Abstract
Background
Obesity is a major public health issue with no definitive treatment. The first-line approach for obesity is lifestyle modification, including a healthy diet. Although the amount of fat has been considered, there is no network meta-analysis (NMA) study investigating the effect of edible oils on body weight. Therefore, we sought to investigate the effect of different edible oils on body weight using a systematic review and NMA study of randomized controlled trials (RCTs).
Method
PubMed, Scopus, ISI Web of Science, and the Cochrane Library were searched from inception to April 2019. RCTs of different edible oils for body weight were included. A frequentist network meta-analysis was conducted to appraise the efficacy of different types of edible oils, and the Surface Under the Cumulative Ranking Curve (SUCRA) was estimated. The GRADE framework was used to assess the certainty of evidence.
Results
Forty-two eligible studies were included. Most of the included trials examined the effect of olive oil compared to canola oil (n = 7 studies), followed by canola oil compared to sunflower oil (n = 6 studies), and olive oil compared to sunflower oil (n = 4 studies). Sesame oil had the highest SUCRA value for reducing weight (SUCRA value = 0.9), followed by the mixture of canola and sesame oil (0.8). Palm oil and soy oil were ranked the lowest (SUCRA value = 0.2).
Conclusion
There is low to moderate certainty of evidence showing that soybean, palm, and sunflower oils were associated with weight gain, while sesame oil produced beneficial anti-obesity effects.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40795-024-00907-0.
Keywords: Dietary fats, Oils, Body weight, Systematic review, Network Meta-analysis
Introduction
Overweight and obesity are prevalent global public health concerns with escalating rates in both developed and developing countries [1]. The strong association between obesity and an elevated risk of chronic diseases and mortality underscores the significance of implementing weight management strategies [2]. Much attention has been directed towards lifestyle habits, particularly dietary intake, implicated in the overweight and obesity epidemics. The impact of various dietary components, such as protein, carbohydrates, and fiber, on body weight and composition has been extensively studied [3–5]. Additionally, the role of fatty acids, whether as part of diets or in isolation, in weight-related outcomes is of considerable interest.
Vegetable oils, also known as plant oils, are important sources of dietary monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs). Olive oil, canola oil, corn oil, soybean oil, peanut oil, safflower oil, and sunflower oil are commonly used as baking ingredients, for frying, or as salad dressings. In contrast, sources of saturated fatty acids (SFAs) include dairy fat (butter), lard (pork), beef tallow, palm oil, palm kernel oil, and coconut oil [6, 7]. Extensive research has focused on the effects of the fatty acid composition of fats and oils on various aspects of health. Evidence suggests that substituting saturated fat with unsaturated fat, particularly PUFAs, is associated with a reduced risk of developing cardiovascular diseases (CVDs) [8].
The contribution of fatty acids and oils to body weight control remains a subject of ongoing debate, with interventional and epidemiological studies yielding mixed results. Studies have shown lower plasma concentrations of n-3 PUFAs in overweight/obese individuals compared to those with a healthy weight [9–12]. The SUN prospective cohort study, involving 7,368 Spanish university graduates, explored the association between olive oil consumption and the risk of weight gain and obesity, reporting that individuals adhering to an olive oil-rich Mediterranean dietary pattern did not have an increased risk of obesity [13]. Conversely, a recent meta-analysis of controlled trials revealed that the intake of canola oil was significantly associated with weight reduction compared to various control groups, including SFAs, olive oil, sunflower oil, dairy fats, fish oil, and safflower oil. However, it should be noted that this weight reduction was only significant when compared to saturated fats and no other control oils, as indicated by further subgroup analysis [14]. Another meta-analysis found no significant weight-reducing effect of flaxseed oil compared to control groups consuming canola oil, corn oil, olive oil, soybean oil, safflower oil, sunflower oil, medium-chain triglyceride oil, or placebo [15].
Although certain pairwise meta-analyses have compared fats and edible oils as described above, it remains unclear which types of fats and oils have more beneficial effects on weight control. Utilizing the methodological approach of network meta-analysis (NMA) is particularly valuable for providing a comprehensive view of the relative efficacy of different fats and oils in modulating body weight. NMA enables the quantification of evidence from direct, indirect, or both types of comparisons of intervention trials simultaneously [16]. To the best of our knowledge, no NMA has been conducted thus far to compare the effectiveness of different types of fats and oils for weight control. Therefore, our objective was to perform a systematic review with NMA of randomized controlled trials (RCTs) to compare the effects of different types of fats and oils on body weight in adults.
Methods
Study design
The study is reported in accordance with the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) extension for NMA [1].
Search strategy
Strategy Electronic databases, including PubMed, Scopus, ISI Web of Science, and the Cochrane Library, were searched up until April 2019 to identify relevant studies using a predefined search strategy (Supplementary Table 1). No restrictions on language or publication date were imposed. Additionally, the reference lists of published systematic reviews were manually screened to identify further potentially relevant studies.
Eligibility criteria
Studies were included if they met the following criteria based on the PICOS framework:
Population: Adult participants aged ≥ 18 years. Pregnant or lactating women, children or adolescents, patients with cancer, AIDS (acquired immunodeficiency syndrome), type 1 diabetes, or critically ill patients were excluded.
Intervention and Comparator: Studies were included if they compared at least two types of edible oils administered orally (liquid or capsule) in an isocaloric exchange status or provided indirect evidence from trials that compared an eligible oil to a comparative control group (non-intervention or placebo). The minimum daily dose required for inclusion was 5 g, with a minimum duration of two weeks. Multicomponent interventions, interventions solely focused on fatty acids, and interventions involving mixed oils (combinations of two or more edible oils) were excluded.
Outcomes: Studies were considered eligible if they reported the mean change from baseline along with the corresponding standard deviation (SD) (or provided sufficient data to calculate these parameters) for body weight in both the experimental and control groups.
Study Design: Randomized controlled trials with either a parallel or crossover design.
Study selection process
Selection Process Reviewers screened all retrieved articles based on titles and abstracts. In the second step, full-text eligibility was assessed according to the predefined criteria. In cases where several publications reported data from the same population, the most comprehensive dataset (e.g., the largest number of study participants or longest study duration) was selected for analysis. The study selection process was cross-checked, and any disagreements were resolved through consensus or by consulting the corresponding author (ASA).
Data extraction
The following information was independently extracted by reviewers using a structured Excel form: study characteristics (author's name, year of publication, study location, study design, follow-up duration, and sample size in each group), participant characteristics (age, gender, health status, and baseline BMI of participants), intervention and comparator description (type of oil, amount of oil intake in grams per day, and calorie intake), and results related to body weight (baseline and endpoint mean values, corresponding SDs, and/or change from baseline in both groups). In cases where a study did not report these values, methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions were used to estimate the missing data if sufficient information was provided [2]. If necessary, missing data from relevant studies were obtained by contacting the corresponding authors. The study selection process and data extraction were cross-checked to minimize potential errors, and any disagreements were resolved through discussion. If no agreement could be reached, the corresponding author made the final decision (ASA).
