Abstract
Chemotherapy in combination with immunotherapy has gradually shown substantial promise to increase T cell infiltration and antitumor efficacy. However, paclitaxel in combination with immune checkpoint inhibitor targeting PD-1/PD-L1 was only used to treat a small proportion of metastatic triple-negative breast cancer (TNBC), and the clinical outcomes was very limited. In addition, this regimen cannot prevent paclitaxel-induced peripheral neuropathy. Therefore, there was an urgent need for a novel target to enhance the antitumor activity of paclitaxel and alleviate chemotherapy-induced peripheral neuropathy in breast cancer. Here, we found that Dickkopf-1 (DKK1) expression was upregulated in multiply subtypes of human breast cancer specimens after paclitaxel-based chemotherapy. Mechanistic studies revealed that paclitaxel promoted DKK1 expression by inducing EGFR signaling in breast cancer cells, and the upregulation of DKK1 could hinder the therapeutic efficacy of paclitaxel by suppressing the infiltration and activity of CD8+ T cells in tumor microenvironment. Moreover, paclitaxel treatment in tumor-bearing mice also increased DKK1 expression through the activation of EGFR signaling in the primary sensory dorsal root ganglion (DRG) neurons, leading to the development of peripheral neuropathy, which is charactered by myelin damage in the sciatic nerve, neuropathic pain, and loss of cutaneous innervation in hindpaw skin. The addition of an anti-DKK1 antibody not only improved therapeutic efficacy of paclitaxel in two murine subtype models of breast cancer but also alleviated paclitaxel-induced peripheral neuropathy. Taken together, our findings providing a potential chemoimmunotherapy strategy with low neurotoxicity that can benefit multiple subtypes of breast cancer patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-024-02067-y.
Keywords: Paclitaxel, DKK1, EGFR, Peripheral neuropathy, Breast cancer
Introduction
Breast cancer is one of the most common causes of death in women globally, with a rising trend in incidence. Paclitaxel (PTX) is widely used as a fundamental chemotherapeutic agent for the treatment of breast cancer [1]. Recently, the combination of paclitaxel and immunotherapy was proposed to be a promising strategy to improve chemotherapy outcomes by enhancing cytotoxic T-cell responses [2]. In particular, paclitaxel plus an immune checkpoint inhibitor targeting PD-1/PD-L1 was suggested to treat breast cancer [3]. Unfortunately, the IMpassion130 clinical trial suggested that only patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) can benefit from this therapeutic strategy [4]. Thus, it is of great significance to explore a new chemoimmunotherapy regimen that can benefit multiple subtypes of breast cancer patients.
Paclitaxel-induced peripheral neuropathy is a progressive and refractory adverse consequence of chemotherapy, which is characterized by chronic pain, a burning sensation, and tingling in the feet and hands in a “glove and stocking” distribution [5]. Neuropathic pain induced by paclitaxel often seriously affects the quality of life in individuals surviving cancer, leading to dose reductions or even to cessation of anticancer therapy [6]. However, there is currently no effective strategy for treating or preventing paclitaxel-induced peripheral neuropathy.
Dickkopf-1 (DKK1), a secreted protein and Wnt signaling pathway regulator, is highly expressed in a wide range of human cancers [7]. Numerous studies have documented that DKK1 can exert immunosuppressive effects within the tumor microenvironment by inducing immunosuppressive polarization of macrophages, fostering the accumulation of myeloid-derived suppressor cells (MDSCs), or stimulating PD-L1 expression in cancer cells, which ultimately suppresses the tumor-killing functions of CD8+ T cells and promotes tumor progression [8, 9]. Recently, a DKK1 neutralizing antibody showed a marked clinical benefit to patients whose tumors overexpress DKK1, suggesting that DKK1 is an ideal target for cancer immunotherapy [10]. Interestingly, DKK1 was reported to be positively correlated with the development of pain in patients with temporomandibular osteoarthritis or Gaucher disease, implying that DKK1 may play an important role in the development of pain [11]. In this study, we aimed to investigate whether targeting DKK1 may enhance the antitumor activity of paclitaxel and alleviate chemotherapy-induced neuropathic pain in breast cancer.
