Abstract
Retroviral vectors for gene therapy are designed to minimize the occurrence of replication-competent retrovirus (RCR); nonetheless, it is possible that a vector-derived RCR could establish an infection in a patient. Since the efficacy of antiretroviral agents can be impacted by interactions between virus, host cell, and drug, five commonly used antiretroviral drugs were evaluated for their abilities to inhibit the replication of a murine leukemia virus (MLV)-derived RCR in human cells. The results obtained indicate that the combination of nucleoside analogs zidovudine and dideoxyinosine with the protease inhibitor indinavir effectively inhibits MLV-derived RCR replication in three human cell lines. In addition, MLV-derived RCR was found to be inherently resistant to the nucleoside analogs lamivudine and stavudine, suggesting that mutations conferring resistance to nucleoside analogs in human immunodeficiency virus type 1 have the same effect even in an alternative viral backbone.
Retroviral vectors designed for use in gene therapy are replication defective and are by definition limited in their potential for insertional mutagenesis and pathogenesis in host cells. Although replication-competent retrovirus (RCR) has never been detected in a gene therapy patient (15, 16), recombination can occur between vector and packaging sequences in vector producer cells or with endogenous retrovirus, and it is possible that an RCR infection could be established. In its native host, murine leukemia virus (MLV) infection normally results in acute viremia followed by clearance; oncogenesis occurs only when newborn or immunosuppressed animals are infected (8, 32). While this suggests that disease due to RCR is not likely to occur in normal humans, the pathological consequences of murine RCR infection remain unknown. Clinical considerations of how to treat such an infection would need to take into account the nature of both the virus and the species of cell involved.
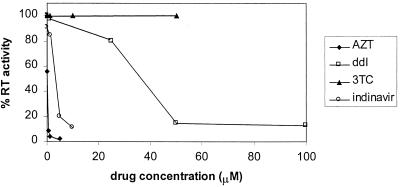
To identify potential treatments for a murine RCR replicating in human cells, five commonly used antiretroviral drugs were tested for the ability to inhibit the establishment and spread of murine RCR infection in human 293 cells. The RCR used for this study resulted from the recombination of vector and packaging sequences in the murine vector producer cell line PA317/G1Na.40 (23). Antiretroviral drugs were added to the cells simultaneously with virus in a medium containing 8 μg of Polybrene per ml, the cells were passaged twice in the presence of drug, and the medium was collected from the cultures 10 days after infection. The amount of virus released into the medium was determined by using commercially available reverse transcriptase (RT) assays according to the manufacturer’s directions (NEN Life Sciences, Boston, Mass.), except that the reaction buffer supplied with the kit was replaced with a buffer containing 25 mM Tris, pH 8.3, and 2.5 mM MnCl2. The sources of antiretroviral agents were as follows: zidovudine (AZT), dideoxyinosine (ddI), and stavudine (d4T) were from Sigma Chemical, St. Louis, Mo., lamivudine (3TC) was supplied by the National Institutes of Health AIDS Reagent Repository, and indinavir was supplied by Merck, Rahway, N.J. Dose response curves for AZT, ddI, 3TC, and indinavir are shown in Fig. 1.
FIG. 1.
Efficacies of antiretroviral drugs against murine RCR replication in 293 cells. 293 cells were infected with G1Na.40 RCR; drugs were added simultaneously with virus to block the establishment and spread of infection. Cultures were passaged in the presence of drug at 4 and 7 days postinfection, and the medium collected 10 days postinfection was precipitated with polyethylene glycol for RT assay. The doses tested were as follows: AZT, 0.01 to 5 μM; ddI, 0.1 to 100 μM; 3TC, 0.1 to 50 μM; indinavir, 0.1 to 10 μM. The relative efficacies of the drugs tested may be arranged as follows: AZT > indinavir > ddI > d4T (data not shown), 3TC.