Quality assessment
Assessment The risk of bias for eligible studies was assessed by an independent reviewer using the Cochrane risk of bias instrument, [3]: which evaluates six domains: (1) sequence generation, (2) allocation sequence concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessors, (5) incomplete outcome data, and (6) selective outcome reporting [4]. Due to the challenge of blinding participants, personnel, and outcome assessors when the oils have distinct flavors and are difficult to disguise, we considered a low risk of bias for the blinding domain in all included studies. Studies were classified as "low risk" if all domains had a low risk of bias, "fair quality" if one domain was not met or two domains were unclear, and "poor quality" if two or more domains were identified as high or unclear risk of bias.
The certainty of evidence for each network estimate was also assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [5].
Statistical analysis
Analysis Data were pooled using random-effects network meta-analysis (NMA) within a frequentist framework to determine the direct and indirect effects of different types of dietary oils on the measurement of body weight. Network plots were used to visualize the indirect comparisons between dietary oil types.
The transitivity assumption was objectively examined using the inconsistency model in both overall and local approaches [6]. To test for potential overall inconsistency, a design-by-treatment interaction model was employed [7, 8]. Local inconsistency was assessed using a side-splitting approach to identify differences between the estimates of direct and indirect comparisons [9]. Consistency was considered to be achieved if the inconsistency was zero, indicating that the results from direct and indirect comparisons were consistent [10].
Heterogeneity between studies was assessed for two comparisons with the top highest number of literatures using the Higgins I2.
A network geometry diagram, illustrating the interactions between interventions, was generated. Each edible oil intervention was represented by nodes, with node size proportionate to the sample size of the corresponding intervention [11]. The effect size, presented as the weighted mean difference (WMD) with a 95% confidence interval (CI) for changes in body weight, was calculated using a random-effects model and presented in a league Table [12]. To rank the hierarchy of intervention oils, the network rank and the surface under the cumulative ranking curve (SUCRA) were used [13, 14]. SUCRA values range from 0 to 100%, indicating the probability of being ranked last and first, respectively.
All analyses were performed using the Network package developed by Ian White in Stata Software version 14 (TX, USA) [15], and the visualizations were created using the network graphs package [16]. A significance threshold of 0.05 was defined.
Assessment of publication biases
We examined small-study effects using comparison-adjusted funnel plots. Contour-enhanced funnel plots were employed to investigate whether funnel plot asymmetry could be attributed to publication bias [17, 18].
Results
Description of the studies
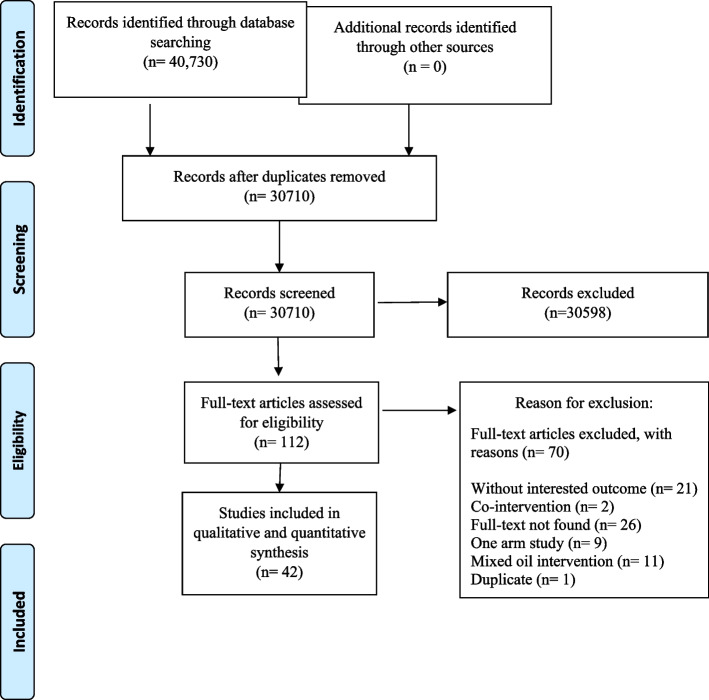
The initial search yielded a total of 40,731 publications, and after the screening process conducted by independent reviewers, 112 publications were assessed in full-text. Finally, 42 publications met the eligibility criteria and were included in the systematic review, following a 2-stage selection process [19–60]. Studies were excluded if they involved the use of mixed oils, included co-interventions in one group, compared low-fat versus high-fat diets, or did not report body weight as a measure (Fig. 1).
Fig. 1.
Flowchart of the selection of studies for the systematic review and meta-analysis
Supplementary Table 2 shows characteristics of the studies were included in analysis. Most of the studies were conducted in Iran (n = 11) [19, 22, 23, 35, 37, 45, 46, 52, 53, 55, 56], followed by Germany (n = 5) [25, 28, 39, 41], Sweden (n = 4) [32, 47, 48, 54], USA (n = 4) [33, 43, 44, 60], Australia (n = 3) [24, 27, 57], Brazil (n = 3) [21, 31, 49], India (n = 2) [42, 58], UK (n = 2) [30, 38], Italy (n = 1) [29], Denmark (n = 1) [59], Malaysia (n = 1) [34], Japan (n = 1) [36], Thailand (n = 1) [26], Finland (n = 1) [50], and Netherland (n = 1) [51]. One study also pooled the results of participants from different countries (Brazil, Ghana, US) [20]. Olive oil was the commonly oil used across the 20 studies [20, 22, 24, 25, 27, 29, 31, 35, 37–39, 41, 44, 46–48, 52, 57, 59]; of which, in four studies participants consumed virgin olive oil [29, 31, 38, 44]. Other oils included sunflower oil (n = 15) [19, 22, 23, 28–30, 32, 39, 42, 51–56], canola (or rapeseed oil) (n = 14) [22, 23, 25, 32, 37, 39, 41, 45, 47, 50, 55, 56, 60], palm oil (n = 6) [27, 30, 34, 54, 58, 59], safflower oil (n = 5) [20, 33, 49, 57, 60], flaxseed oil (n = 5) [19, 24, 36, 51, 53], soy bean oil (n = 5) [21, 26, 31, 34, 49], coconut oil (n = 5) [21, 33, 38, 43, 49], corn oil (n = 4) [36, 43, 44, 48], sesame oil (n = 3) [45, 46, 58], rice bran oil (n = 3) [26, 42, 56], butter (n = 3), almond (n = 1) [35], chia oil (n = 1) [49], echium oil (n = 1) [28], linseed oil (n = 1) [28], microalgae oil (n = 1) [28], peanut oil (n = 1) [20], walnut oil (n = 1) [35] and a mixture of sesame and canola oils (n = 1) [45]. Approximately, 62% of the studies were enrolled both male and female participants [19, 20, 25–30, 32, 34, 35, 39, 42–48, 51–55, 57, 58]; while, other studies were conducted on males (n = 7) [24, 36, 41, 50, 59, 60], or females (n = 7) [21–23, 31, 33, 37, 49, 56], exclusively. Studies were enrolled participants with dyslipidemia (n = 11) [26, 28, 32, 35, 42–44, 46–48, 55], obesity (n = 8) [21, 41, 49, 51–54], diabetes (n = 3) [22, 56, 58], metabolic syndrome (n = 3) [19, 25, 50], hypertension (n = 2) [29, 58], osteoporosis (n = 1) [23], and patients with at least one classic cardiovascular risk factor (n = 1) [37]. Other studies conducted on healthy participants (n = 14) [20, 24, 27, 30, 31, 33, 34, 36, 38, 39, 45, 57, 59, 60]. Most of the included studies focused on middle aged participants (35–60 years old) (n = 27), and nine studies were conducted on young adults (18–35 years old). The remaining six studies were enrolled a wide range of age from young adults to elderly. The length of intervention ranged from 3 to 26 weeks, and the mean of oil prescribed varied from 5 to 55 g/day.