Results
Paclitaxel can promote DKK1 expression in breast cancer
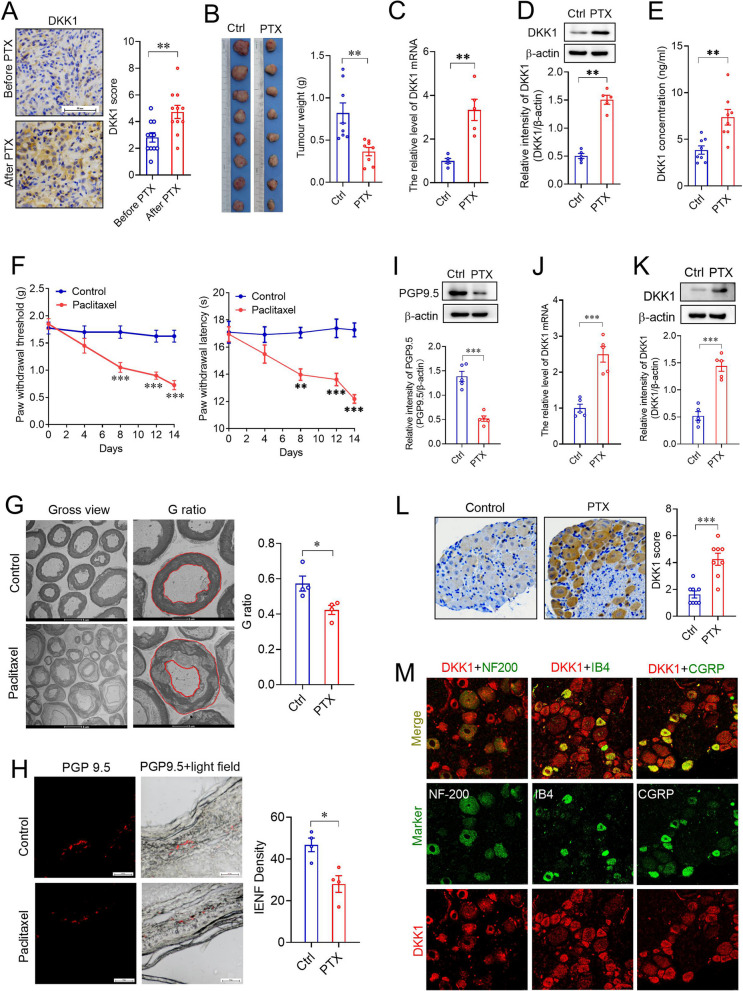
To identify whether DKK1 is a potential target to enhance sensitivity to paclitaxel in breast cancer, we first examined DKK1 expression in 11 human breast cancer tissues before and after neoadjuvant chemotherapy, including paclitaxel. The results of the IHC assay showed that DKK1 expression was significantly upregulated in breast cancer tissues after paclitaxel-based chemotherapy (Fig. 1A). Because these breast cancer patients received other drug therapies in addition to paclitaxel, we then established a mouse xenograft model using 4T1 mouse breast cancer cells to examine the effects of paclitaxel alone on tumor growth and DKK1 expression. As the results showed, although tumor growth was delayed in the mice treated with paclitaxel (8 mg/kg) every other day for 2 weeks (Fig. 1B), paclitaxel promoted the expression of DKK1 mRNA and protein in the tumors (Fig. 1C, D). As DKK1 is a secretory glycoprotein, we measured DKK1 levels in the serum from vehicle (CREMO)- and paclitaxel-treated tumor-bearing mice. The results showed that PTX treatment significantly increased serum DKK1 levels (Fig. 1E).
Fig. 1.
