction mixture and incubated at 30°C for 1, 5, 10, 15, 20, 25, 30, 35, 40, 50, or 60 additional min. The amount of 32P-labeled ssRNA which was bound to HCV NS3 helicase was shown.
TABLE 1.
Structure-based mutagenesis of RNA-binding residues of HCV NS3 helicase
| Helicase | % activity fora:
|
|||
|---|---|---|---|---|
| Basal ATPase | Poly(U)-stimulated ATPase | ssRNA binding | Duplex nucleic acid unwinding | |
| wt | 100 | 823 | 100 | 100 |
| Ser-231>Ala | 260 | 709 | 121 | 100 |
| Thr-269>Ala | 60 | 47 | 21 | 1 |
| Ser-370>Ala | 104 | 694 | 124 | 109 |
| Thr-411>Ala | 274 | 205 | 24 | <1 |
| Trp-501>Phe | 99 | 197 | 100 | 112 |
| Trp-501>Leu | 114 | 47 | 21 | <1 |
| Trp-501>Ala | 101 | 49 | 40 | <1 |
The wt or mutated HCV NS3 helicase was tested for basal ATPase, poly(U)-stimulated ATPase, ssRNA binding, and duplex nucleic acid unwinding activities. The activities of each mutant were converted to a percentage relative to the corresponding activity of the wt HCV helicase (taken as 100%) except for the poly(U)-stimulated ATPase activity, for which the activity of the wt or mutated helicase was compared to the basal ATPase activity of the wt HCV helicase.
ssRNA binding of the HCV NS3 helicase protein.
We measured several parameters of ssRNA binding to the purified HCV helicase by using a filter-binding assay. The association of 32P-labeled ssRNA to the HCV NS3 helicase was close to completion within a few minutes after mixing (data not shown). As shown in Fig. 2C, binding of ssRNA to the HCV helicase was protein concentration dependent. The amount of ssRNA bound was a linear function of the HCV helicase protein concentration up to 8 nM (Fig. 2C, insert), and 0.454 molecule of ssRNA was bound for every molecule of HCV NS3 helicase present in the reaction mixture. The maximal amount of ssRNA binding achieved in this reaction was close to 95%. The apparent Kd of the ssRNA-HCV NS3 helicase complex, at which 50% of maximal binding of ssRNA to the HCV NS3 helicase domain was observed, was calculated to be 5.22 nM. We also measured the off-rate constant of preformed ssRNA-HCV NS3 helicase complex (Fig. 2D). In this case, 32P-labeled ssRNA was incubated with the HCV NS3 helicase protein for 15 min to allow formation of the 32P-labeled ssRNA-HCV NS3 helicase complex. Then a 50-fold excess of 3H-labeled ssRNA with the same sequence was added to the mixture to prevent de novo association of the RNA-free HCV NS3 helicase with 32P-labeled ssRNA. The dissociation rate was determined to be 1.52 × 10−2 min−1, which is consistent with the apparent Kd value of 5.22 nM if the association is limited only by physical diffusion. We also examined effects of pH and addition of monovalent (Na+) or divalent (Mn2+) cation on the interaction between ssRNA and HCV helicase. In comparison to the unwinding activity, ssRNA binding to the HCV NS3 helicase was less sensitive to change in pH (data not shown). Optimal binding was observed at pH 7.0, although ssRNA binding dropped only slightly at pH 6.5 or 8.0. NaCl and MnCl2 had inhibitory effects on ssRNA binding, although this curve for inhibition as a function of salt concentration was not as sharp as that for unwinding activity (data not shown).
Polynucleotide-stimulated ATPase of the HCV NS3 helicase.
ATPase activity of the purified HCV NS3 helicase was also examined by using a thin-layer chromatography method. As shown previously by others (45), this HCV helicase protein had a basal ATPase activity (activity in the absence of any polynucleotide), and the ATPase activity was stimulated severalfold in the presence of poly(U) (data not shown). The order of ATPase stimulation by polynucleotides was poly(U)>poly(C)>poly(A)>poly(G) (data not shown).
Structure-based mutagenesis study of the HCV NS3 helicase.
