Abstract
Background
Extranodal natural killer/T-cell lymphoma (ENKTL) with tonsil involvement is not common, especially in children.
Case presentation
A 13-year-old girl presented with an unexplained sore throat for more than 2 months, together with intermittent fever and suppurative tonsilitis. Nasopharyngoscopy revealed a pharyngeal mass. Enhanced computed tomography (CT) scan showed tonsillar hypertrophy and punctate calcification. Chronic pyogenic granulomatous inflammation with pseudoepithelial squamous epithelial hyperplasia was observed in left tonsil, and pyogenic granulomatous inflammation and a small number of T-lymphoid cells were detected in the right tonsil. The immunohistochemical results showed CD2+, CD3+, CD4+, CD5+, CD8+, granzyme B+, and TIA-1+. The Ki-67 proliferation index was 20%. The case showed T cell receptor gene rearrangement. Finally, the case was diagnosed as ENKTL of stage II with tonsil involvement. The patient received 6 cycles of chemotherapy with SMILE regimen, and showed complete response with no recurrence in the follow-up.
Conclusion
We presented a rare case of ENKTL with tonsil involvement in a child. The patient showed complete response to the SMILE chemotherapy with no recurrence.
Keywords: Natural killer/T-cell lymphoma, ENKTL, Tonsils, SMILE chemotherapy, Case report
Introduction
Natural killer/T-cell lymphoma (NKTL) is an aggressive malignant tumor of NK cell or cytotoxic T cell lineage origin [1]. The disease is more prevalent in Asia, Central and South America than in Western countries [2, 3]. In China, NKTL is one of the most common lymphoma types, second only to diffuse large B-cell lymphoma [4].
Extranodal NKTL (ENKTL), designated as a typical type of NKTL, most often occurs in non-lymphatic sites such as nose [5], nasopharynx [6], and upper aerodigestive tract in adults [7]. Additionally, the skin and gastrointestinal tract may also be involved in patients of an advanced stage [8]. To our best knowledge, few or even no cases with tonsil involvement have been reported in children and adolescents. Here, we present a rare case of ENKTL with tonsil involvement in a child.
Case presentation
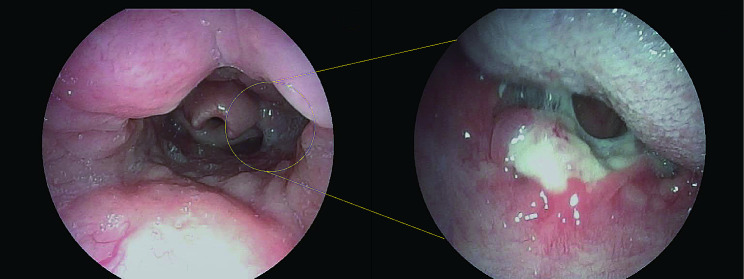
A 13-year-old girl complained about sore throat for more than 2 months accompanied by intermittent fever. She was diagnosed with suppurative tonsillitis in a local hospital before admitting to our hospital, and denied history of convulsions or coma, nausea, vomiting, or difficulty swallowing. Since the onset of the disease, she showed a poor appetite and poor-quality sleep. Nasopharyngoscopy revealed an ulcer lesion mass in pharyngeal region (Fig. 1). Contrast enhanced computed tomography (CT) showed slightly swollen uvula and bilateral tonsillar enlargement (Fig. 2). In the absence of surgical contraindications, tonsil biopsy was performed under general anesthesia after obtaining the written informed consent from her parents.
Fig. 1.
Nasopharyngoscopy indicated an ulcer lesion in pharyngeal region
Fig. 2.