Paclitaxel promoted tumoral DKK1 expression in vitro and in vivo. A IHC staining of DKK1 in breast cancer tissues before and after neoadjuvant chemotherapy including paclitaxel. **p < 0.01, a two-tailed unpaired t test, n = 11. Scale bar, 50 µm. B Effects of paclitaxel on tumor growth in a mouse xenograft model. **p < 0.01, a two-tailed unpaired t test, n = 8. C, D Effects of paclitaxel on DKK1 mRNA and protein expression in xenograft tumors. C Real-time analysis of DKK1 mRNA expression; D Western blot of DKK1 protein expression. **p < 0.01, a two-tailed unpaired t test, n = 5. F Effects of paclitaxel on mechanical allodynia (Left) and thermal hyperalgesia (right) of xenograft mice. **p < 0.01, ***p < 0.001, two-way ANOVA, n = 8. G Transmission electron micrographs of the sciatic nerves and quantification of the G-ratio in mouse sciatic nerves. *p < 0.05, a two-tailed unpaired t test, n = 4. H Immunofluorescence staining of PGP9.5 in the mouse hind paw intraplantar skin and quantification analysis of intraepidermal nerve fibers (IENF). *p < 0.05, a two-tailed unpaired t test, n = 4. I Western blot of PGP9.5 in the mouse hind paw intraplantar skin from control or paclitaxel-treated xenograft mice. ***p < 0.001, a two-tailed unpaired t test, n = 5. J, K Effects of paclitaxel on the expression of DKK1 mRNA (J) and protein (K) expression in L4-L5 DRGs from xenograft mice. ***p < 0.001, a two-tailed unpaired t test, n = 5. L IHC staining of DKK1 expression in L4-L5 DRGs from xenograft mice treated with vehicle (Ctrl) or paclitaxel (PTX). ***p < 0.001, a two-tailed unpaired t test, n = 8. M Analysis of DKK1 mRNA expression in L4-L5 DRG neurons in paclitaxel-treated xenograft mice by FISH combined with immunofluorescence, n = 3
Considering that the 4T1 breast carcinoma model is triple-negative breast cancer (TNBC), we next used a luminal B breast cancer model, MMTV-PyMT mice bearing spontaneous breast tumors, to investigate the effects of paclitaxel on tumor growth and DKK1 expression. Similarly, paclitaxel treatment for 2 weeks significantly suppressed tumor growth and promoted tumoral DKK1 expression in the PyMT spontaneous model (Figure S1A-D). ELISA showed that serum DKK1 levels were also increased in paclitaxel-treated PyMT mice (Figure S1E).
To determine whether paclitaxel induced DKK1 expression in breast cancer cells, we incubated four distinct breast cancer cell lines, namely, MCF-7 (ER+, PR+, HER2−), BT-474 (ER+, PR+, HER2+), MDA-MB-231 (ER−, PR−, HER2−) and SKBR3 (ER−, PR−, HER2+) cells, with 200 nM paclitaxel. As expected, the mRNA and protein expression of DKK1 was increased in these paclitaxel-treated breast cancer cells (Figure S1F, G). By measuring DKK1 levels in cell culture medium, we also found that paclitaxel could promote the secretion of DKK1 from breast cancer cells (Figure S1H). Our results suggest that paclitaxel can promote DKK1 expression in different subtypes of breast cancer cells.
DKK1 is a potential target to enhance sensitivity to paclitaxel in breast cancer
To investigate whether paclitaxel-induced DKK1 overexpression could attenuate chemotherapeutic efficacy by inhibiting the infiltration and function of CD8+ T cells in breast cancer, we first established stable DKK1-overexpressing and DKK1-knockdown 4T1 cells. The construction efficiency was confirmed by Western blotting (Figure S2A). Then, we injected DKK1-overexpressing 4T1 cells subcutaneously into BALB/c mice and found that DKK1 significantly promoted tumor growth (Figure S2B). Using flow cytometry, we confirmed that both the infiltration and activity (GZMB+) of CD8+ T cells were dramatically reduced in DKK1-overexpressing tumors (Figure S2C-E).