Site-directed mutagenesis experiments were performed to help elucidate the roles of several residues which appear to stabilize the interaction between HCV helicase and ss nucleic acid as assessed based on our recently published X-ray crystal structure of an HCV NS3 helicase-(dU)8 oligonucleotide complex (Fig. 3B) (20). Individual substitutions were introduced at the following residues: Thr-269, Thr-411, Ser-231, Ser-370, and Trp-501 (Table 1). The γ-OH groups of Thr-269 and Thr-411 form direct hydrogen bonds with phosphate oxygens of the bound oligonucleotide in our structure of the binary complex, while the γ-OH groups of Ser-231 and Ser-370 make water-mediated contacts with phosphate oxygens (Fig. 3B). Mutant enzyme bearing either the substitution of Ala at Ser-231 or the substitution of Ala at Ser-370 was indistinguishable from wt HCV helicase with regard to basal or poly(U)-stimulated ATPase and unwinding activities as well as ssRNA binding (Table 1). Alanine substitution at Thr-269 or Thr-411 decreased RNA binding to 20% of wt levels, indicating that the γ-OH group of each threonine residue is required for efficient binding of nucleic acid substrate. Additionally, both mutants bearing substitution of Ala for Thr were completely deficient in poly(U)-stimulated ATPase and nucleic acid unwinding activities (Table 1). Since these two mutated proteins retained levels of basal ATPase activity similar to those of the wt HCV helicase, the reduction in RNA binding ability was unlikely to have been caused by a deleterious conformational change in the mutant protein and was probably caused by the loss of a single hydrogen bond to a backbone phosphate of the nucleic acid.
In our structure of the HCV helicase-(dU)8 oligonucleotide binary complex, Trp-501 forms a stacking interaction with the 3′ uridine base of the (dU)8 oligonucleotide (Fig. 3B) (20). Replacement of this tryptophan with either leucine or alanine decreased RNA binding and abolished unwinding activity and enhancement of ATPase activity by poly(U) (Table 1). Again, the presence of wt levels of basal ATPase activity in these mutated proteins indicated that the loss of other helicase activities was unlikely to have been due to gross disruption of protein structure upon introduction of these mutations. The mutant enzyme bearing Phe at Trp-501 displayed wt levels of basal ATPase and unwinding activities as well as ssRNA binding ability. These results indicate that ring-to-ring stacking of Trp-501 with a base of the nucleic acid substrate is required for the unwinding activity of this helicase. Interestingly, the mutant bearing Phe at Trp-501 was less sensitive than the wt helicase to stimulation of ATPase activity by poly(U) but not by other polynucleotides, such as poly(C) (data not shown). The reasons for this are unclear.
DISCUSSION
Gorbalenya and Koonin have proposed that helicases be classified into three superfamilies (SF1, SF2, and SF3) and two (smaller) families (6). SF1 and SF2 are the two largest superfamilies of helicase proteins that contain all seven conserved motifs, although the sequences of these motifs differ between the two superfamilies (Fig. 4A). Motifs I and II, the so-called “Walker motifs A and B” (47), are shared by all helicases as well as a wide variety of other NTP-utilizing proteins. The roles of these two motifs in NTP binding and hydrolysis are well documented, while the functions of residues in the remaining motifs are less clear. Recently published X-ray crystal structures of three helicases, the DNA helicases PcrA (44) and Rep (22) from SF1 and an RNA helicase, the HCV NS3 protein (20, 48), from SF2, demonstrated that, for all three helicases, most of the seven conserved motifs are located at similar positions along a cleft separating two structurally similar domains (Fig. 3A). In addition, the X-ray crystal structures of Rep helicase (22) and the HCV NS3 helicase (20) contain an ss oligonucleotide, which binds in a groove oriented perpendicularly to the NTP-binding cleft (Fig. 3A). This orientation of the bound oligonucleotide is roughly perpendicular to that in models proposed by two other groups based on their X-ray crystal structures of HCV helicase without oligonucleotide (3, 48). These models for nucleic acid binding were influenced largely by an earlier mutagenesis study of the eukaryotic translation initiation factor eIF-4A (34) and the observation of the presence in domain 2 of HCV helicase of three arginine residues of motif VI (Fig. 4), which line the cleft separating domains 1 and 2 (Fig. 3A).
The mutagenesis results described here demonstrate that replacement of HCV NS3 residue Thr-269 or Thr-411 with alanine leads to abolishment of stimulation of ATPase activity by poly(U) and complete loss of unwinding activity. Binding of HCV helicase to ssRNA is also greatly diminished in both mutated proteins. These results support our earlier structural observation that these two threonine residues play a critical role in binding of HCV helicase to nucleic acid. Thr-269 (in domain 1) makes a hydrogen bond to a phosphate oxygen near the 3′ end of the bound oligonucleotide, while Thr-411 (in domain 2) participates in a very similar interaction near the 5′ end of the oligonucleotide. Each interaction is stabilized by a hydrogen bond between the threonine γ-oxygen and an NH group in the main chain of the protein (the NH group of Lys-272 bonds with Thr-269 and the NH group of Ala-413 bonds with Thr-411). Superimposition of domains 1 and 2 of HCV helicase shows that these two threonine residues are indeed very similar in their positions and interactions with nucleic acids (see Fig. 2 in reference 20).