Quality assessment
The methodological quality assessment of the included studies is provided in Supplementary Table 3. Overall, two randomized controlled trials (RCTs) were rated as low risk [53, 54], one RCT was rated as poor quality [59], and the remaining studies were judged to be of fair quality [19–39, 41–52, 55–58, 60]. The main concern in the studies was related to the "random sequence generation" and "allocation concealment process," which were not adequately explained. Two studies were also assessed to have a high risk of bias in the "incomplete outcome data" domain [52, 59] due to factors such as the study not being registered or missing participants exceeding 10% of the initial recruitment. However, data was analyzed using an intention-to-treat approach.
The assessment of the certainty of evidence is also shown using a color scheme in Table 1. The main concerns contributing to the risk of bias were imprecision (wide confidence intervals crossing the minimal important difference) and inconsistency (due to evidence of very serious incoherence).
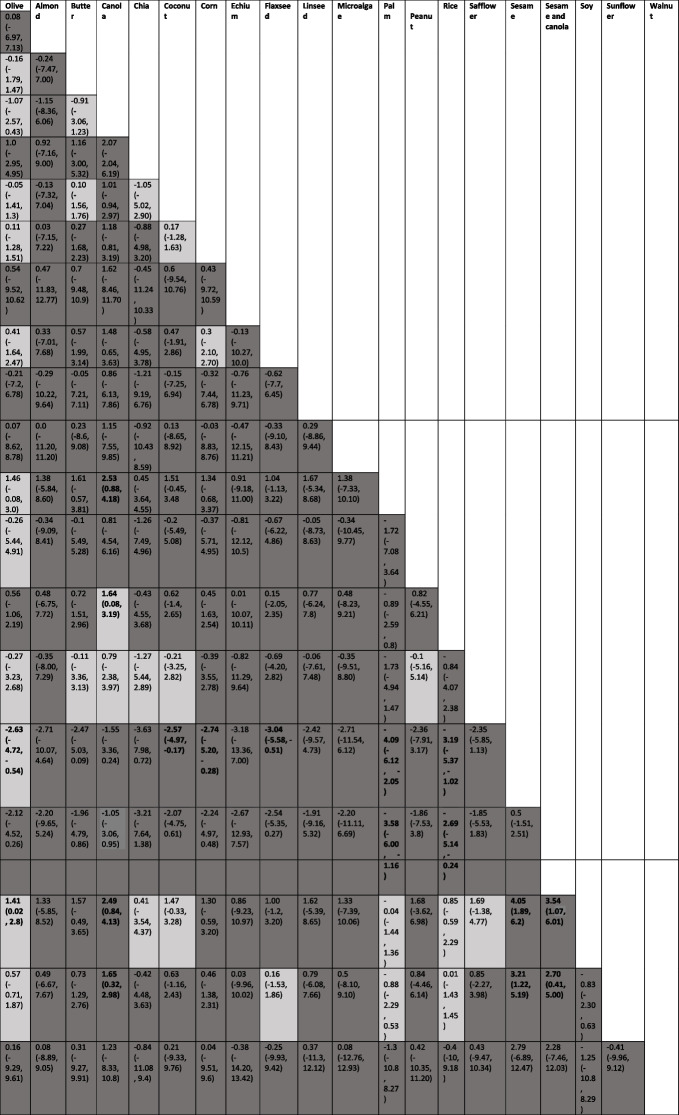
Table 1.
The effect of different edible oils on body weight, using network meta-analysis sorted based on GRADE certainty of evidencea
aThe columns show the oils in the comparisons, and the rows show mean differences and confidence intervals
![]()
Network Meta-analysis
The network diagram depicting the direct comparisons for body weight is presented in Fig. 2. As shown by the edges of the diagram, the majority of the included trials focused on comparing the effects of olive oil versus canola oil (n = 7 studies), followed by canola oil versus sunflower oil (n = 6 studies), and olive oil versus sunflower oil (n = 4 studies).
Fig. 2.
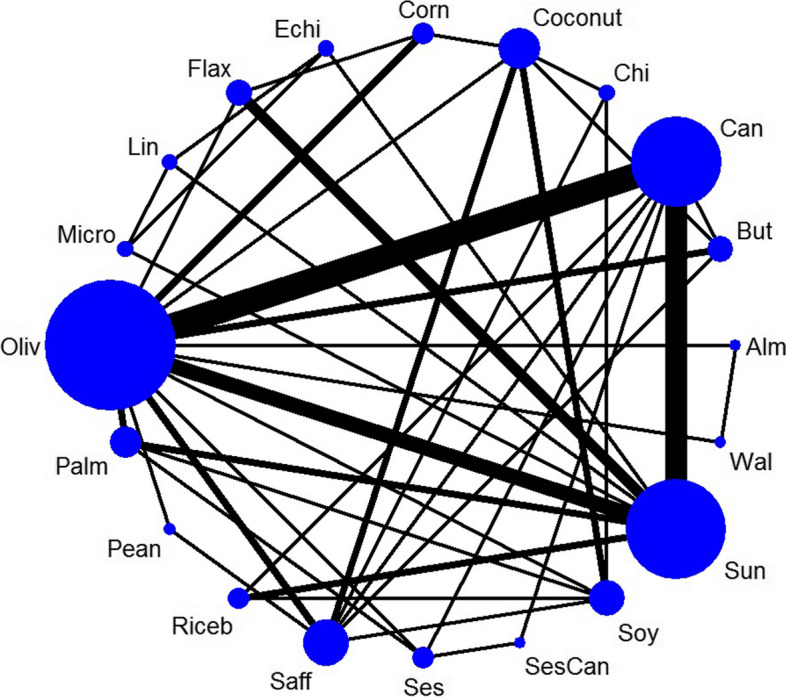
Network diagrams for body weight: The size of the nodes is proportional to the total number of participants allocated to intervention and the thickness of the lines proportional to the number of studies evaluating each direct comparison
Inconsistency
We did not find any significant global inconsistency based on the design-by-treatment model (P = 0.07). However, significant inconsistency was observed in the following comparisons: canola oil versus sunflower oil, canola oil versus sesame oil, canola oil versus a mixture of sesame and canola oil, palm oil versus sesame oil, and sesame oil versus a mixture of sesame and canola oil, as assessed using the side-splitting approach (Supplementary Table 4). Furthermore, the loop-specific approach revealed some evidence of inconsistency within the loop formed by canola, palm, sesame oil, and sunflower oil (Supplementary Fig. 1). The between study heterogeneity also was assessed for canola versus sunflower (n = 6 studies) and canola versus olive oil (n = 7 studies), and the results showed no evidence of heterogeneity (I2 = 0%).