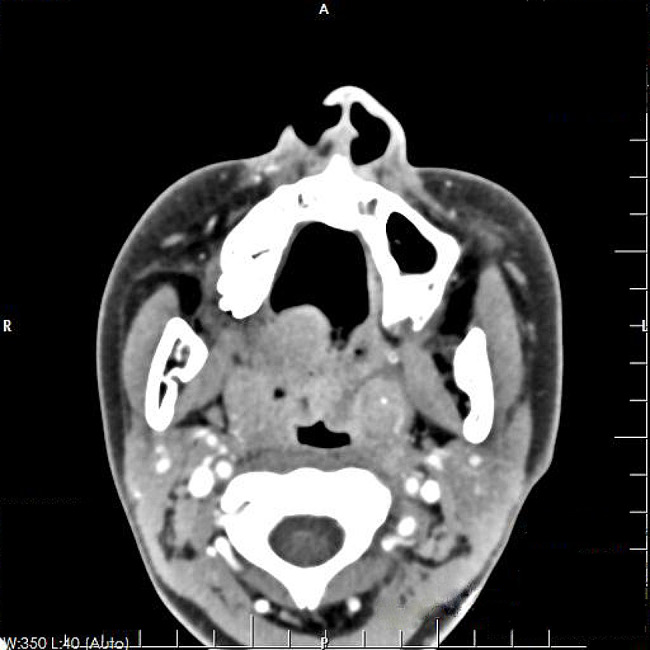
Contrast enhanced CT showed a slightly swollen uvula, bilateral tonsillar enlargement, and punctate calcification of the left tonsil
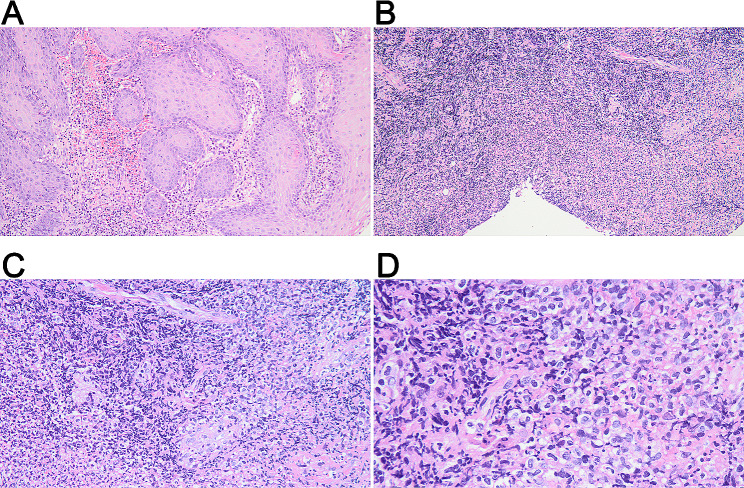
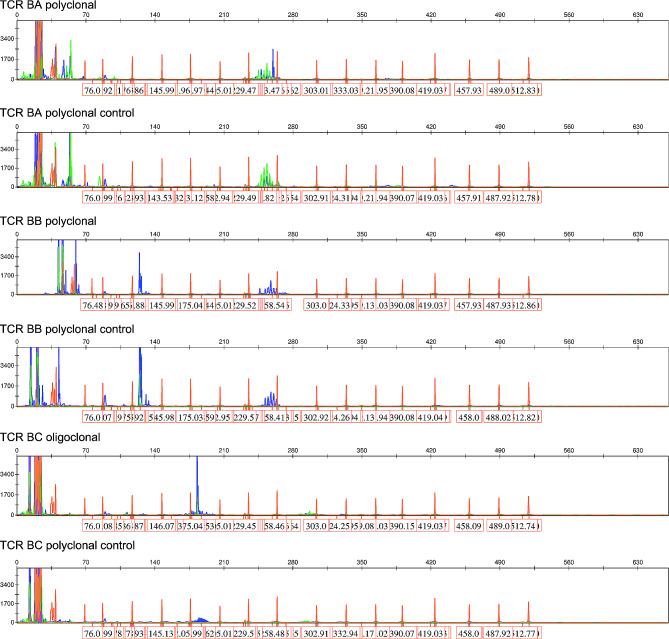
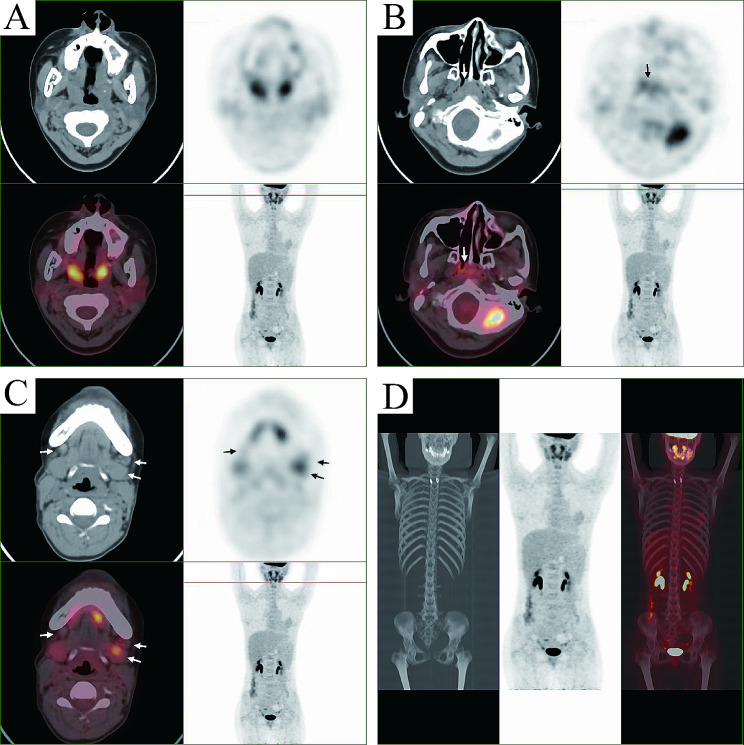
Pathological examination indicated tonsillar ulcer and necrosis, underlying blood vessels and lymphocytes proliferation. Chronic pyogenic granulomatous inflammation was observed together with pseudoepithelial squamous epithelial hyperplasia in left tonsil, and pyogenic granulomatous inflammation and a small number of T-lymphoid cells were detected in the right tonsil. Some lymphocytes showed bright cytoplasm, round, oval or slightly irregular nuclei, together with multiple lymphocytes and neutrophils infiltration, as well as presence of a few glands. Lymphocyte infiltration was seen in a few vessel walls. In addition, the tonsillar surface showed stratified squamous epithelial pseudoepithelial hyperplasia, vascular hyperplasia, necrosis, and acute and chronic inflammatory cell infiltration (Fig. 3). Immunohistochemically, the tumor cells were positive for CD2 (Fig. 4A), CD3 (Fig. 4B), CD4 (Fig. 4C), CD5 (Fig. 4D), CD7 (Fig. 4E), CD8 (Fig. 4F), granzyme B (Fig. 4G), and TIA-1 (Fig. 4H), and were negative for CD20, CD57, CD34 and SMA (data not shown). The Ki-67 proliferation index was 20% (Fig. 4I). On this basis, NKTL was highly suspected, and then genetic test was performed. T cell receptor (TCR) gene rearrangement was detected (Fig. 5). PET/CT showed infiltration mainly to the tonsil and posterior pharyngeal wall (Fig. 6) with the maximal SUV of 8.3 and 7.5, respectively. Finally, the patient was diagnosed with stage II ENKTL with tonsil involvement.