Thr-269 is part of the “TxGx” motif, which was originally identified by Pause and Sonenberg as a unique motif for a subgroup of so-called DEAD, DEAH, and DExH helicases within SF2 (35). The equivalent threonine residue can be identified in other SF2 helicases, such as the eukaryotic translation initiation factor eIF-4A (Fig. 4A), and CI protein of potyviruses, a group of plant viruses (Fig. 4B). This threonine is also well conserved in the homologous NS3 protein of HGV (or GBV-C), which is the most closely related to HCV among the members of the Flaviviridae family (Fig. 4B). Interestingly, members of the third group of the Flaviviridae family, the Pestivirus genus, have a serine at the equivalent position (Fig. 4B), which presumably can participate in a similar hydrogen bond interaction with nucleic acid substrate. The TxGx motif is not strictly conserved in the NS3 RNA helicase of the Flavivirus genus, which has the least homology to HCV of any member of the Flaviviridae family. It appears that members of the flavivirus group do contain a homologous threonine residue as deduced based on the predicted secondary structure (Thr-269 of HCV helicase is located in a turn between a β-strand and an α-helix) and distance to the conserved motifs I, Ia, and II (Fig. 4B). However, for the flaviviruses, a threonine or valine residue is found at the glycine position of the TxGx motif (Fig. 4B). The role of the glycine residue in the TxGx motif has not yet been clearly defined. While the TxGx motif has not been identified in SF1 helicases, comparison of the E. coli Rep helicase-oligonucleotide structure with the structure we have identified suggests that Thr-83 of the E. coli Rep helicase is homologous to Thr-269 of the HCV NS3 helicase (Fig. 4A). Thr-83 of Rep helicase also uses its γ-OH group to form a hydrogen bond with a phosphate oxygen of the bound oligonucleotide (22). Additionally, this threonine residue is located in a turn between a β-strand and an α-helix in domain 1A of Rep helicase (which is structurally homologous to domain 1 of HCV NS3 helicase), as is the case for Thr-269 of HCV helicase. This threonine residue is well conserved as part of the TFH sequence, located downstream of motif Ia, in several SF1 helicases (Fig. 4A).
Thr-411 is located in motif V (6), which has the signature sequence TxxxxxG and is absolutely conserved in both SF1 and SF2 (Figs. 4A and 4B). Korolev and coworkers found that Thr-556 of Rep helicase, which is equivalent to Thr-411 of HCV helicase, also forms a hydrogen bond with a backbone phosphate of oligonucleotide through its γ-OH group (22). Our mutagenesis results, combined with sequence alignments and a structural comparison of these two helicase-nucleic acid complexes, strongly indicate that the interactions between these two threonine residues and the phosphate backbone of the nucleic acid substrate are critical for proper helicase function.
In the HCV NS3 helicase-oligonucleotide binary complex, Ser-231 and Ser-370 also have interactions with the nucleic acid backbone through their γ-OH groups (20). Ser-231 makes a water-mediated hydrogen bond to a phosphate oxygen at the domain 1-nucleic acid interface, while Ser-370 makes a water-mediated interaction at the domain 2-nucleic acid interface. Both of these residues are absolutely conserved within various HCV strains. Ser-231 is part of motif Ia, which has been identified in members of both SF1 and SF2 (6) (Figs. 4A and 4B). The homologous residue in E. coli Rep helicase is Thr-56 as determined based on a structural alignment of domain 1A of Rep with domain 1 of the HCV helicase. Thr-56 of Rep helicase forms a direct hydrogen bond with the phosphate backbone of the bound oligonucleotide through its γ-OH group (22). However, many helicases in members of SF1 and SF2 do not contain a serine or threonine at the corresponding position of motif Ia (Fig. 4B). For example, two SF2 helicases, i.e., the NS3 helicase of bovine viral diarrhea virus (Fig. 4B) and the NPH-II helicase of vaccinia virus, contain a leucine at this position instead. Our findings that substitution of alanine for serine at either position 231 or position 370 results in helicase enzymes which display wt levels of unwinding and ATPase activities as well as ssRNA binding suggest that these two serine residues may not be required for proper helicase function. In the HCV helicase, two amino acids immediately following either Ser-231 (Val-232 and Ala-233 in domain 1) or Ser-370 (Lys-371 and Lys-372 in domain 2) may be more important for stabilizing the interaction with ss nucleic acid. In each instance, these two adjacent amino acids make a direct (Val-232 or Lys-371) and a water-mediated (Ala-233 or Lys-372) hydrogen bond with phosphate oxygens of the bound oligonucleotide through their main-chain NH groups. In both domains 1 and 2, these residues form the beginnings of α-helices whose N-termini point directly toward phosphate groups of the bound oligonucleotide. These helix dipole-phosphate interactions appear to be more important for stabilization of the binary complex than the water-mediated interactions involving Ser-231 and Ser-370.