Subsequently, we established a mouse xenograft model using stably DKK1-knockdown or vector-transfected 4T1 cells. After 1 week, the tumor-bearing mice were injected intraperitoneally with paclitaxel (8 mg/kg) or vehicle every other day for 2 weeks. The results showed that knockdown of DKK1 significantly enhanced the antitumor growth activity of paclitaxel in vivo (Figure S2F). Moreover, flow cytometry assays suggested that DKK1 knockdown greatly increased the infiltration and activity of CD8+ T cells in paclitaxel-treated xenograft tumors (Figure S2G-I). These results indicate that DKK1 is a potential immunotherapeutic target to enhance sensitivity to paclitaxel in breast cancer.
EGFR signaling participates in paclitaxel-induced DKK1 upregulation in breast cancer
We have demonstrated that the activation of EGFR promotes DKK1 expression through parallel MEK-ERK and PI3K-Akt signaling pathways in hepatocellular carcinoma [12]. Hence, we hypothesized that EGFR signaling participates in the paclitaxel-induced upregulation of DKK1 in breast cancer. To test our hypothesis, we first analyzed EGFR expression in 11 human breast cancer specimens before and after neoadjuvant chemotherapy. IHC staining of tumor tissues showed that paclitaxel-based chemotherapy dramatically enhanced EGFR expression in breast cancer (Figure S3A). Next, we determined the effects of paclitaxel on EGFR expression in tumors from xenograft mice and MMTV-PyMT mice. IHC staining and Western blot assays suggested that paclitaxel treatment significantly promoted EGFR expression in the tumors (Figure S3B-E). By stimulating MCF-7 and MDA-MB-231 cells with 200 nM paclitaxel, we found that paclitaxel could promote EGFR protein expression in these cells (Figure S3F).
Subsequently, we assessed the effects of exogenous EGFR on DKK1 mRNA and protein levels in breast cancer cells. The results showed that ectopic expression of EGFR markedly promoted DKK1 mRNA and protein expression in MCF-7 and MDA-MB-231 cells (Figure S3G, H). Moreover, we evaluated the effects of the EGFR tyrosine kinase inhibitor AG1478, the Akt inhibitor MK2206, and the MEK inhibitor PD98059 on paclitaxel-induced expression of DKK1 in breast cancer cells. As expected, when breast cancer cells were pretreated with AG1478 (20 µM), MK2206 (10 µM), or PD98059 (20 µM) for 10 min followed by coincubation with paclitaxel (200 nM) for 24 h, the paclitaxel-induced increase in DKK1 mRNA and protein expression could be dramatically blocked by each of these inhibitors (Figure S3I-L). Together, these results suggested that paclitaxel could promote DKK1 expression by activating the EGFR signaling pathway in breast cancer cells.
DKK1 contributes to paclitaxel-induced peripheral neuropathy
Paclitaxel-induced peripheral neuropathy seriously affects patient quality of life [13]. Consistent with previous reports [5, 13], our results showed that paclitaxel treatment could produce mechanical allodynia and thermal hyperalgesia in xenograft tumor-bearing mice (Fig. 1F). Furthermore, paclitaxel caused myelin damage in the sciatic nerves of tumor-bearing mice (Fig. 1G). To assess myelination integrity in sciatic nerves, we calculated the G-ratios of myelinated fibers and found that paclitaxel treatment markedly reduced this (Fig. 1G). Next, we examined the expression of the general nerve marker PGP9.5 in hindpaw skin. Through immunofluorescence and Western blot, we observed that paclitaxel significantly inhibited the expression of PGP9.5 in the intraplantar epidermis (Fig. 1H, I), indicating that paclitaxel caused the loss of intraepidermal nerve fibers.