The comparison between dietary oils
The effects of different edible oils on body weight are presented in Table 1. The network meta-analysis (NMA) revealed that palm oil (low certainty of evidence; MD: 2.53 kg; 95% CI: 0.88, 4.18), rice bean oil (moderate certainty of evidence; MD: 1.64 kg; 95% CI: 0.08, 3.19), soybean oil (low certainty of evidence; MD: 2.49 kg; 95% CI: 0.84, 4.13), and sunflower oil (low certainty of evidence; MD: 1.65 kg; 95% CI: 0.32, 2.98) were associated with a significant weight gain compared to canola oil. Soybean oil also resulted in weight gain when compared to olive oil (moderate certainty of evidence; MD: 1.41 kg; 95% CI: 0.02, 2.8), sesame oil (low certainty of evidence; MD: 4.05 kg; 95% CI: 1.89, 6.2), and a mixture of sesame and canola oil (low certainty of evidence; MD: 3.54 kg; 95% CI: 1.07, 6.01). Furthermore, sunflower oil was found to lead to weight gain compared to sesame oil (low certainty of evidence; MD: 3.21 kg; 95% CI: 1.22, 5.19) and a mixture of sesame and canola oil (low certainty of evidence; MD: 2.70 kg; 95% CI: 0.41, 5.00).
On the other hand, sesame oil demonstrated beneficial effects on body weight compared to olive oil (moderate certainty of evidence; MD: -2.63 kg; 95% CI: -4.72, -0.54), coconut oil (low certainty of evidence; MD: -2.57 kg; 95% CI: -4.97, -0.17), corn oil (low certainty of evidence; MD: -2.74 kg; 95% CI: -5.20, -0.28), flaxseed oil (low certainty of evidence; MD: -3.04 kg; 95% CI: -5.58, -0.51), palm oil (low certainty of evidence; MD: -4.09 kg; 95% CI: -6.12, -2.05), and rice bran oil (low certainty of evidence; MD: -3.19 kg; 95% CI: -5.37, -1.02). Additionally, a mixture of canola and sesame oil resulted in significant weight loss compared to palm oil (low certainty of evidence; MD: -3.58 kg; 95% CI: -6.00, -1.16) and rice bran oil (low certainty of evidence; MD: -2.69 kg; 95% CI: -5.14, -0.24).
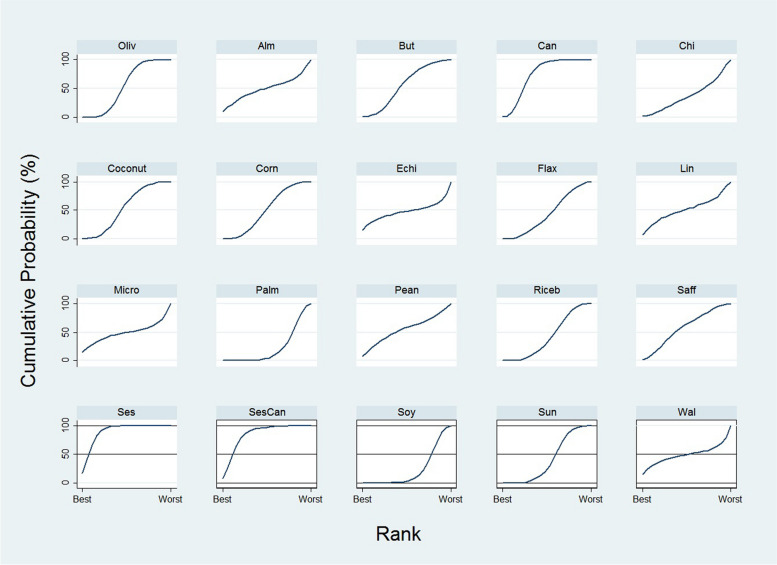
Ranking
Figure 3 presents the ranking probabilities indicating the effectiveness of each oil in weight loss. Sesame oil had the highest SUCRA value for reducing weight (SUCRA value = 0.9), followed by the mixture of canola and sesame oil (0.8), indicating that these oils are the most effective for body weight control. On the other hand, palm oil and soybean oil were ranked as the least effective (SUCRA value = 0.2) (Supplementary Table 5).
Fig. 3.
The ranking probabilities indicating the effectiveness of each oil in weight loss
Publication bias
The visual inception of funnel plot for body weight showed no evidence of asymmetry (Supplementary Fig. 2).
Discussion
The present systematic review and NMA incorporated direct and indirect evidence from 42 studies to evaluate the effect of different edible oils (olive oil, almond oil, butter, canola oil, chia oil, coconut oil, corn oil, echium oil, flaxseed oil, linseed oil, microalgae oil, palm oil, peanut oil, rice bran oil, safflower oil, sesame oil, a mixture of sesame and canola oils, soybean oil, sunflower oil, and walnut oil) on body weight. The available evidence, with low to moderate certainty, indicated that regardless of calorie and total fat intakes, soybean oil, palm oil, and sunflower oil were significantly associated with weight gain, while sesame oil demonstrated beneficial effects in combating obesity compared to various control oils. These findings are supported by the SUCRA values.
Numerous studies have explored the health-related outcomes of dietary fatty acids. Saturated fatty acids (SFA), primarily found in animal sources as well as palm and coconut oil, have been associated with metabolic disorders [61], cardiovascular diseases [62], cancers [63], immune system dysfunction [63], and allergy [64]. In contrast, fish oil and vegetable oils containing monounsaturated fatty acids (MUFA) or omega-3 polyunsaturated fatty acids (ω-3 PUFAs) have been recommended as replacements for SFA [65]. However, generating nutritional recommendations based on findings regarding dietary fatty acids can be challenging. Edible oils consist of a combination of various types of fatty acids, and it can be difficult to identify the predominant fatty acid in a specific oil. Additionally, the presence of antioxidants in certain oils may modulate their health effects related to fatty acids. Hence, our study aimed to identify the best and worst edible oils for weight loss and translate these findings into nutritional recommendations.