The side chain of Trp-501 of the HCV helicase appears to have an important stacking interaction with a base at the 3′ end of the bound oligonucleotide in the binary structure, and observation of its position in the complex led to a hypothesis for how the HCV helicase and related helicases function in unwinding of duplex nucleic acid (20). This model was based on the ability of the aromatic side chain of Trp-501 to intercalate between bases of the unwound nucleic acid product, thereby maintaining unidirectional movement of the enzyme along the nucleic acid. This model is consistent with the 3′-to-5′ unwinding activity of HCV NS3 helicase (10, 12, 18, 33, 46). Therefore, it was not surprising that replacement of Trp-501 with alanine led to complete loss of unwinding activity. Only in the presence of another aromatic side chain (phenylalanine), and not in the presence of an aliphatic, hydrophobic side chain (leucine), at this position was the HCV helicase able to retain unwinding activity. These results support the model in which a stacking interaction between the aromatic ring of this amino acid and a nucleotide base of bound nucleic acid is essential for movement of the enzyme along the polynucleotide substrate. Korolev et al. observed that Phe-183 of Rep helicase has a similar stacking interaction with a base of the bound DNA oligonucleotide (22). Both Phe-183 of E. coli Rep helicase and Trp-501 of the HCV helicase are located in a turn between two α-helices. Our structural alignment analysis suggests that Phe-183 of E. coli Rep helicase also acts as a gatekeeper residue to maintain the unidirectional movement of this enzyme along the DNA substrate. Although Trp-501 of HCV helicase and Phe-183 of Rep helicase are located in completely different positions in the corresponding primary sequences (Fig. 4A), they are located in similar positions in the three-dimensional structures (Fig. 3B; also see Fig. 6 in reference 22). The lack of homology in primary sequences surrounding this aromatic gatekeeper amino acid in both SF1 and SF2 helicases makes it difficult to accurately predict the homologous residues in other viral SF2 helicases (Fig. 4B).
Despite elegant kinetic analysis of E. coli helicase II (UvrD) and Rep helicase (reviewed in reference 30 and references therein), a detailed understanding of how ATP binding and hydrolysis are coupled to unwinding of double-stranded nucleic acid has yet to emerge. Based on mechanistic and structural studies of Rep DNA helicase, Lohman and coworkers have classified possible mechanisms for unwinding as either active or passive (30). In the passive unwinding model, helicase binds preferentially to ss nucleic acid and captures newly-formed ss regions only as they become exposed due to “breathing” of the double strands at the unwinding fork. In the active unwinding model, helicase binds to both ss and double-stranded regions and actively separates the strands, through an unknown mechanism, in an NTP-dependent reaction. Both models were proposed based on observations of oligomeric DNA helicases, such as simian virus 40 T antigen and E. coli DnaB helicase, which form hexamers, and E. coli Rep helicase, HeLa cell DNA helicase, and herpes simplex virus (HSV) UL9, which form dimers.
These models do not provide a mechanism for RNA helicases, such as human helicase A, vaccinia virus NPH-II helicase, or HCV NS3 helicase (38), for which there is no evidence for dimerization or oligomerization. Based on observed packing interfaces in crystals of the HCV type 1b helicase domain, Cho and coworkers proposed that the functional form of HCV helicase is a dimer (3). Both Yao et al. (48) and Cho et al. (3) hypothesized that motif VI (QRRGRxGR) of domain 2 functions as the RNA-binding motif and that ssRNA binds in the cleft between domains 1 and 2. Three sets of data do not support these models: (i) there is no evidence suggesting that HCV helicase is a dimer in solution (38); (ii) in the crystal structure presented by Kim et al., the oligonucleotide does not bind in the cleft between domains 1 and 2 (20); and (iii) substitution for the last two arginine residues of motif VI (Arg-464 and Arg-467) with alanine leads to loss of ATPase and unwinding activities but not RNA binding ability (19, 25).
