Abstract
Acute appendicitis is a common surgical emergency. It is commonly caused by obstruction of the appendiceal lumen due to fecaliths, tumors, or lymphoid hyperplasia. For over a century, appendectomy has been the primary treatment for acute appendicitis. Abraham Groves performed the first open appendectomy in 1883. In 1983, Kurt Semm completed the first laparoscopic appendectomy, heralding a new era in appendectomy. However, appendectomy is associated with certain complications and a rate of negative appendectomies. Studies have suggested controversy over the impact of appendectomy on the development of inflammatory bowel disease and Parkinson’s disease, but an increasing number of studies indicate a possible positive correlation between appendectomy and colorectal cancer, gallstones, and cardiovascular disease. With the recognition that the appendix is not a vestigial organ and the advancement of endoscopic te-chnology, Liu proposed the endoscopic retrograde appendicitis therapy. It is an effective minimally invasive alternative for treating uncomplicated acute appendicitis. Our team has developed an appendoscope with a disposable digital imaging system operated through the biopsy channel of a colonoscope and successfully applied it in the treatment of appendicitis. This article provides an overview of the progress in endoscopic treatment for acute appendicitis and offers a new perspective on the future direction of appendiceal disease treatment.
Keywords: Acute appendicitis, Endoscopic technology, Endoscopic retrograde appendicitis therapy, Appendoscope, Appendiceal disease treatment
Core Tip: In this article, our team has developed an appendoscope with a disposable digital imaging system operated through the biopsy channel of a colonoscope and successfully applied it in the treatment of appendicitis. It provides an overview of the progress in endoscopic treatment for acute appendicitis and offers a new perspective on the future direction of appendiceal disease treatment.
INTRODUCTION
Acute appendicitis is a common surgical emergency, most frequently occurring in the 10-20 age group, with a male-to-female ratio of 1.4:1.0[1]. It is commonly caused by obstruction of the appendiceal lumen due to fecaliths, tumors, or lymphoid hyperplasia[2]. The diagnosis of acute appendicitis still lacks a gold standard and requires a combination of clinical presentation (Alvarado score)[3] and imaging modalities such as ultrasound or computed tomography (CT)/magnetic resonance imaging (MRI) suggestive of appendicitis[4,5]. Liu[6] defines acute uncomplicated appendicitis as an appendiceal diameter greater than 6mm, excluding appendiceal perforation and appendiceal tumors. For pregnant women, children, and patients planning pregnancy who refuse CT scans, colonoscopy can be combined to confirm appendicitis, with endoscopic findings including mucosal edema and the presence of pus or fecaliths at the appendiceal orifice[7]. Acute appendicitis is classified into acute simple appendicitis, acute suppurative appendicitis, acute gangrenous and perforated appendicitis, and inflammatory masses or periappendiceal abscesses formed by omental wrapping[5]. Clinically, acute simple appendicitis and acute suppurative appendicitis are collectively referred to as acute uncomplicated appendicitis; gangrenous and perforated appendicitis or those with periappendiceal abscesses are collectively referred to as acute complicated appendicitis[8].
Traditional treatments for appendicitis include conservative antibiotic therapy and appendectomy, the latter comprising open appendectomy (OA) and laparoscopic appendectomy (LA). Harris[9] performed the first OA in 1883. In 1983, Semm[10] completed the first LA, heralding a new era in appendectomy. For over a century, appendectomy has been the primary treatment for acute appendicitis. However, appendectomy is associated with a series of complications such as wound infection, incisional hernia, intra-abdominal infection, intestinal obstruction, interstitial pneumonia, urinary tract infection, cardiovascular accidents, etc[11-13]. Excessive surgical treatment can also lead to negative appendectomies, with recent studies reporting rates as high as 8%-30%[14,15]. Studies have suggested controversy over the impact of appendectomy on the development of inflammatory bowel disease and Parkinson’s disease[16,17], but an increasing number of studies indicate a possible positive correlation between appendectomy and colorectal cancer, gallstones, and cardiovascular disease[18-20].
Some scholars have proposed that the appendix is not a vestigial organ but can produce various immunoglobulins. Moreover, due to its unique shape and position, the appendix is considered a reservoir or safe house for intestinal microbiota, playing an important role in regulating the gut flora[21]. With the development of endoscopic minimally invasive technology, Liu et al[22] proposed the endoscopic retrograde appendicitis therapy (ERAT). Our team has developed an appendoscope, which has been successfully applied in clinical practice[23]. Both ERAT and the appendoscope can achieve the goal of treating acute appendicitis while preserving the appendix.