Nucleoside analogs such as AZT have been reported to have a broad spectrum of antiretroviral activity (10, 27–29, 31); however, efficacy has been shown to vary with the species and cell type or tissue tested (1–4, 22, 24). We found that AZT was highly effective in inhibiting RCR replication in 293 cells, with a 98% reduction in RT activity at a concentration of 5 μM (Fig. 1). ddI also inhibited murine RCR, although much higher doses were required for effective inhibition. Two other nucleoside analogs tested, d4T (data not shown) and 3TC, had no effect on detectable levels of virus even at a concentration of 50 μM, which was the highest concentration of these drugs at which toxic effects were not observed. In addition to the nucleoside analogs, the protease inhibitor indinavir was also tested. Although structural similarities have been reported between the proteases of MLV and human immunodeficiency virus type 1 (HIV-1) (18, 20), differences in protease substrate specificity between murine retroviruses and HIV-1 have been demonstrated (19). We examined the efficacy of indinavir in doses ranging from 0.01 to 10 μM and found that it effectively inhibited murine RCR replication in 293 cells (Fig. 1). To ensure that the reductions in infectivity observed were not due to toxic effects of the drugs on the cell line being assayed, the cell doubling time in the presence of the highest dose of each drug was determined; no difference between control and drug-treated cells was observed (data not shown).
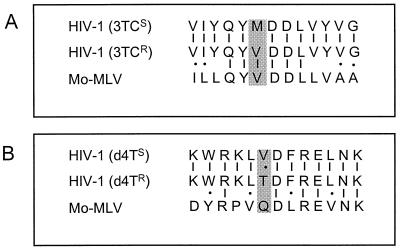
The results shown in Fig. 1 demonstrate that murine RCR was resistant to two nucleoside analogs frequently used against HIV-1 in the clinic. Clinical HIV isolates resistant to 3TC have been well characterized and most frequently result from a mutation at residue 184 of the HIV-1 RT B chain from a methionine to a valine or isoleucine (M184[V/I]) (11, 17, 25, 30). A comparison of the sequence of wild-type MLV RT with that of HIV-1 indicates that in the region of this mutation, the sequences of the two viruses are highly conserved (Fig. 2A). The HIV-1 M184 residue occurs in a highly conserved domain of the RT active site (YMDD); as in 3TC-resistant HIV-1, this residue is a valine in the RT of wild-type MLV. The M184V mutation in HIV-1 has also been associated with low-level resistance to ddI in in vitro studies (11, 12). While ddI did have an effect the replication of murine RCR in our studies, the concentration needed to achieve 50% inhibition of the viral activity was 40 μM, which was 500-fold greater than that required for AZT. Resistance to d4T is much more unusual in the clinic (14, 17); however, a single mutation (from valine to threonine) at residue 75 of HIV-1 RT associated with d4T resistance in vitro has been identified (13). The examination of the region surrounding this mutation in the MLV RT reveals that a glutamine occurs at the homologous position and that in general this region is not well conserved between MLV and HIV-1 (Fig. 2B). The relative resistance of the MLV-derived RCR to both 3TC and d4T that we observed in vitro can therefore be accounted for by the sequence of the RT. Our experiments demonstrate a good correlation between the presence of amino acid changes in HIV-1 causing resistance to particular drugs and resistance of MLV to these drugs, even in the context of a different viral backbone.
FIG. 2.
Alignment of MLV and HIV-1 RT sequences. The sequences of Moloney MLV (Mo-MLV) and HIV-1 RT were aligned in regions flanking mutations known to confer resistance to one or more drugs. The murine RCR used in these studies was sequenced and found to be identical to the Moloney sequence in these regions (data not shown). (A) 3TC resistance mutation occurs at amino acid 184 of HIV (shaded box); the sequence of the Moloney RT in this region is identical to that of 3TC-resistant HIV-1. (B) d4T resistance mutation occurs at amino acid 75 of HIV (shaded box). The sequence of MLV RT in the homologous position differs from wild-type HIV at several residues in this region.
Combinations of RT inhibitors and protease inhibitors are the most commonly used clinical treatment for HIV infection (26).