The mutagenesis results reported here, along with those of mutagenesis studies of motif VI residues of the HCV NS3 helicase (19, 25), support the published structure of the NS3 helicase-oligonucleotide complex and accompanying model for unwinding of duplex nucleic acid (Fig. 5; also see reference 20). In this model, the 3′ ss tail of a duplex nucleic acid substrate binds in the groove that separates domain 3 from domains 1 and 2 in HCV helicase. ATP binds to the helicase through interaction with motifs I and II on domain 1. The phosphate binding loop or motif I, GxGKS/T, is responsible for binding of the β-phosphate group of ATP, while Asp-290 of motif II binds to Mg2+ or Mn2+ and helps orient the ATP-Mg2+ or ATP-Mn2+ complex for water-mediated hydrolysis. Binding of ATP leads to closure of the cleft between domains 1 and 2, which is driven by the interaction of Arg-464 and Arg-467 of motif VI in domain 2 with the γ- and α-phosphate groups, respectively, of the bound ATP molecule. Based on multiple crystal forms of the enzyme, domains 1 and 3 of the HCV helicase consistently form a rigid unit, while domain 2 remains flexible relative to the other two domains (2, 20, 48). This type of NTP-dependent movement of two domains has been observed in other enzymes utilizing NTP, such as adenylate kinase (1, 41) and mRNA capping enzyme (11). Closure of the ATP-binding cleft is predicted to lead to translocation of domains 1 and 3 in a 3′-to-5′ direction along the bound polynucleotide strand. It seems that the role of Trp-501, the gatekeeper amino acid in the RNA-binding channel, is to prevent the bound polynucleotide strand from slipping back in the opposite direction. This results in a unidirectional movement of the helicase in a 3′-to-5′ direction along the bound polynucleotide. Val-432, which disrupts nucleotide base stacking of the bound oligonucleotide at the 5′ end of the nucleic acid binding site, may also contribute to this gatekeeper function. The location of the other polynucleotide strand, released by unwinding, has not yet been determined. Mutagenesis studies (19, 25), however, indicate that it is unlikely to be bound to the arginine-rich motif, motif VI, as proposed by Yao et al. (48) or Cho et al. (3).
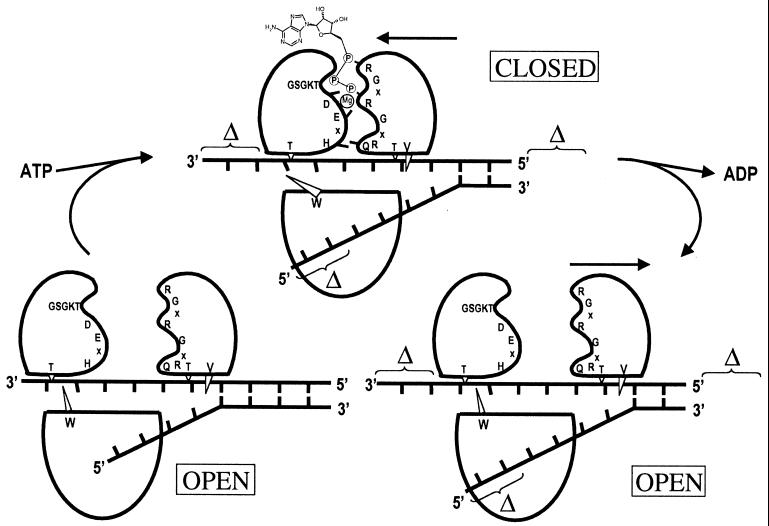
FIG. 5.
Diagram of the unwinding model proposed by Kim et al. (20). See text for a detailed explanation of this proposed model of unwinding. Three domains of HCV helicase are shown in the same orientation as that shown in Fig. 3A. Also shown are three conserved motifs (I [GSGKT], II [DExH], and VI [QRxGRxGR]), as well as four conserved residues (Thr-269 of domain 1, Thr-411 and Val-432 of domain 2, and Trp-501 of domain 3). The putative location of two antiparallel strands of duplex nucleic acid substrate is also shown. (Bottom left) In the absence of ATP, HCV helicase adopts an open form, in which the interdomain cleft between domains 1 and 2 is wide open. (Top) Upon ATP binding, domain 2 closes onto domains 1 and 3, largely due to the interactions between ATP-Mg2+ and conserved residues of motifs I, II, and VI. The formation of this closed configuration results in movement of domains 1 and 3 along the bound polynucleotide chain in a 3′-to-5′ direction and concurrent unwinding of a certain number of base pairs into the duplex substrate. (Bottom right) After movement of domains 1 and 3 and hydrolysis of the bound ATP molecule, ADP and phosphate are released and domain 2 moves to the right to restore the open form of HCV helicase.