Fig. 3.
Representative HE-staining images of tonsil mass. (A) Perilesional pseudoepitheliomatous hyperplasia, under a magnification of 100×; (B) Massive necrosis and vascular proliferation, under a magnification of 100×; (C) Small foci of tumor cells distributed in the necrotic background and invading blood vessels, under a magnification of 200×; (D) Tumor cells have medium, translucent cytoplasm and irregular, distorted nuclei, under a magnification of 400×
Fig. 4.
Immunohistochemical results of tonsil mass. The tumor cells were positive for CD2 (A), CD3 (B), CD4 (C), CD5 (D), CD7 (E), CD8 (F), granzyme B (G), and TIA-1 (H). (I). Ki-67 expression. The images were observed under a magnification of 200×
Fig. 5.
A positive gene scan result of T cell receptor (TCR) gene rearrangement. The clonal rearrangement test utilized PCR-capillary electrophoresis analysis to detect the size distribution of TCR gene amplification. For TCRB-C test, the size range of the PCR amplification product was distributed in the two intervals of 170–210 bp and 285–325 bp. The judgment of monoclonality was based on whether there was a single prominent peak or multiple peaks of similar height within these two ranges. If it was obviously single, it was judged as positive. This TCRB-C obviously had a single blue prominent peak and was judged as positive. For the TCRB-B reaction, the judgment range was 240–285 bp. Within the range, there were multiple blue peaks, and there was no particularly prominent peak. Therefore, it was considered to be negative. NC, negative control
Fig. 6.