ERAT
Inspired by the clinical application of endoscopic retrograde cholangiopancreatography (ERCP), Liu et al[22] first proposed ERAT in 2012. The procedure is as follows: A colonoscope with a transparent cap at the tip is inserted into the cecum, using the transparent cap to push aside the Gerlach’s valve at the appendiceal orifice, and the Seldinger technique is used for appendiceal cannulation; after successful cannulation, aspiration of the appendiceal lumen is performed to reduce luminal pressure; under X-ray guidance, a contrast agent is injected for appendiceal lumen imaging; appendiceal lumen irrigation and/or lithotripsy are performed; and a stent is placed for drainage. Liu et al[24] conducted a multicenter clinical study in 2015, involving 34 patients with a definitive diagnosis of acute uncomplicated appendicitis, one of whom failed cannulation, resulting in a 97% success rate for ERAT. One patient (3%) underwent appendectomy due to perforation within 48 hours postoperatively, and there were no long-term complications at 12-month follow-up, while two patients (6.2%) underwent appendectomy due to recurrent abdominal pain.
To evaluate the clinical efficacy of ERAT compared to LA in the treatment of acute appendicitis, retrospective studies have been conducted. Yang et al[25] included 422 patients (ERAT 79; LA 343) and found that the cure rate within one year and the pain score 6 hours postoperatively in the ERAT group were significantly higher than in the LA group. Moreover, compared to the LA group, the ERAT group had significantly shorter median operative time and median hospital stay. There was no significant difference between the two groups in terms of median time to recurrence and incidence of adverse events at one year. Ding et al[26] divided 210 patients with acute appendicitis into ERAT, LA, and OA groups. The results showed that the operative time in the ERAT group was significantly shorter than in the LA and OA groups. The postoperative hospital stay, postoperative bed rest time, surgery-related complications, and hospitalization costs were all significantly lower in the ERAT group compared to the latter two groups.
To compare the efficacy of ERAT with antibiotic treatment for acute uncomplicated appendicitis, Li et al[27] conducted a multicenter retrospective study. By comparing treatment success rates, median hospital stays, pain relief rate within 24 hours, and one-year follow-up recurrence rate, the ERAT group outperformed the antibiotic group. This may be because fecaliths, the most common cause of acute appendicitis, can be endoscopically removed during ERAT, and a stent can be placed to relieve luminal pressure. Pata et al[28] conducted a meta-analysis comparing ERAT with appendectomy or antibiotic treatment for acute uncomplicated appendicitis. The results showed no significant differences in technical success rates and one-year follow-up treatment efficacy among the three, but ERAT had advantages in postoperative pain relief and hospital stay duration.
ERAT is an effective minimally invasive alternative for treating uncomplicated acute appendicitis. However, current clinical studies are mostly from China. To further evaluate the safety and efficacy of ERAT, a comprehensive, international, multicenter, randomized controlled prospective study is urgently needed.
NON-X-RAY-ASSISTED ERAT FOR SPECIAL POPULATIONS WITH ACUTE UNCOMPLICATED APPENDICITIS
The ERAT procedure requires X-ray assistance, which carries a certain radiation risk. There have been successful cases of ERAT performed without X-ray assistance. Kang et al[29] used contrast-enhanced ultrasound instead of endoscopic retrograde appendiceal imaging in a prospective, randomized controlled trial to compare the efficacy of modified ERAT (mERAT) with antibiotic treatment for children with acute uncomplicated appendicitis. The results showed that the overall success rate of mERAT treatment (100.0%) was significantly higher than that of antibiotics (80.9%). The median discharge time in the mERAT group was significantly shorter than that in the antibiotic treatment group (6.00 days ± 1.76 days). mERAT provides a new treatment option for children with acute uncomplicated appendicitis. Liu et al[30] reported a case of a pregnant woman at 18 weeks of gestation with acute appendicitis who successfully completed ERAT without anesthesia and X-ray assistance. The patient’s abdominal pain was immediately relieved postoperatively, and the pain was completely relieved the next day. The patient was discharged quickly without antibiotic treatment during hospitalization. Thus, non-X-ray-assisted ERAT is an effective treatment method for special populations such as children or pregnant women with acute appendicitis.