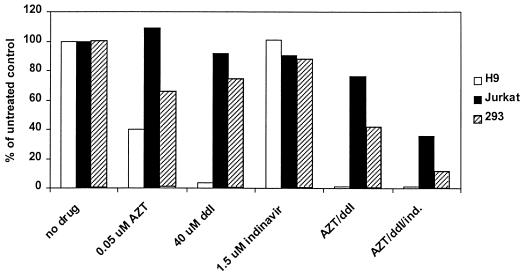
The studies described above demonstrate that three clinically relevant antiretroviral agents are effective in inhibiting the replication of murine RCR in 293 cells. To ensure that the drugs identified were also active in other human cell types, these drugs were retested alone and in combination in 293 cells as well as two human cell lines of lymphoid origin, Jurkat and H9 cells. In order to be able to detect decreases in viral infectivity for the combination treatments, an intermediate dose of drug was chosen for the combination therapies tested; each drug was tested singly in the same experiment so that any additive effects of the combination treatments could clearly be assessed. H9 and Jurkat cells were infected and passaged under the same conditions as those described previously for 293 cells. For increased sensitivity of detection, infectivity was measured by a quantitative PCR assay for the presence of viral envelope sequences. This RCR PCR assay amplifies a 712-bp region of the envelope sequences contained in pPAM3, the packaging sequence present in PA317 cells (21). The primers used for amplification were 5′-TTGTCCACCACGGTGCTCAAT-3′ and 5′-GGCTCGTACTCTATAGGCTTC-3. Amplification products were analyzed by gel electrophoresis and Southern blotting and were analyzed on a Storm imaging system (Molecular Dynamics, Sunnyvale, Calif.). The results of these experiments are shown in Fig. 3.
FIG. 3.
Efficacy of combination therapy in murine RCR-infected human cells. Cell lines were infected with G1Na.40 RCR in the presence of drugs and passaged twice, and cell pellets were harvested 10 days after infection for RCR PCR. The concentrations of drugs chosen for this experiment were based upon the single drug doses in 293 cells (0.05 uM AZT, 40 μM ddI, and 1.5 μM indinavir [ind.]). The efficacies of the drugs varied between cell types; however, in each case the combination treatments were more effective than any single drug treatment.
The efficacy of AZT, ddI, and indinavir alone varied between cell types. For example, H9 cells were more sensitive to both nucleoside analogs than either 293 or Jurkat cells and appeared to be extremely sensitive to ddI (96.6% inhibited at 40 μM). In contrast, infectivity in Jurkat cells appeared to be unaffected by 0.05 μM AZT and was only slightly affected by 40 μM ddI. These results support previous studies demonstrating that antiretroviral efficacy can vary between cell types, even in an in vitro assay system in which identical virus and conditions were used to establish infection. However, in all three cell lines, the combinations of two and three drugs were more effective than any single drug. In the most resistant cell line assayed, Jurkat, the AZT-ddI combination inhibited approximately 24% and the addition of the protease inhibitor indinavir further reduced activity to 36% of the untreated control cultures. In H9 and 293 cells, infectivity in the triple combination-treated cells was reduced to 1.1 and 11.3% of that of the untreated control cells, respectively. The approximate 50% inhibitory concentrations (IC50s) of AZT and indinavir in 293 cells were 0.08 and 3.0 μM, respectively, which are similar to IC50s reported in in vitro studies against HIV-1 and well within the achievable concentrations of these drugs in vivo (maximum concentration of drug in serum [Cmax] for AZT, 3.4 μM; Cmax for indinavir, 12.6 μM) (4–6, 9). While the IC50 for ddI determined in 293 cells (approximately 40 μM) exceeds the Cmax in vivo (8.2 μM), the increased sensitivity of H9 cells to this drug suggests that ddI may have efficacy against some cell types in vivo. These results demonstrate that the use of the AZT-ddI-indinavir combination therapy yields a good inhibition of murine RCR replication in three different human cell types.
Although distribution studies and patient monitoring indicate that retroviral vectors can potentially be found in a number of tissues, it is not clear what cell type, if any, might be particularly susceptible to RCR infection. In the one study in which murine RCR infection was established in immunosuppressed primates (7), three of eight monkeys died of T-cell lymphoma; the surviving monkeys have remained positive for viral sequences at very low levels in peripheral blood lymphocyte samples. It is worth noting that monkeys that survived the infection developed an antibody response to the RCR but those that died did not. Individual animal variability in the degree of immune system suppression and/or immune system recovery following transplantation may be responsible for differences in RCR pathogenesis. An early intervention with the combination of antiretroviral drugs identified in this study might limit the viral load in multiple cell types and allow sufficient time for immune system recovery and long-term survival.
Acknowledgments
We thank David Onions and Tyler Martin for helpful comments on these studies. We are grateful to Merck and Co. for providing indinavir.