Peripheral sensory neurons in dorsal root ganglia (DRG) are highly susceptible to paclitaxel accumulation owing to the devoid of the blood–brain barrier [14]. Therefore, we first measured DKK1 expression in the dorsal root ganglia (DRGs) of tumor-bearing mice. As the results showed, paclitaxel treatment dramatically promoted DKK1 mRNA and protein expression in L4-L5 DRGs (Fig. 1J-L). Through FISH combined with immunofluorescence, we found that DKK1 mRNA was universally colocalized with NF200-, IB4- and CGRP-positive DRG neurons in paclitaxel-treated tumor-bearing mice (Fig. 1M).
To investigate whether DKK1 was involved in paclitaxel-induced peripheral neuropathy, we virally expressed DKK1 in the DRGs and examined whether DKK1 overexpression could induce peripheral neuropathy in normal mice (Figure S4A). As expected, the expression of exogenous DKK1 triggered a significant decrease in both the mechanical withdrawal threshold and thermal withdrawal latency (Figure S4B). Moreover, we also observed that DKK1 overexpression in the DRGs caused myelin damage, a decline in the G-ratio of sciatic nerves, and the loss of intraepidermal nerve fibers in normal mice (Figure S4C-E). Then, we intrathecally injected lentivirus-loaded DKK1 shRNA to knock down DKK1 in L4-L5 DRGs in normal mice. One week later, these mice were treated with paclitaxel (8 mg/kg) or vehicle every other day for 2 weeks (Figure S4F). The results showed that knockdown of DKK1 almost completely blocked paclitaxel-induced mechanical allodynia and thermal hyperalgesia (Figure S4G). In addition, DKK1 depletion attenuated paclitaxel-induced myelin damage in the sciatic nerve and loss of cutaneous innervation (Figure S4H-K).
EGFR signaling is involved in paclitaxel-induced DKK1 upregulation in DRGs
To investigate whether EGFR signaling was involved in paclitaxel-mediated upregulation of DKK1 in DRGs, we first examined the distribution of EGFR in DRG neurons. Immunofluorescence staining showed that EGFR was widely expressed in NF200-, IB4- and CGRP-positive DRG neurons in paclitaxel-treated tumor-bearing mice (Figure S5A). Then, we assessed the effects of paclitaxel on EGFR signaling in the DRGs. Through Western blot and IHC staining, we found that paclitaxel treatment can promote the expression of EGFR protein in the DRGs in xenograft tumor-bearing mice (Figure S5B, C). Pretreatment with the EGFR inhibitor AG1478 blocked paclitaxel-induced upregulation of DKK1 mRNA and protein in DRGs in tumor-bearing mice (Figure S5D-F). Furthermore, AG1478 treatment could attenuate paclitaxel-induced neuropathic pain, myelin damage in the sciatic nerve, and loss of cutaneous innervation (Figure S5E-G). Similarly, AKT inhibitor MK2206 and MEK inhibitor PD98059 also blocked paclitaxel-induced upregulation of DKK1 expression in DRGs and nerve injury in tumor-bearing mice (Figure S6). Together, these results suggested that EGFR signaling contributed to paclitaxel-induced nerve injury and DKK1 upregulation in DRGs in tumor-bearing mice.