Despite providing the same energy content, different oils have been reported to have varying effects on body weight. In line with our findings, soybean oil, sunflower oil, and palm oil were associated with increased body weight, while sesame oil exhibited anti-obesity effects. This suggests the involvement of factors beyond calorie content in influencing body weight. Consistent with our findings, a meta-analysis demonstrated that sesame oil could reduce body weight and BMI [66]. Furthermore, pooling data from 23 trials revealed that canola oil led to weight loss without significant effects on other anthropometric measures [67]. However, some other studies have failed to find significant effects of palm oil [68] or coconut oil [69] on body weight.
The properties of oils are primarily attributed to their fatty acid composition. In this study, the oils categorized as obesogenic oils contained saturated fatty acids (SFA) and pro-inflammatory fatty acids. Palm oil, for instance, is known to have high levels of SFA, particularly palmitic acid (49 g/100 g) [70], soybean [71] and sunflower oil [72] are also rich in omega-6 polyunsaturated fatty acids (PUFAs) (> 50% linoleic acid in both oils). Both omega-6 PUFA and SFA-rich oils have been associated with inflammation, elevated blood cholesterol levels, and cardiovascular disease events [62, 73, 74]. However, previous studies have demonstrated no significant association between palm oil consumption and obesity [68] or cardiovascular diseases [75]. Moreover, sesame oil is rich in linoleic acid, which is the most abundant fatty acid in this oil [76, 77]. Our NMA revealed that sesame oil has a clinically significant anti-obesity effect, resulting in weight loss ranging from 2.57 to 4.09 kg. This effect may be attributed to the high flavonoid content, antioxidant activity, and the presence of lignans such as sesamin, episesamin, and sesamolin in sesame oil [78–80]. Sesamin has been proposed to exert its anti-obesity effects by inhibiting adenosine A1 receptors [81], hepatic fatty acid synthase [82], and intestinal cholesterol absorption [83]. Furthermore, antioxidant agents have been suggested to increase leptin secretion from adipose tissue, suppress lipogenesis, and reduce weight [84, 85]. However, it should be noted that the number of included studies investigating the effect of sesame oil on body weight is limited, which limits the certainty of the conclusions drawn.
To our knowledge, this is the first systematic review and NMA conducted to assess the effect of different edible oils on body weight, using a comprehensive search strategy. We also assessed the methodological quality of the included studies and the quality of evidence using standard tools.
However, several limitations need to be addressed. The most significant limitation is the lack of adjustment for oil consumption based on energy intake since energy intake was not specified in all the included studies. Additionally, there was considerable variation in macronutrient intakes among the studies, raising questions about whether the observed findings are primarily related to the type of edible oils or the overall dietary composition of macronutrients. The dose–response relationship was not examined, making it difficult to determine the specific amount of oil responsible for the observed effects.
Furthermore, dietary habits, physical activity levels, and baseline BMI were not consistently reported in all the included studies. The method of using the intervention oil (e.g., frying, cooking, or as a salad dressing) was not specified, which could also impact calorie intake. Subgroup analysis could not be performed due to the limited number of studies, despite observing inconsistent results in some associations. The observed inconsistency for the canola versus sunflower comparison may be related to the wide variation of characteristics of the participants (including diabetes, osteoporosis, hyperlipidemic, and healthy). Moreover, the mean age of participants also varied between 29 to 59 years old, which may affect the results.
Moreover, body weight was the only anthropometric measure assessed in this study. While body weight can serve as a suitable indicator of fat mass in the general population, it is recommended to consider multiple anthropometric measures in future studies. Additionally, body weight was a secondary outcome in most of the included studies, suggesting that the sample sizes may not have been adequately powered to detect significant differences. Participant compliance is another important limitation to consider. We assumed the same amount of intervention oil was consumed across studies, but this may not be accurate, especially for aromatic oils like olive oil. The different taste and color of the oils may have influenced participant and personnel blinding, despite our decision to rate low risk of bias for this domain during the quality assessment process. The short-term duration of the included studies may also impact the results, as the longest follow-up duration was 26 weeks in the study by Ferrara et al. [29], and the long-term effects of the oils remain unknown. Finally, the certainty of evidence was assessed as low to moderate, mainly due to the limited number of included studies in certain nodes, wide confidence intervals crossing the minimal important difference, and evidence of significant incoherence. Therefore, additional future evidence could potentially alter our findings.
Conclusion
In conclusion, our study demonstrated that soybean, palm, and sunflower oils were associated with weight gain; and, sesame oil exhibited beneficial anti-obesity effects. It is important to note that these findings were supported by evidence of low to moderate certainty. Furthermore, subgroup analysis to examine other intervention details could not be performed due to the limited number of included studies in each subgroup. Therefore, future research should aim to strictly control macronutrient and energy intakes to isolate the effects of oil type on weight changes.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
SA contributed to literature search, data extraction, contributed to manuscript drafting and approving the final manuscript. SS contributed to the study conception, literature search, data extraction, quality assessment, and manuscript drafting and approving the final manuscript. NRJ contributed to the literature search, data extraction, quality assessment and manuscript drafting. MM contributed to the literature search, data extraction, quality assessment and manuscript drafting. SSh contributed to the literature search, data extraction. EL contributed to the literature search, data extraction. ASA contributed to the study conception, data analysis, and manuscript drafting and approving the final manuscript. All authors acknowledge full responsibility for the analyses and interpretation of the report. All authors have read and approved the final manuscript. ASA is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
Not applicable.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 2.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. London: Wiley; 2011.