REFERENCES
- 1.Balzarini J, Pauwels R, Baba M, Herdewijn P, DeClercq E, Broder S, Johns D G. The in vitro and in vivo anti-retrovirus activity and intracellular metabolism of 3′-azido-2′,3′-dideoxythymidine and 2′,3′-dideoxycytidine are highly dependent on the cell species. Biochem Pharmacol. 1988;37:897–903. doi: 10.1016/0006-2952(88)90178-5. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini J, Matthes E, Meeus P, Johns D G, DeClercq E. The antiretroviral and cytostatic activity, and metabolism of 3′-azido-2′,3′-dideoxythymidine, 3′-fluoro-2′,3′-dideoxythymidine and 2′,3′-dideoxycytidine are highly cell type-dependent. Adv Exp Med Biol. 1989;253B:407–413. doi: 10.1007/978-1-4684-5676-9_60. [DOI] [PubMed] [Google Scholar]
- 3.Chow H-H, Li P, Brookshier G, Tang Y. In vivo tissue disposition of 3′-azido-3′-deoxythymidine and its anabolites in control and retrovirus-infected mice. Drug Metab Dispos. 1997;25:412–422. [PubMed] [Google Scholar]
- 4.Coates J A V, Cammack N, Jenkinson H J, Jowett A J, Jowett M I, Pearson B A, Penn C R, Rouse P L, Viner K C, Cameron J M. (−)-2′Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992;36:733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks S G, Smith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 6.Deminie C A, Bechtold C M, Stock D, Alam M, Djang F, Balch A H, Chou T-C, Prichard M, Colonno R J, Lin P-F. Evaluation of reverse transcriptase and protease inhibitors in two-drug combinations against human immunodeficiency virus replication. Antimicrob Agents Chemother. 1996;40:1346–1351. doi: 10.1128/aac.40.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donahue R E, Kessler S W, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R, Bacher J, Zsebo K M, Nienhuis A W. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan H. Leukemogenesis by Moloney murine leukemia virus: a multi-step process. Trends Microbiol. 1997;5:74–82. doi: 10.1016/S0966-842X(96)10076-7. [DOI] [PubMed] [Google Scholar]
- 9.Flexner C, Hendrix C. Pharmacology of anti-retroviral agents. In: DeVito V T Jr, Hellman S, Rosenberg S A, editors. AIDS: biology, diagnosis, and prevention. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 479–493. [Google Scholar]
- 10.Fox B A, Woffendin C, Yang Z-Y, San H, Ranga U, Gordon D, Osterholzer J, Nabel G J. Genetic modification of human peripheral blood lymphocytes with a transdominant negative form of Rev: safety and toxicity. Hum Gene Ther. 1995;6:997–1004. doi: 10.1089/hum.1995.6.8-997. [DOI] [PubMed] [Google Scholar]
- 11.Gao Q, Gu Z, Parniak M A, Cameron J, Cammack N, Boucher C, Wainberg M A. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1993;37:1390–1392. doi: 10.1128/aac.37.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Z, Gao Q, Li X, Parniak M A, Wainberg M A. Novel mutation in the human immunodeficiency virus type 1 reverse transcriptase gene that encodes cross-resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine. J Virol. 1992;66:7128–7135. doi: 10.1128/jvi.66.12.7128-7135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacey S F, Larder B A. Novel mutation (V75T) in human immunodeficiency virus type 1 reverse transcriptase confers resistance to 2′,3′-didehydro-2′,3′-dideoxythymidine in cell culture. Antimicrob Agents Chemother. 1994;38:1428–1432. doi: 10.1128/aac.38.6.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lea A P, Faulds D. Stavudine: a review of its pharmcodynamic and pharmacokinetic properties and clinical potential in HIV infection. Drugs. 1996;5:846–864. doi: 10.2165/00003495-199651050-00009. [DOI] [PubMed] [Google Scholar]
- 15.Long Z, Li L-P, Grooms T, Lockey C, Nader K, Mychkovsky I, Mueller S, Burimski I, Ryan P, Kikuchi G, Ennist D, Marcus S, Otto E, McGarrity G. Biosafety monitoring of patients receiving intracerebral injections of murine retroviral vector producer cells. Hum Gene Ther. 1998;9:1165–1172. doi: 10.1089/hum.1998.9.8-1165. [DOI] [PubMed] [Google Scholar]