HCV helicase represents a potential target for development of therapeutic drugs effective against hepatitis C. Potential HCV helicase-specific inhibitors could act through one of the following mechanisms: (i) inhibition of ATPase activity by interference with ATP binding, (ii) inhibition of ATP hydrolysis or ADP release by blocking the opening or the closing of the interface between domains 1 and 2, (iii) inhibition of RNA binding, (iv) inhibition of unwinding by sterically blocking translocation of helicase along the polynucleotide chain, and (v) inhibition of the coupling of ATP hydrolysis to unwinding. In addition, disruption of the interaction between HCV NS3 helicase and other viral or cellular proteins in a replication complex could also inhibit HCV replication. Recently, proof that helicase inhibitors act as antiviral agents was obtained for HSV (reviewed in reference 15). Using high throughput screening, two groups identified the same class of aminothiazole compounds, which inhibited the HSV UL5-UL8-UL52 helicase-primase complex (29, 42, 43). Optimization of the screening hits resulted in inhibitors that blocked HSV growth in cell culture (29, 42, 43) and were orally active in an animal model of disease caused by HSV (29). The mechanism of antiviral action was confirmed when both groups independently selected resistant viruses with single point mutations in the UL5 DNA helicase gene (29, 43). This demonstrates that it is possible to develop selective, potent inhibitors of viral helicases for use as antiviral agents. The study of the crystal structure of the HCV helicase combined with these mutagenesis studies has identified key residues and binding pockets that are essential for enzyme function. An understanding of the mechanism of action, in combination with the knowledge of the three-dimensional structure of the enzyme, provides the basis for structure-assisted drug design.
ACKNOWLEDGMENTS
We thank Ann Kwong and Lynn Zuchowski for their interest and helpful discussions during the course of this study. The expert technical assistance of Brett O’Hare with oligonucleotide synthesis and DNA automatic sequencing is gratefully acknowledged. We are indebted to Steve Bellon, Christian Gross, Ann Kwong, Andrew Marks, Vicki Sato, Jeffrey Saunders, Michael Su, and John Thomson for critically reading the manuscript.
REFERENCES
- 1.Bilderback T, Fulmer T, Mantulin W W, Glaser M. Substrate binding causes movement in the ATP binding domain of Escherichia coli adenylate kinase. Biochemistry. 1996;35:6100–6106. doi: 10.1021/bi951833i. [DOI] [PubMed] [Google Scholar]
- 2.Bryant G L, Harris M S, Baldwin E T, Tandeske L, Shoemaker K R, Finzel B C. 1999. Annual American Crystallographic Association Meeting, Buffalo, N.Y. 1999. HCV helicase RNA-binding-domain flexibility quantified by comparison of multiple crystal forms; p. 83. [Google Scholar]
- 3.Cho H-S, Ha N-C, Kang L-W, Chung K M, Back S H, Jang S K, Oh B-H. Crystal structure of RNA helicase from genotype 1b hepatitis C virus. J Biol Chem. 1998;273:15045–15052. doi: 10.1074/jbc.273.24.15045. [DOI] [PubMed] [Google Scholar]
- 4.Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 5.Ellis N A. DNA helicases in inherited human disorders. Curr Opin Genet Dev. 1997;7:354–363. doi: 10.1016/s0959-437x(97)80149-9. [DOI] [PubMed] [Google Scholar]
- 6.Gorbalenya A E, Koonin E V. Helicases: amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol. 1993;3:419–429. [Google Scholar]
- 7.Grakoui A, McCourt D W, Wychowski C, Feinstone S M, Rice C M. Characterization of the hepatitis C virus-encoded serine proteinase: determination of proteinase-dependent polyprotein cleavage sites. J Virol. 1993;67:2832–2843. doi: 10.1128/jvi.67.5.2832-2843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grakoui A, McCourt D W, Wychowski C, Feinstone S M, Rice C M. A second hepatitis C virus-encoded proteinase. Proc Natl Acad Sci USA. 1993;90:10583–10587. doi: 10.1073/pnas.90.22.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grakoui A, Wychowski C, Lin C, Feinstone S M, Rice C M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67:1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwack Y, Kim D W, Han J H, Choe J. Characterization of RNA binding activity and RNA helicase activity of the hepatitis C virus NS3 protein. Biochem Biophys Res Commun. 1996;225:654–659. doi: 10.1006/bbrc.1996.1225. [DOI] [PubMed] [Google Scholar]