- 3.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. London: Wiley; 2019. [DOI] [PMC free article] [PubMed]
- 4.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brignardello-Petersen R, Florez ID, Izcovich A, Santesso N, Hazlewood G, Alhazanni W, et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. Bmj. 2020;371:m3900. 10.1136/bmj.m3900 [DOI] [PubMed] [Google Scholar]
- 6.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9:e99682. 10.1371/journal.pone.0099682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins J, Jackson D, Barrett J, Lu G, Ades A, White I. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med. 2014;33:3639–54. 10.1002/sim.6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dias S, Welton N, Caldwell D, Ades A. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–44. 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 10.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3:80–97. 10.1002/jrsm.1037 [DOI] [PubMed] [Google Scholar]
- 11.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 13.Salanti G, Ades A, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 14.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:1–9. 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White IR. Network meta-analysis. Stata J. 2015;15:951–85. 10.1177/1536867X1501500403 [DOI] [Google Scholar]
- 16.Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stand Genomic Sci. 2015;15:905–50. [Google Scholar]
- 17.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–6. 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Mavridis D, Welton NJ, Sutton A, Salanti G. A selection model for accounting for publication bias in a full network meta-analysis. Stat Med. 2014;33:5399–412. 10.1002/sim.6321 [DOI] [PubMed] [Google Scholar]
- 19.Akrami A, Nikaein F, Babajafari S, Faghih S, Yarmohammadi H. Comparison of the effects of flaxseed oil and sunflower seed oil consumption on serum glucose, lipid profile, blood pressure, and lipid peroxidation in patients with metabolic syndrome. J Clin Lipidol. 2018;12:70–7. 10.1016/j.jacl.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 20.Akuamoah-Boateng L, Iyer SS, Sales RL, Lokko P, Lartey A, Monteiro JBR, et al. Effect of peanut oil consumption on energy balance. J Appl Res. 2007;7:185–95. [Google Scholar]
- 21.Assuncao ML, Ferreira HS, dos Santos AF, Cabral CR Jr, Florencio TM. Effects of dietary coconut oil on the biochemical and anthropometric profiles of women presenting abdominal obesity. Lipids. 2009;44:593–601. 10.1007/s11745-009-3306-6 [DOI] [PubMed] [Google Scholar]
- 22.Atefi M, Pishdad GR, Faghih S. The effects of canola and olive oils on insulin resistance, inflammation and oxidative stress in women with type 2 diabetes: a randomized and controlled trial. J Diabetes Metab Disord. 2018;17:85–91. [DOI] [PMC free article] [PubMed]
- 23.Azemati M, Shakerhosseini R, Hekmatdos A, Alavi-Majd H, Hedayati M, Houshiarrad A, et al. Comparison of the effects of canola oil versus sunflower oil on the biochemical markers of bone metabolism in osteoporosis. J Res Med Sci. 2012;17:1137–43. [PMC free article] [PubMed] [Google Scholar]
- 24.Barden AE, Croft KD, Durand T, Guy A, Mueller MJ, Mori TA. Flaxseed oil supplementation increases plasma F1-phytoprostanes in healthy men. J Nutr. 2009;139:1890–5. 10.3945/jn.109.108316 [DOI] [PubMed] [Google Scholar]
- 25.Baxheinrich A, Stratmann B, Lee-Barkey YH, Tschoepe D, Wahrburg U. Effects of a rapeseed oil-enriched hypoenergetic diet with a high content of alpha-linolenic acid on body weight and cardiovascular risk profile in patients with the metabolic syndrome. Br J Nutr. 2012;108:682–91. 10.1017/S0007114512002875 [DOI] [PubMed] [Google Scholar]
- 26.Bumrungpert A, Chongsuwat R, Phosat C, Butacnum A. Rice Bran Oil Containing Gamma-Oryzanol Improves Lipid Profiles and Antioxidant Status in Hyperlipidemic Subjects: A Randomized Double-Blind Controlled Trial. J Altern Complement Med. 2018;25:353–8. [DOI] [PubMed]
- 27.Choudhury N, Tan L, Truswell AS. Comparison of palmolein and olive oil: effects on plasma lipids and vitamin E in young adults. Am J Clin Nutr. 1995;61:1043–51. 10.1093/ajcn/61.5.1043 [DOI] [PubMed] [Google Scholar]
- 28.Dittrich M, Jahreis G, Bothor K, Drechsel C, Kiehntopf M, Bluher M, et al. Benefits of foods supplemented with vegetable oils rich in alpha-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: a double-blind, randomized, controlled trail. Eur J Nutr. 2015;54:881–93. 10.1007/s00394-014-0764-2 [DOI] [PubMed] [Google Scholar]
- 29.Ferrara LA, Raimondi AS, D’Episcopo L, Guida L, Dello Russo A, Marotta T. Olive oil and reduced need for antihypertensive medications. Arch Intern Med. 2002;160:837–42. 10.1001/archinte.160.6.837 [DOI] [PubMed] [Google Scholar]
- 30.Filippou A, Teng KT, Berry SE, Sanders TA. Palmitic acid in the sn-2 position of dietary triacylglycerols does not affect insulin secretion or glucose homeostasis in healthy men and women. Eur J Clin Nutr. 2014;68:1036–41. 10.1038/ejcn.2014.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GalvaoCandido F, Xavier Valente F, da Silva LE, GoncalvesLeao Coelho O, Gouveia Peluzio MDC, GoncalvesAlfenas RC. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: a randomized, double-blinded, placebo-controlled clinical trial. Eur J Nutr. 2018;57:2445–55. 10.1007/s00394-017-1517-9 [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson IB, Vessby B, Ohrvall M, Nydahl M. A diet rich in monounsaturated rapeseed oil reduces the lipoprotein cholesterol concentration and increases the relative content of n-3 fatty acids in serum in hyperlipidemic subjects. Am J Clin Nutr. 1994;59:667–74. 10.1093/ajcn/59.3.667 [DOI] [PubMed] [Google Scholar]
- 33.Harris M, Hutchins A, Fryda L. The impact of virgin coconut oil and high-oleic safflower oil on body composition, lipids, and inflammatory markers in postmenopausal women. J Med Food. 2017;20:345–51. 10.1089/jmf.2016.0114 [DOI] [PubMed] [Google Scholar]
- 34.Karupaiah T, Chuah KA, Chinna K, Matsuoka R, Masuda Y, Sundram K, et al. Comparing effects of soybean oil- and palm olein-based mayonnaise consumption on the plasma lipid and lipoprotein profiles in human subjects: a double-blind randomized controlled trial with cross-over design. Lipids Health Dis. 2016;15:131. 10.1186/s12944-016-0301-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaseb F, Rashidi M, Afkhami-Ardekani M, Fallahzadeh H. Effect of olive, almond and walnut oil on cardiovascular risk factors in type 2 diabetic patients. Int J Diabetes Developing Countries. 2013;33:115–9. 10.1007/s13410-012-0108-9 [DOI] [Google Scholar]