Positron emission tomography/computed tomography (PET/CT) indicated infiltration to the throat (A), maxillary (B), and submandibular glands (C). (D) No obvious distant metastasis was detected
For the treatment, the patient received 6 cycles of chemotherapy using SMILE regimen (Table 1). The patient showed complete response with no recurrence or adverse events in the 12-month follow-up.
Table 1.
SMILE chemotherapy scheme
| Agent | Standard dose/day | Actual dose/day | Day |
|---|---|---|---|
| Inpatient medication | |||
| Methotrexate | 2 g/m2 | 5.6 g/m2 | 1 |
| Leucovorin | 15 mg | SD | 2, 3, 4 |
| Ifosfamide | 1.5 g/m2 | 1.65 g/m2 | 2, 3, 4 |
| Mesna | 300 mg/m2 | SD | 2, 3, 4 |
| Dexamethasone | 40 mg | SD | 2, 3, 4 |
| Etoposide | 100 mg/m2 | 110 mg/m2 | 2, 3, 4 |
| Outpatient medication | |||
| L-asparaginase | 6000 U/m2 | SD | 8, 10, 12, 14, 16, 18, 20 |
The chemotherapy lasted for 28 days. SD, standard dose; SMILE, steroid (dexamethasone), methotrexate, ifosfamide, L-asparaginase, and etoposide
Discussion
ENKTL is rarely reported in children and adolescents [9], with most cases showing nasopharyngeal involvement. Occasionally, extranasal involvement has been reported, including skin, lungs, intestinal tract, testis, and bone marrow [10]. However, tonsil involvement in children has been rarely reported. We presented a 13-year-old girl of ENKTL with tonsils involvement, and reported our experiences on it with an aim to provide enhanced understanding on it.
Given that ENKTL mostly occurred in the nasopharynx, patients often presented with nasal congestion, epistaxis, fever, and swollen lymph nodes [11]. As its early clinical manifestations are atypical, it is usually misdiagnosed as lymphatic diseases. For the differential diagnosis, ENKTL should be distinguished from indolent T-cell lymphoproliferative disease. Indolent T-cell lymphoma showed low proliferation and no tendency of metastasis. In contrast, ENKTL showed a high possibility of metastasis with fast proliferation speed. Pathologically, T lymphocyte infiltration was the major feature for indolent T-cell lymphoma, while that for the ENKTL usually involved diffused lymphocyte infiltration and lymphoid follicle hyperplasia. At the same time, lymphoma gene rearrangement can also be used to identify these two conditions. In clinical practice, giant lymph node hyperplasia is characterized by lymphatic enlargement with unknown etiology, which mainly invades the chest cavity, hilum and lungs. Chronic lymphadenitis often has obvious lesions, concurrent with localized lymphadenopathy, headache, and tenderness. Such possibility was excluded as the patient showed no related symptoms.
The diagnosis of ENKTL mainly relies on biopsy and immunohistochemical staining, as well as TCR gene rearrangement. The histological phenotypes of ENKTL were often diffuse and permeative lymphocytic proliferation with an angiocentric and angiodestructive growth pattern, as well as fibrinoid changes [12]. ENKTL is typically composed of intermediate-sized cells with focal transformed cells or mixed small and large cells [13], which show irregular nuclear contours, inconspicuous nucleoli, and granular chromatin [14]. Consistent with previous reports, the pathological findings of our case included proliferation in blood vessels and lymphocytes, a portion of bright lymphocyte cytoplasm, profuse infiltration of lymphocytes and neutrophils, as well as round, oval or slightly irregular nuclei. In terms of immunophenotype, the typical phenotype of ENKTL was CD2+, cytoplasmic CD3 epsilon+, and cytotoxic markers (granzyme B, TIA-1, and perforin) (+) [3, 15]. Consistently, the immunohistochemical results for the tonsillar samples in this study were CD2+, CD3+, CD4+, CD5+, CD8+, granzyme B+, and TIA-1+. The expression rates of these markers reported in previous studies conducted using large cohorts were CD2 (93%), cCD3 (84%), CD4 (10%), CD5 (27%), CD8 (22%), granzyme B (83%), TIA1 (90%), and perforin (86%) [16–18]. Ki-67 proliferation index has been reported to be highly correlated with disease progression [19]. The moderate Ki-67 index (20%) in the case indicated moderate progression and moderate risk of the disease. In most cases, TCR genes were in germ-line configuration, but a small proportion of cases may show clonal TCR gene rearrangement that indicated T-cell derivation [20, 21]. Finally, the patient was diagnosed as ENKTL with tonsil involvement.