Anti-DKK1 antibody enhances the antitumor efficacy of paclitaxel and alleviates paclitaxel-induced peripheral neuropathy in vivo
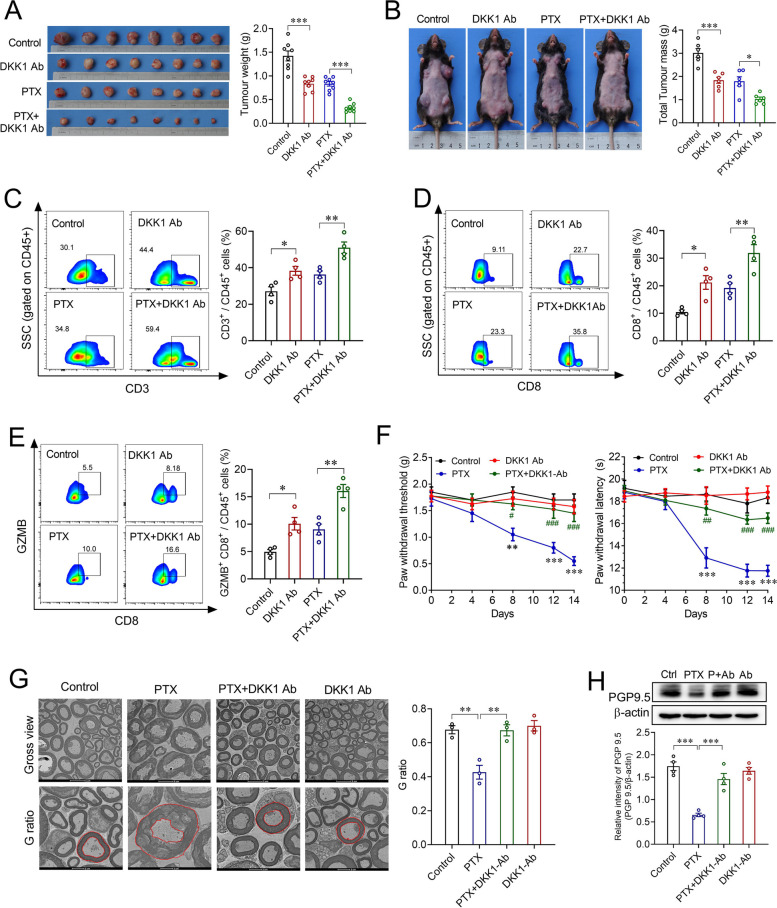
Multiple DKK1-neutralizing antibodies have shown promising benefits in patients whose tumors overexpress DKK1 in clinical trials [15]. Therefore, we first asked whether the addition of an anti-DKK1 antibody would enhance the antitumor activity of paclitaxel in breast cancer. To test this hypothesis, an anti-DKK1 antibody and/or pacliatxel was intraperitoneally administered to 4T1 xenograft mice and MMTV-PyMT mice bearing spontaneous breast tumors. As expected, tumor growth in the paclitaxel combined with anti-DKK1 antibody group was dramatically decreased compared to that in the paclitaxel group (Fig. 2A, B). Using flow cytometry, we confirmed a substantial increase in the infiltration and activity (GZMB+) of CD8+ T cells in the paclitaxel combined with anti-DKK1 antibody group compared to the paclitaxel group in xenografted tumors (Fig. 2C-E).
Fig. 2.
The DKK1 antibody enhanced the antitumor activity of paclitaxel and attenuated chemotherapy-induced peripheral neuropathy. A, B Effects of DKK1 antibody on the antitumor activity of paclitaxel in xenograft mice (A) and MMTV-PyMT mice (B). **p < 0.01, ***p < 0.001, one-way ANOVA, n = 6/8. C-E) Flow cytometry of CD3+ (C), CD8+ (D), and GZMB+CD8+ (E) CD45+ cells in xenograft mice treated with vehicle, PTX, PTX plus DKK1 antibody, or DKK1 antibody. *p < 0.05, **p < 0.01, one-way ANOVA, n = 4. F Effects of the DKK1 antibody on paclitaxel-induced mechanical allodynia (left) and thermal hyperalgesia (right) in xenograft mice. **p < 0.01, ***p < 0.001 compared with control group; ##p < 0.01, ###p < 0.001 compared with PTX group; two-way ANOVA, n = 8. G Transmission electron micrographs of the sciatic nerves and quantification of the G-ratio in mouse sciatic nerves from xenograft mice treated with vehicle, PTX, PTX plus DKK1 antibody, or DKK1 antibody. **p < 0.01, one-way ANOVA, n = 3. H Western blot of PGP9.5 in the hind paw intraplantar skin from xenograft mice treated with vehicle, PTX, PTX plus DKK1 antibody, or DKK1 antibody. ***p < 0.001, one-way ANOVA, n = 4
Subsequently, we wondered whether the anti-DKK1 antibody could attenuate paclitaxel-induced nerve injury and neuropathic pain. The behavioral test revealed that the anti-DKK1 antibody significantly attenuated paclitaxel-induced mechanical allodynia and thermal hypoesthesia in 4T1-xenograft mice (Fig. 2F). By examining the ultrastructure of sciatic nerves, we found that the anti-DKK1 antibody dramatically alleviated paclitaxel-induced myelin damage in 4T1-xenograft mice (Fig. 2G). Additionally, Western blot analysis of hindpaw skins showed that the anti-DKK1 antibody could reverse paclitaxel-induced downregulation of PGP9.5 expression in 4T1-xenograft mice (Fig. 2H), implying that the anti-DKK1 antibody ameliorated the decreases in skin innervation caused by paclitaxel. Together, these findings demonstrated that the severity of paclitaxel-induced peripheral neuropathy could be improved by anti-DKK1 antibody in vivo.