- 36.Kawakami Y, Yamanaka-Okumura H, Naniwa-Kuroki Y, Sakuma M, Taketani Y, Takeda E. Flaxseed oil intake reduces serum small dense low-density lipoprotein concentrations in Japanese men: a randomized, double blind, crossover study. Nutr J. 2015;14:39. 10.1186/s12937-015-0023-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandouzi N, Zahedmehr A, Nasrollahzadeh J. Effects of canola or olive oil on plasma lipids, lipoprotein-associated phospholipase A2 and inflammatory cytokines in patients referred for coronary angiography. Lipids Health Dis. 2020;19:1–10. 10.1186/s12944-020-01362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khaw KT, Sharp SJ, Finikarides L, Afzal I, Lentjes M, Luben R, et al. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open. 2018;8:e020167. 10.1136/bmjopen-2017-020167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kratz M, von Eckardstein A, Fobker M, Buyken A, Posny N, Schulte H, et al. The impact of dietary fat composition on serum leptin concentrations in healthy nonobese men and women. J Clin Endocrinol Metab. 2002;87:5008–14. 10.1210/jc.2002-020496 [DOI] [PubMed] [Google Scholar]
- 40.Kruse M, Kemper M, Gancheva S, Osterhoff M, Dannenberger D, Markgraf D, et al. Dietary rapeseed oil supplementation reduces hepatic steatosis in obese men—a randomized controlled trial. Mole Nutr Food Res. 2020. 10.1002/mnfr.202000419. [DOI] [PubMed]
- 41.Kruse M, von Loeffelholz C, Hoffmann D, Pohlmann A, Seltmann AC, Osterhoff M, et al. Dietary rapeseed/canola-oil supplementation reduces serum lipids and liver enzymes and alters postprandial inflammatory responses in adipose tissue compared to olive-oil supplementation in obese men. Mol Nutr Food Res. 2015;59:507–19. [DOI] [PubMed]
- 42.Kuriyan R, Gopinath N, Vaz M, Kurpad AV. Use of rice bran oil in patients with hyperlipidaemia. Natl Med J India. 2005;18:292–6. [PubMed] [Google Scholar]
- 43.Maki KC, Hasse W, Dicklin MR, Bell M, Buggia MA, Cassens ME, et al. Corn oil lowers plasma cholesterol compared with coconut oil in adults with above-desirable levels of cholesterol in a randomized crossover trial. J Nutr. 2018;148:1556–63. 10.1093/jn/nxy156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maki KC, Lawless AL, Kelley KM, Kaden VN, Geiger CJ, Dicklin MR. Corn oil improves the plasma lipoprotein lipid profile compared with extra-virgin olive oil consumption in men and women with elevated cholesterol: results from a randomized controlled feeding trial. J Clin Lipidol. 2015;9:49–57. 10.1016/j.jacl.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 45.Moghtaderi F, Amiri M, Zimorovat A, Raeisi-Dehkordi H, Rahmanian M, Hosseinzadeh M, et al. The effect of canola, sesame and sesame-canola oils on body fat and composition in adults: a triple-blind, three-way randomised cross-over clinical trial. Int J Food Sci Nutr. 2021;72:226–35. 10.1080/09637486.2020.1786024 [DOI] [PubMed] [Google Scholar]
- 46.Namayandeh SM, Kaseb F, Lesan S. Olive and sesame oil effect on lipid profile in hypercholesterolemic patients, which better? Int J Prev Med. 2013;4:1059–62. [PMC free article] [PubMed] [Google Scholar]
- 47.Nydahl M, Gustafsson IB, Ohrvall M, Vessby B. Similar effects of rapeseed oil (canola oil) and olive oil in a lipid-lowering diet for patients with hyperlipoproteinemia. J Am Coll Nutr. 1995;14:643–51. 10.1080/07315724.1995.10718554 [DOI] [PubMed] [Google Scholar]
- 48.Nydahl MC, Gustafsson IB, Vessby B. Lipid-lowering diets enriched with monounsaturated or polyunsaturated fatty acids but low in saturated fatty acids have similar effects on serum lipid concentrations in hyperlipidemic patients. Am J Clin Nutr. 1994;59:115–22. 10.1093/ajcn/59.1.115 [DOI] [PubMed] [Google Scholar]
- 49.Oliveira-de-Lira L, Santos EMC, de Souza RF, Matos RJB, Silva MCD, Oliveira LDS, et al. Supplementation-dependent effects of vegetable oils with varying fatty acid compositions on anthropometric and biochemical parameters in obese women. Nutrients. 2018;10:932. 10.3390/nu10070932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palomaki A, Pohjantahti-Maaroos H, Wallenius M, Kankkunen P, Aro H, Husgafvel S, et al. Effects of dietary cold-pressed turnip rapeseed oil and butter on serum lipids, oxidized LDL and arterial elasticity in men with metabolic syndrome. Lipids Health Dis. 2010;9:137. 10.1186/1476-511X-9-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pieters DJ, Zock PL, Fuchs D, Mensink RP. Effect of alpha-linolenic acid on 24-h ambulatory blood pressure in untreated high-normal and stage I hypertensive subjects. Br J Nutr. 2019;121:155–63. 10.1017/S0007114518003094 [DOI] [PubMed] [Google Scholar]
- 52.Rezaei S, Akhlaghi M, Sasani MR, Barati BR. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Nutr (Burbank, Los Angeles County, Calif). 2019;57:154–61. 10.1016/j.nut.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 53.Rezaei S, Sasani MR, Akhlaghi M, Kohanmoo A. Flaxseed oil in the context of a weight loss programme ameliorates fatty liver grade in patients with non-alcoholic fatty liver disease: a randomised double-blind controlled trial. Br J Nut. 2020;123:994–1002. 10.1017/S0007114520000318 [DOI] [PubMed] [Google Scholar]
- 54.Rosqvist F, Kullberg J, Ståhlman M, Cedernaes J, Heurling K, Johansson H-E, et al. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: a randomized trial. J Clin Endocrinol Metab. 2019;104:6207–19. 10.1210/jc.2019-00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saedi S, Noroozi M, Khosrotabar N, Mazandarani S, Ghadrdoost B. How canola and sunflower oils affect lipid profile and anthropometric parameters of participants with dyslipidemia. Med J Islam Repub Iran. 2017;31:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salar A, Faghih S, Pishdad GR. Rice bran oil and canola oil improve blood lipids compared to sunflower oil in women with type 2 diabetes: A randomized, single-blind, controlled trial. J Clin Lipidol. 2016;10:299–305. 10.1016/j.jacl.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 57.Sanders K, Johnson L, Odea K, Sinclair AJ. The effect of dietary-fat level and quality on plasma-lipoprotein lipids and plasma fatty-acids in normocholesterolemic subjects. Lipids. 1994;29:129–38. 10.1007/BF02537152 [DOI] [PubMed] [Google Scholar]
- 58.Sankar D, Rao MR, Sambandam G, Pugalendi KV. A pilot study of open label sesame oil in hypertensive diabetics. J Med Food. 2006;9:408–12. 10.1089/jmf.2006.9.408 [DOI] [PubMed] [Google Scholar]