To date, there is still no standard treatment regimen for ENKTL. As previously described, the most successful treatment option is the non-anthracycline-containing regimens, especially with L-asparaginase [22, 23]. Up to 90% of the patients of stage I and II would show remission after treatment [8]. In a retrospective cohort study included 336 NKTL patients, cases received asparaginase-containing chemotherapy showed higher overall response rates and complete remission rates than the counterparts received no asparaginase-containing regimens. Consistently, our case underwent asparaginase-based SMILE chemotherapy showed good outcome with complete response.
Conclusion
We reported a 13-year-old ENKTL case with tonsil involvement based on CT, pathological analysis and TCR gene rearrangement. The patient showed complete response after SMILE chemotherapy. We hope that this case will be of interest to readers and helpful to the diagnosis, treatment, risk assessment, and management of ENKTL.
Acknowledgements
Not applicable.
Abbreviations
- CT
computed tomography
- ENKTL
extranodal natural killer/T-cell lymphoma
- NKTL
natural killer/T-cell lymphoma
- TCR
T cell receptor
Author contributions
Conceptualization and Methodology: JM and TZ. Resources: YG, and KL. Investigation: WC, and KZ. Formal analysis: YX, and XZ. Writing - Original Draft: YX, and XZ. Writing - Review & Editing: all authors. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
Not applicable.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study protocol was reviewed and approved by Ethical Committee of Kunming Children’s Hospital. Informed consent was obtained from the patient’s parents included in the study.
Consent for publication
Written informed consent for the publication of the personal or clinical details along with any identifying images was obtained from the patient’s parents.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Xiao and Xing Zhang contributed equally to this work and share first authorship.
Contributor Information
Jing Ma, Email: majing@etyy.cn.
Tiesong Zhang, Email: km62502023@126.com.
References
- 1.Reneau JC, Shindiapina P, Braunstein Z, Youssef Y, Ruiz M, Farid S, et al. Extranodal Natural Killer/T-Cell lymphomas: current approaches and future directions. J Clin Med. 2022;11(10):2699. 10.3390/jcm11102699. 10.3390/jcm11102699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. 10.1016/s1470-2045(18)30864-7. 10.1016/s1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima M. Aggressive mature natural killer cell neoplasms: from epidemiology to diagnosis. Orphanet J Rare Dis. 2013;8:95. 10.1186/1750-1172-8-95. 10.1186/1750-1172-8-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429–34. 10.1309/ajcp7yltqpusdq5c. 10.1309/ajcp7yltqpusdq5c [DOI] [PubMed] [Google Scholar]
- 5.van Doesum JA, Niezink AGH, Huls GA, Beijert M, Diepstra A, van Meerten T. Extranodal Natural Killer/T-cell lymphoma, nasal type: diagnosis and treatment. Hemasphere. 2021;5(2):e523. 10.1097/hs9.0000000000000523. 10.1097/hs9.0000000000000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harabuchi Y, Takahara M, Kishibe K, Nagato T, Kumai T. Extranodal Natural Killer/T-Cell Lymphoma, nasal type: Basic Science and Clinical Progress. Front Pediatr. 2019;7:141. 10.3389/fped.2019.00141. 10.3389/fped.2019.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931–7. 10.1182/blood-2008-10-185256. 10.1182/blood-2008-10-185256 [DOI] [PubMed] [Google Scholar]
- 8.Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. 2017;10(1):85. 10.1186/s13045-017-0452-9. 10.1186/s13045-017-0452-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li QH, Yang P, Wang JJ, Fei D, Tian L, Wan W, et al. Analysis on clinical characteristics and prognosis of 47 patients with Extranodal NK/T Cell Lymphoma. J Experimental Hematol. 2021;29(1):86–90. [DOI] [PubMed] [Google Scholar]
- 10.Wang YC, Wang L, Yin CY et al. Clinicopathologic analysis of NK/T cell lymphoma in 11 children Chinese Journal of Cancer Prevention and Treatment. 2020;27(5):393-7.