Conclusion
Our study provides the first evidence that paclitaxel promotes DKK1 expression through the activation of EGFR signaling in breast cancer cells and DRG neurons. On the one hand, the upregulation of DKK1 in breast cancer cells could hinder the therapeutic efficacy of paclitaxel by suppressing the infiltration and activity of CD8+ T cells. On the other hand, the upregulation of DKK1 in DRG neurons contributes to paclitaxel-induced peripheral neurotoxicity. More importantly, the addition of an anti-DKK1 antibody not only enhances the therapeutic efficacy of paclitaxel in two murine subtype models of breast cancer but also attenuates paclitaxel-induced peripheral neurotoxicity. Taken together, our study suggested that paclitaxel in combination with DKK1 may be a potential chemoimmunotherapy strategy with low neurotoxicity that can benefit multiple subtypes of breast cancer patients.
Supplementary Information
Acknowledgements
This study was supported by the National Key R&D Program of China (2021YFA0909600), Key projects of Henan Science and Technology Department (232102311115, 242102311032, 242102311072), Natural Science Foundation of Henan (232300420050). This work was also supported by the Open Projects in the Key Laboratory for Neuroscience of Peking University.
Authors’ contributions
H. Shi, H. Tao, J. He, F. Zhu, C. Xie, and Y. Cheng performed experiments. J. He, H. Sun, and D. Fang performed conceptualization and experimental design. L. Hou, and D. Fang wrote the paper. C. Qin and S. Xie supervised the entire project.
Funding
This study was supported by the National Key R&D Program of China (2021YFA0909600), Key projects of Henan Science and Technology Department (232102311115, 242102311032, 242102311072), Natural Science Foundation of Henan (232300420050). This work was also supported by the Open Projects in the Key Laboratory for Neuroscience of Peking University.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All human tissues were collected from The First Affiliated Hospital of Henan University. Written informed consent was obtained from each patient. The study was approved by the Ethics Committee of Henan University. All animal procedures were conducted strictly following the protocols approved by the Institutional Animal Care and Use Committee at Henan University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong-Xiang Shi, Hang-Tian Tao and Jin-Jin He contributed equally to this work.
Contributor Information
Chang-Jiang Qin, Email: hhyyqcj@163.com.
Dong Fang, Email: fangdong@henu.edu.cn.
Song-Qiang Xie, Email: xiesq@henu.edu.cn.