- 16.Martineau D, Klump W M, McCormack J E, DePolo N J, Kamantigue E, Petrowski M, Hanlon J, Jolly D J, Mento S J, Sajjadi N. Evaluation of PCR and ELISA assays for screening clinical trial subjects for replication-competent retrovirus. Hum Gene Ther. 1997;8:1231–1241. doi: 10.1089/hum.1997.8.10-1231. [DOI] [PubMed] [Google Scholar]
- 17.Mayers D L. Prevalence and incidence of resistance to zidovudine and other anti-retroviral drugs. Am J Med. 1997;102:70–75. doi: 10.1016/s0002-9343(97)00067-3. [DOI] [PubMed] [Google Scholar]
- 18.Menéndez-Arias L, Weber I T, Oroszlan S. Mutational analysis of the substrate binding pocket of murine leukemia virus protease and comparison with human immunodeficiency virus protease. J Biol Chem. 1995;270:29162–29168. doi: 10.1074/jbc.270.49.29162. [DOI] [PubMed] [Google Scholar]
- 19.Menendez-Arias L, Weber I T, Soss J, Harrison R W, Gotte D, Oroszlan S. Kinetic and modeling studies of subsites S4-S3′ of moloney murine leukemia virus protease. J Biol Chem. 1994;269:16795–16801. [PubMed] [Google Scholar]
- 20.Menéndez-Arias L, Gotte D, Oroszlan S. Moloney murine leukemia virus protease: bacterial expression and characterization of the purified enzyme. Virology. 1993;196:557–563. doi: 10.1006/viro.1993.1511. [DOI] [PubMed] [Google Scholar]
- 21.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolas F, De Sousa G, Thomas P, Placidi M, Lorenzon G, Rahmani R. Comparative metabolism of 3′-azido-3′-deoxythymidine in cultured hepatocytes from rats, dogs, monkeys, and humans. Drug Metab Dispos. 1995;23:308–313. [PubMed] [Google Scholar]
- 23.Otto E, Jones-Trower A, Vanin E F, Stambaugh K, Mueller S N, Anderson W F, McGarrity G J. Characterization of a replication-competent retrovirus resulting from recombination of packaging and vector sequences. Hum Gene Ther. 1994;5:567–575. doi: 10.1089/hum.1994.5.5-567. [DOI] [PubMed] [Google Scholar]
- 24.Patel B A, Boudinot F D, Schinazi R F, Gallo J M, Chu C K. Comparative pharmacokinetics and interspecies scaling of 3′-azido-3′-deoxy-thymidine (AZT) in several mammalian species. J Pharmacobiodyn. 1990;13:206–211. doi: 10.1248/bpb1978.13.206. [DOI] [PubMed] [Google Scholar]
- 25.Quan Y, Gu Z, Li X, Li Z, Morrow C D, Wainberg M A. Endogenous reverse transcription assays reveal high-level resistance to the triphosphate of (−)2′-dideoxy-3′-thiacytidine by mutated M184V human immunodeficiency virus type 1. J Virol. 1996;70:5642–5645. doi: 10.1128/jvi.70.8.5642-5645.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richman D D. HIV therapeutics. Science. 1996;272:1886–1888. doi: 10.1126/science.272.5270.1886. [DOI] [PubMed] [Google Scholar]
- 27.Sidwell R W, Hitchcock M, Okleberry K M, Burger R A, Warren R P, Morrey J D. Suppression of murine retroviral disease by 2′,3′-didehydro-2′,3′-dideoxythymidine (d4T) Antivir Res. 1992;19:313–324. doi: 10.1016/0166-3542(92)90012-t. [DOI] [PubMed] [Google Scholar]
- 28.Sin J-I, Specter S. The role of interferon-gamma in antiretroviral activity of methionine enkephalin and AZT in a murine cell culture. J Pharmacol Exp Ther. 1996;279:1268–1273. [PubMed] [Google Scholar]
- 29.Specter S, Plotnikoff N, Bradley W G, Goodfellow D. Methionine enkephalin combined with AZT therapy reduce murine retrovirus-induced disease. Int J Immunopharmacol. 1994;16:911–917. doi: 10.1016/0192-0561(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 30.Tisdale M, Kemp S D, Parry N R, Larder B A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tókés S, Aradi J. Inhibition of Moloney murine leukemia virus reverse transcriptase by partially 5-thiolated polyuridylic acid. Biochim Biophys Acta. 1995;1261:115–120. [PubMed] [Google Scholar]
- 32.Tsichlis P N, Lazo P A. Virus-host interactions and the pathogenesis of murine and human oncogenic retroviruses. Curr Top Microbiol Immunol. 1991;171:95–171. doi: 10.1007/978-3-642-76524-7_5. [DOI] [PubMed] [Google Scholar]