- 11.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P, et al. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol. 2013;37(1):14–23. 10.1097/PAS.0b013e31826731b5. 10.1097/PAS.0b013e31826731b5 [DOI] [PubMed] [Google Scholar]
- 13.Quintanilla-Martinez L, Kremer M, Keller G, Nathrath M, Gamboa-Dominguez A, Meneses A, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095–105. 10.1016/s0002-9440(10)63061-1. 10.1016/s0002-9440(10)63061-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKelvie PA, Climent F, Krings G, Hasserjian RP, Abramson JS, Pilch BZ, et al. Small-cell predominant extranodal NK/T cell lymphoma, nasal type: clinicopathological analysis of a series of cases diagnosed in a western population. Histopathology. 2016;69(4):667–79. 10.1111/his.12990. 10.1111/his.12990 [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Ko YH. The pathologic and genetic characteristics of Extranodal NK/T-Cell Lymphoma. Life (Basel). 2022;12(1). 10.3390/life12010073. [DOI] [PMC free article] [PubMed]
- 16.Ng SB, Lai KW, Murugaya S, Lee KM, Loong SL, Fook-Chong S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol. 2004;17(9):1097–107. 10.1038/modpathol.3800157. 10.1038/modpathol.3800157 [DOI] [PubMed] [Google Scholar]
- 17.Schwartz EJ, Molina-Kirsch H, Zhao S, Marinelli RJ, Warnke RA, Natkunam Y. Immunohistochemical characterization of nasal-type extranodal NK/T-cell lymphoma using a tissue microarray: an analysis of 84 cases. Am J Clin Pathol. 2008;130(3):343–51. 10.1309/v561qtm6854w4wav. 10.1309/v561qtm6854w4wav [DOI] [PubMed] [Google Scholar]
- 18.Li YX, Wang H, Feng XL, Liu QF, Wang WH, Lv N, et al. Immunophenotypic characteristics and clinical relevance of CD56 + and CD56- extranodal nasal-type natural killer/T-cell lymphoma. Leuk Lymphoma. 2011;52(3):417–24. 10.3109/10428194.2010.543718. 10.3109/10428194.2010.543718 [DOI] [PubMed] [Google Scholar]
- 19.Tang YL, Zhou Y, Cheng LL, Su YZ, Wang CB. BCL2/Ki-67 index predict survival in germinal center B-cell-like diffuse large B-cell lymphoma. Oncol Lett. 2017;14(3):3767–73. 10.3892/ol.2017.6577. 10.3892/ol.2017.6577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36(4):481–99. 10.1097/PAS.0b013e31824433d8. 10.1097/PAS.0b013e31824433d8 [DOI] [PubMed] [Google Scholar]
- 21.Lipford EH Jr., Margolick JB, Longo DL, Fauci AS, Jaffe ES. Angiocentric immunoproliferative lesions: a clinicopathologic spectrum of post-thymic T-cell proliferations. Blood. 1988;72(5):1674–81. 10.1182/blood.V72.5.1674.1674 [DOI] [PubMed] [Google Scholar]
- 22.Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121(25):4997–5005. 10.1182/blood-2013-01-453233. 10.1182/blood-2013-01-453233 [DOI] [PubMed] [Google Scholar]
- 23.Jiang M, Zhang H, Jiang Y, Yang Q, Xie L, Liu W, et al. Phase 2 trial of sandwich L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012;118(13):3294–301. 10.1002/cncr.26629. 10.1002/cncr.26629 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.