- 11.Hakansson K, Doherty A J, Shuman S, Wigley D B. X-ray crystallography reveals a large conformational change during guanyl transfer by mRNA capping enzymes. Cell. 1997;89:545–553. doi: 10.1016/s0092-8674(00)80236-6. [DOI] [PubMed] [Google Scholar]
- 12.Hong Z, Ferrari E, Wright-Minogue J, Chase R, Risano C, Seelig G, Lee C-G, Kwong A D. Enzymatic characterization of hepatitis C virus NS3/4A complexes expressed in mammalian cells by using the herpes simplex virus amplicon system. J Virol. 1996;70:4261–4268. doi: 10.1128/jvi.70.7.4261-4268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houghton M. Hepatitis C viruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 1035–1058. [Google Scholar]
- 14.Jin L, Peterson D L. Expression, isolation, and characterization of the hepatitis C virus ATPase/RNA helicase. Arch Biochem Biophys. 1995;323:47–53. doi: 10.1006/abbi.1995.0008. [DOI] [PubMed] [Google Scholar]
- 15.Jones P S. Strategies for antiviral drug discovery. Antivir Chem Chemother. 1998;9:283–302. [PubMed] [Google Scholar]
- 16.Kadaré G, Haenni A-L. Virus-encoded RNA helicases. J Virol. 1997;71:2583–2590. doi: 10.1128/jvi.71.4.2583-2590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanai A, Tanabe K, Kohara M. Poly (U) binding activity of hepatitis C virus NS3 protein, a putative RNA helicase. FEBS Lett. 1995;376:221–224. doi: 10.1016/0014-5793(95)01283-x. [DOI] [PubMed] [Google Scholar]
- 18.Kim D W, Gwack Y, Han J H, Choe J. C-terminal domain of the hepatitis C virus NS3 protein contains an RNA helicase activity. Biochem Biophys Res Commun. 1995;215:160–166. doi: 10.1006/bbrc.1995.2447. [DOI] [PubMed] [Google Scholar]
- 19.Kim D W, Kim J, Gwack Y, Han J H, Choe J. Mutational analysis of the hepatitis C virus RNA helicase. J Virol. 1997;71:9400–9409. doi: 10.1128/jvi.71.12.9400-9409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J L, Morgenstern K A, Griffith J P, Dwyer M D, Thomson J A, Murcko M A, Lin C, Caron P R. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure. 1998;6:89–100. doi: 10.1016/s0969-2126(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 21.Koonin E V, Dolja V V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. . (Erratum, 28:546.) [DOI] [PubMed] [Google Scholar]
- 22.Korolev S, Hsieh J, Gauss G H, Lohman T M, Waksman G. Major domain swiveling revealed by the crystal structures of complexes of E. coli Rep helicase bound to single-stranded DNA and ADP. Cell. 1997;90:635–647. doi: 10.1016/s0092-8674(00)80525-5. [DOI] [PubMed] [Google Scholar]
- 23.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo G, Choo Q-L, Alter H J, Gitnick G L, Redeker A G, Purcell R H, Miyamura T, Dienstag J L, Alter M J, Stevens C E, Tegtmeier G E, Bonino F, Colombo M, Lee W-S, Kuo C, Berger K, Shuster J R, Overby L R, Bradley D W, Houghton M. An assay for circulating antibodies to a major etiologic virus of human non-A non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 25.Lin, C. Unpublished data.
- 26.Lin C, Chambers T J, Rice C M. Mutagenesis of conserved residues at the yellow fever virus 3/4A and 4B/5 dibasic cleavage sites: effects on cleavage efficiency and polyprotein processing. Virology. 1993;192:596–604. doi: 10.1006/viro.1993.1076. [DOI] [PubMed] [Google Scholar]
- 27.Lin C, Lindenbach B D, Prágai B M, McCourt D W, Rice C M. Processing in the hepatitis C virus E2-NS2 region: identification of p7 and two distinct E2-specific products with different C termini. J Virol. 1994;68:5063–5073. doi: 10.1128/jvi.68.8.5063-5073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C, Prágai B M, Grakoui A, Xu J, Rice C M. Hepatitis C virus NS3 serine proteinase: trans-cleavage requirements and processing kinetics. J Virol. 1994;68:8147–8157. doi: 10.1128/jvi.68.12.8147-8157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liuzzi M, Crute J J, Grygon C A, Hargrave K D, Simoneau B, Faucher A-M, Bolger G, Duan J, Kibler P, Cordingley M G. Aminothiazolyl-phenyl-based inhibitors of HSV helicase-primase: a novel class of orally active antiherpetic agents. Antivir Res. 1998;37:A42. [Google Scholar]
- 30.Lohman T M, Bjornson K P. Mechanisms of helicase-catalyzed DNA unwinding. Annu Rev Biochem. 1996;65:169–214. doi: 10.1146/annurev.bi.65.070196.001125. [DOI] [PubMed] [Google Scholar]