References
- 1.Skubník J, Pavlícková VS, Ruml T, Rimpelová S. Autophagy in cancer resistance to paclitaxel: development of combination strategies. Biomed Pharmacother. 2023;161:114458. 10.1016/j.biopha.2023.114458 [DOI] [PubMed] [Google Scholar]
- 2.Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. New Engl J Med. 2022;387(3):217–26. 10.1056/NEJMoa2202809 [DOI] [PubMed] [Google Scholar]
- 3.Pusztai L, Yau C, Wolf DM, Han HS, Du LL, Wallace AM, String-Reasor E, Boughey JC, Chien AJ, Elias AD, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell. 2021;39(7):989-+. [DOI] [PMC free article] [PubMed]
- 4.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. 10.1016/S1470-2045(19)30689-8 [DOI] [PubMed] [Google Scholar]
- 5.Vermeer CJC, Hiensch AE, Cleenewerk L, May AM, Eijkelkamp N. Neuro-immune interactions in paclitaxel-induced peripheral neuropathy. Acta Oncol. 2021;60(10):1369–82. 10.1080/0284186X.2021.1954241 [DOI] [PubMed] [Google Scholar]
- 6.Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014;10(12):694–707. 10.1038/nrneurol.2014.211 [DOI] [PubMed] [Google Scholar]
- 7.Jaschke N, Hofbauer LC, Göbel A, Rachner TD. Evolving functions of Dickkopf-1 in cancer and immunity. Cancer Lett. 2020;482:1–7. 10.1016/j.canlet.2020.03.031 [DOI] [PubMed] [Google Scholar]
- 8.Shi T, Zhang YP, Wang Y, Song XR, Wang HB, Zhou XY, Liang KJ, Luo YT, Che KY, Wang X, et al. DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol Res. 2022;10(12):1506–24. 10.1158/2326-6066.CIR-22-0218 [DOI] [PubMed] [Google Scholar]
- 9.Sui Q, Liu D, Jiang W. Dickkopf 1 impairs the tumor response to PD-1 blockade by inactivating CD8+ T cells in deficient mismatch repair colorectal cancer. J Immunother Cancer. 2021;9(8):e001498. 10.1136/jitc-2020-001498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, Ishikawa N, Kohno N, Ito H, Miyamoto M, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Can Res. 2010;70(13):5326–36. 10.1158/0008-5472.CAN-09-3879 [DOI] [PubMed] [Google Scholar]
- 11.Srikanth MP, Feldman RA. Elevated Dkk1 mediates downregulation of the canonical Wnt pathway and lysosomal loss in an iPSC model of neuronopathic gaucher disease. Biomolecules. 2020;10(12):1630. 10.3390/biom10121630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu J, Li W, Liang C, Wang X, Yao X, Yang RH, Zhang ZS, Liu HF, Liu FY, Pei SH, et al. EGF promotes transcription in hepatocellular carcinoma by enhancing the phosphorylation and acetylation of histone H3. Sci Signal. 2020;13(657):eabb5727. 10.1126/scisignal.abb5727 [DOI] [PubMed] [Google Scholar]
- 13.Chen LH, Yeh YM, Chen YF, Hsu YH, Wang HH, Lin PC, Chang LY, Lin CCK, Chang MS, Shen MR. Targeting interleukin-20 alleviates paclitaxel-induced peripheral neuropathy. Pain. 2020;161(6):1237–54. 10.1097/j.pain.0000000000001831 [DOI] [PubMed] [Google Scholar]
- 14.Xu YH, Jiang ZS, Chen XW. Mechanisms underlying paclitaxel-induced neuropathic pain: channels, inflammation and immune regulations. Eur J Pharmacol. 2022;933:175288. 10.1016/j.ejphar.2022.175288 [DOI] [PubMed] [Google Scholar]
- 15.Klempner SJ, Bendell JC, Villaflor VM, Tenner LL, Stein SM, Rottman JB, Naik GS, Sirard CA, Kagey MH, Chaney MF, et al. Safety, efficacy, and biomarker results from a phase Ib study of the anti-DKK1 antibody DKN-01 in combination with pembrolizumab in advanced esophagogastric cancers. Mol Cancer Ther. 2021;20(11):2240–9. 10.1158/1535-7163.MCT-21-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.