- 59.Tholstrup T, Hjerpsted J, Raff M. Palm olein increases plasma cholesterol moderately compared with olive oil in healthy individuals. Am J Clin Nutr. 2011;94:1426–32. 10.3945/ajcn.111.018846 [DOI] [PubMed] [Google Scholar]
- 60.Wardlaw GM, Snook JT, Lin MC, Puangco MA, Kwon JS. Serum-lipid and apolipoprotein concentrations in healthy-men on diets enriched in either canola oil or safflower oil. Am J Clin Nutr. 1991;54:104–10. 10.1093/ajcn/54.1.104 [DOI] [PubMed] [Google Scholar]
- 61.Cascio G, Schiera G, Di Liegro I. Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev. 2012;8:2–17. 10.2174/157339912798829241 [DOI] [PubMed] [Google Scholar]
- 62.Briggs MA, Petersen KS, Kris-Etherton PM. Saturated fatty acids and cardiovascular disease: replacements for saturated fat to reduce cardiovascular risk. Healthcare. 2017. 10.3390/healthcare5020029. [DOI] [PMC free article] [PubMed]
- 63.Ford JH. Saturated fatty acid metabolism is key link between cell division, cancer, and senescence in cellular and whole organism aging. Age. 2010;32:231–7. 10.1007/s11357-009-9128-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kankaanpää P, Sütas Y, Salminen S, Licbtenstein A, Isolauri E. Dietary fatty acids and allergy. Ann Med. 1999;31:282–7. 10.3109/07853899908995891 [DOI] [PubMed] [Google Scholar]
- 65.Kris-Etherton PM, Krauss RM. Public health guidelines should recommend reducing saturated fat consumption as much as possible: YES. Am J Clin Nutr. 2020;112:13–8. 10.1093/ajcn/nqaa110 [DOI] [PubMed] [Google Scholar]
- 66.Raeisi-Dehkordi H, Mohammadi M, Moghtaderi F, Salehi-Abargouei A. Do sesame seed and its products affect body weight and composition? A systematic review and meta-analysis of controlled clinical trials. J Funct Foods. 2018;49:324–32. 10.1016/j.jff.2018.08.036 [DOI] [Google Scholar]
- 67.Raeisi-Dehkordi H, Amiri M, Humphries KH, Salehi-Abargouei A. The effect of canola oil on body weight and composition: a systematic review and meta-analysis of randomized controlled clinical trials. Adv Nutr. 2019;10:419–32. 10.1093/advances/nmy108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muhamad NA, Mustapha N, Baharin MF, Mutalip MHA, Malek MA, Salleh R, et al. Impact of palm oil versus other oils on weight changes: a systematic review. Food Nutr Sci. 2018;9:915. [Google Scholar]
- 69.Duarte AC, Spiazzi BF, Zingano CP, Merello EN, Wayerbacher LF, Teixeira PP, et al. The effects of coconut oil on the cardiometabolic profile: a systematic review and meta-analysis of randomized clinical trials. Lipids Health Dis. 2022;21:1–15. 10.1186/s12944-022-01685-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chowdhury K, Banu LA, Khan S, Latif A. Studies on the fatty acid composition of edible oil. Bangladesh J Sci Ind Res. 2007;42:311–6. 10.3329/bjsir.v42i3.669 [DOI] [Google Scholar]
- 71.Jokić S, SudaR R, Svilović S, Vidović S, Bilić M, Velić D, et al. Fatty acid composition of oil obtained from soybeans by extraction with supercritical carbon dioxide. Czech J Food Sci. 2013;31:116–25. 10.17221/8/2012-CJFS [DOI] [Google Scholar]
- 72.Akkaya MR. Prediction of fatty acid composition of sunflower seeds by near-infrared reflectance spectroscopy. J Food Sci Technol. 2018;55:2318–25. 10.1007/s13197-018-3150-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karupaiah T, Chuah K-A, Chinna K, Matsuoka R, Masuda Y, Sundram K, et al. Comparing effects of soybean oil-and palm olein-based mayonnaise consumption on the plasma lipid and lipoprotein profiles in human subjects: a double-blind randomized controlled trial with cross-over design. Lipids Health Dis. 2016;15:1–11. 10.1186/s12944-016-0301-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karupaiah T, Noor MI, Sundram K. Dietary fatty acids and their influence on blood lipids and lipoproteins. Healthful lipids. New York: AOCS Publishing; 2019. p. 171–203.
- 75.Mancini A, Imperlini E, Nigro E, Montagnese C, Daniele A, Orrù S, et al. Biological and nutritional properties of palm oil and palmitic acid: effects on health. Molecules. 2015;20:17339–61. 10.3390/molecules200917339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agidew MG, Dubale AA, Atlabachew M, Abebe W. Fatty acid composition, total phenolic contents and antioxidant activity of white and black sesame seed varieties from different localities of Ethiopia. Chem Biol Technol Agric. 2021;8:1–10. 10.1186/s40538-021-00215-w [DOI] [Google Scholar]
- 77.Ryan E, Galvin K, O’Connor TP, Maguire AR, O’Brien NM. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum Nutr. 2007;62:85–91. 10.1007/s11130-007-0046-8 [DOI] [PubMed] [Google Scholar]
- 78.Bigoniya P, Nishad R, Singh CS. Preventive effect of sesame seed cake on hyperglycemia and obesity against high fructose-diet induced Type 2 diabetes in rats. Food Chem. 2012;133:1355–61. 10.1016/j.foodchem.2012.01.112 [DOI] [Google Scholar]
- 79.Pathak N, Rai A, Kumari R, Bhat K. Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacogn Rev. 2014;8:147. 10.4103/0973-7847.134249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sukumar D, Arimboor R, Arumughan C. HPTLC fingerprinting and quantification of lignans as markers in sesame oil and its polyherbal formulations. J Pharm Biomed Anal. 2008;47:795–801. 10.1016/j.jpba.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 81.Yuliana ND, Iqbal M, Jahangir M, Wijaya CH, Korthout H, Kottenhage M, et al. Screening of selected Asian spices for anti obesity-related bioactivities. Food Chem. 2011;126:1724–9. 10.1016/j.foodchem.2010.12.066 [DOI] [PubMed] [Google Scholar]
- 82.Ide T, Ashakumary L, Takahashi Y, Kushiro M, Fukuda N, Sugano M. Sesamin, a sesame lignan, decreases fatty acid synthesis in rat liver accompanying the down-regulation of sterol regulatory element binding protein-1. Biochimi Biophys Acta. 2001;1534:1–13. 10.1016/S1388-1981(01)00167-6 [DOI] [PubMed] [Google Scholar]
- 83.Hirose N, Inoue T, Nishihara K, Sugano M, Akimoto K, Shimizu S, et al. Inhibition of cholesterol absorption and synthesis in rats by sesamin. J Lipid Res. 1991;32:629–38. 10.1016/S0022-2275(20)42050-4 [DOI] [PubMed] [Google Scholar]
- 84.Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Investig. 1997;100:2777–82. 10.1172/JCI119824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baboota RK, Bishnoi M, Ambalam P, Kondepudi KK, Sarma SM, Boparai RK, et al. Functional food ingredients for the management of obesity and associated co-morbidities–A review. J Funct Foods. 2013;5:997–1012. 10.1016/j.jff.2013.04.014 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.