- 31.Lüking A, Stahl U, Schmidt U. The protein family of RNA helicases. Crit Rev Biochem Mol Biol. 1998;33:259–296. doi: 10.1080/10409239891204233. [DOI] [PubMed] [Google Scholar]
- 32.Matson S W, Bean D W, George J W. DNA helicases: enzymes with essential roles in all aspects of DNA metabolism. Bioessays. 1994;16:13–22. doi: 10.1002/bies.950160103. [DOI] [PubMed] [Google Scholar]
- 33.Morgenstern K A, Landro J A, Hsiao K, Lin C, Yong G, Su M S-S, Thomson J A. Polynucleotide modulation of the protease, nucleoside triphosphatase, and helicase activities of a hepatitis C virus NS3-NS4A complex isolated from transfected COS cells. J Virol. 1997;71:3767–3775. doi: 10.1128/jvi.71.5.3767-3775.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pause A, Methot N, Sonenberg N. The HRIGRXXR region of the DEAD box RNA helicase eukaryotic translation initiation factor 4A is required for RNA binding and ATP hydrolysis. Mol Cell Biol. 1993;13:6789–6798. doi: 10.1128/mcb.13.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pause A, Sonenberg N. Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. EMBO J. 1992;11:2643–2654. doi: 10.1002/j.1460-2075.1992.tb05330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter D J. Inhibition of the hepatitis C virus helicase-associated ATPase activity by the combination of ADP, NaF, MgCl2, and poly(rU). Two ADP binding sites on the enzyme-nucleic acid complex. J Biol Chem. 1998;273:7390–7396. doi: 10.1074/jbc.273.13.7390. [DOI] [PubMed] [Google Scholar]
- 37.Porter D J. A kinetic analysis of the oligonucleotide-modulated ATPase activity of the helicase domain of the NS3 protein from hepatitis C virus. The first cycle of interaction of ATP with the enzyme is unique. J Biol Chem. 1998;273:14247–14253. doi: 10.1074/jbc.273.23.14247. [DOI] [PubMed] [Google Scholar]
- 38.Porter D J, Short S A, Hanlon M H, Preugschat F, Wilson J E, Willard D H, Jr, Consler T G. Product release is the major contributor to kcat for the hepatitis C virus helicase-catalyzed strand separation of short duplex DNA. J Biol Chem. 1998;273:18906–18914. doi: 10.1074/jbc.273.30.18906. [DOI] [PubMed] [Google Scholar]
- 39.Preugschat F, Averett D R, Clarke B E, Porter D J T. A steady-state and pre-steady-state kinetic analysis of the NTPase activity associated with the hepatitis C virus NS3 helicase domain. J Biol Chem. 1996;271:24449–24457. doi: 10.1074/jbc.271.40.24449. [DOI] [PubMed] [Google Scholar]
- 40.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 931–960. [Google Scholar]
- 41.Schulz G E. Induced-fit movement in adenylate kinases. Faraday Discuss. 1992;93:85–93. doi: 10.1039/fd9929300085. [DOI] [PubMed] [Google Scholar]
- 42.Spector F C, Liang L, Giordano H, Sivaraja M, Peterson M G. Inhibition of herpes simplex virus replication by a 2-amino thiazole via interactions with the helicase component of the UL5-UL8-UL52 complex. J Virol. 1998;72:6979–6987. doi: 10.1128/jvi.72.9.6979-6987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spector F C, Liang L, Giordano H, Sivaraja M, Peterson M G. T157602, a 2-amino-thiazole inhibits HSV replication by interacting with the UL5 component of the UL5/8/52 helicase primase complex. Antivir Res. 1998;37:A43. doi: 10.1128/jvi.72.9.6979-6987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanya H S, Bird L E, Brannigan J A, Wigley D B. Crystal structure of a DExx box DNA helicase. Nature. 1996;384:379–383. doi: 10.1038/384379a0. [DOI] [PubMed] [Google Scholar]
- 45.Suzich J A, Tamura J K, Palmer-Hill F, Warrener P, Grakoui A, Rice C M, Feinstone S M, Collett M S. Hepatitis C virus NS3 protein polynucleotide-stimulated nucleoside triphosphatase and comparison with the related pestivirus and flavivirus enzymes. J Virol. 1993;67:6152–6158. doi: 10.1128/jvi.67.10.6152-6158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai C-L, Chi W-K, Chen D-S, Hwang L-H. The helicase activity associated with hepatitis C virus nonstructural protein 3 (NS3) J Virol. 1996;70:8477–8484. doi: 10.1128/jvi.70.12.8477-8484.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao N, Hesson T, Cable M, Hong Z, Kwong A D, Le H V, Weber P C. Structure of the hepatitis C virus RNA helicase domain. Nat Struct Biol. 1997;4:463–467. doi: 10.1038/nsb0697-463. [DOI] [PubMed] [Google Scholar]