ERAT FOR ACUTE COMPLICATED APPENDICITIS
Although ERAT is not routinely used for treating acute complicated appendicitis, there have been successful clinical case reports to date. Song et al[31] reported a case of a 73-year-old elderly woman diagnosed with periappendiceal and subhepatic abscesses. Due to poor baseline conditions and no surgical indications after multidisciplinary discussions, the patient underwent ERAT with stent placement. Follow-up CT after 2 months showed no abscess, and there was no recurrence after 6 years of follow-up. Li et al[32] reported a case of a 34-year-old woman diagnosed with acute appendicitis complicated by a giant appendiceal abscess and intestinal obstruction. The patient underwent ERAT with stent placement, and the intestinal obstruction was relieved postoperatively. A follow-up CT after 2 months showed complete resolution of the abscess. Cui et al[33] from our team performed ERAT on 9 patients diagnosed with appendiceal abscesses, and the patients had good prognoses postoperatively. Therefore, ERAT is an effective treatment method for periappendiceal abscesses, but more extensive clinical studies are needed to confirm this.
FUNNEL-HOOD-ASSISTED ERAT
Luo et al[34] performed ERAT using an independently developed funnel-shaped hood with a small-diameter tip. A 33-year-old male patient diagnosed with acute appendicitis experienced immediate relief of abdominal pain symptoms after undergoing Funnel-hood-assisted ERAT, and the patient was discharged three days later. Funnel-hood-assisted ERAT is a technological innovation that can reduce the difficulty of cannulating the appendiceal lumen, and it is expected to improve the success rate of ERAT treatment and promote its clinical application.
SPY-GLASS DS ASSISTED ERAT
The SpyGlass DS is a second-generation cholangioscopy system with an outer sheath diameter of 3.3 mm, equipped with one biopsy channel (diameter 1.2 mm), one fiber optic channel (1.0 mm), and two irrigation channels. It allows direct visualization of the biliary tract and is used for the diagnosis of biliary and pancreatic duct strictures, treatment of difficult stones, and radiofrequency ablation of cholangiocarcinoma. The procedure can be completed without the need for X-ray or ultrasound guidance.
Kong et al[35] reported on 14 cases of acute uncomplicated appendicitis treated with SpyGlass DS-assisted ERAT, achieving a 100% success rate. The average operation time was 37.8 minutes ± 22.0 minutes. All patients experienced immediate postoperative relief of abdominal pain. The average postoperative hospital stay was 1.9 days ± 0.7 days. No recurrences were observed during a follow-up period of 2 months to 24 months. Additionally, Kong et al[36] successfully performed SpyGlass DS-assisted ERAT on a patient diagnosed with acute appendicitis at 14 weeks + 2 days of pregnancy without anesthesia, and the patient eventually gave birth at full term. Similarly, Wang et al[37] performed ERAT using a digital single-operator cholangioscopy system in a pregnant woman. The patient’s abdominal pain was significantly relieved postoperatively, and she was discharged without antibiotics. SpyGlass DS-assisted ERAT is a safe and effective alternative for diagnosing and treating acute uncomplicated appendicitis, providing a treatment option for special populations such as pregnant women who need to avoid or refuse X-ray exposure.
APPENDOSCOPIC TREATMENT OF ACUTE APPENDICITIS
Inspired by the SpyGlass DS, our team has successfully developed an appendoscope specifically designed for the diagnosis and treatment of appendiceal diseases, which has passed ethical review and clinical trials and obtained a national patent. The appendoscope is a disposable digital imaging system operated through the biopsy channel of a colonoscope; it is equipped with an LED light source system and has two models with outer sheath diameters of 3.3 mm or 2.6 mm, featuring a biopsy channel (diameter 2.0 mm or 1.2 mm) and two irrigation channels. The distal end of the outer sheath can be adjusted in multiple directions for ease of operation. The appendoscope avoids complications related to X-rays and contrast agents and provides more accurate and intuitive observation under direct endoscopic vision, which is more conducive to the diagnosis and treatment of appendicitis. Compared to the SpyGlass DS, the appendoscope has a more stable imaging system, wider biopsy and irrigation channels, and a price of around 5000 RMB, which is significantly more affordable than the former (approximately 12000 RMB domestically).
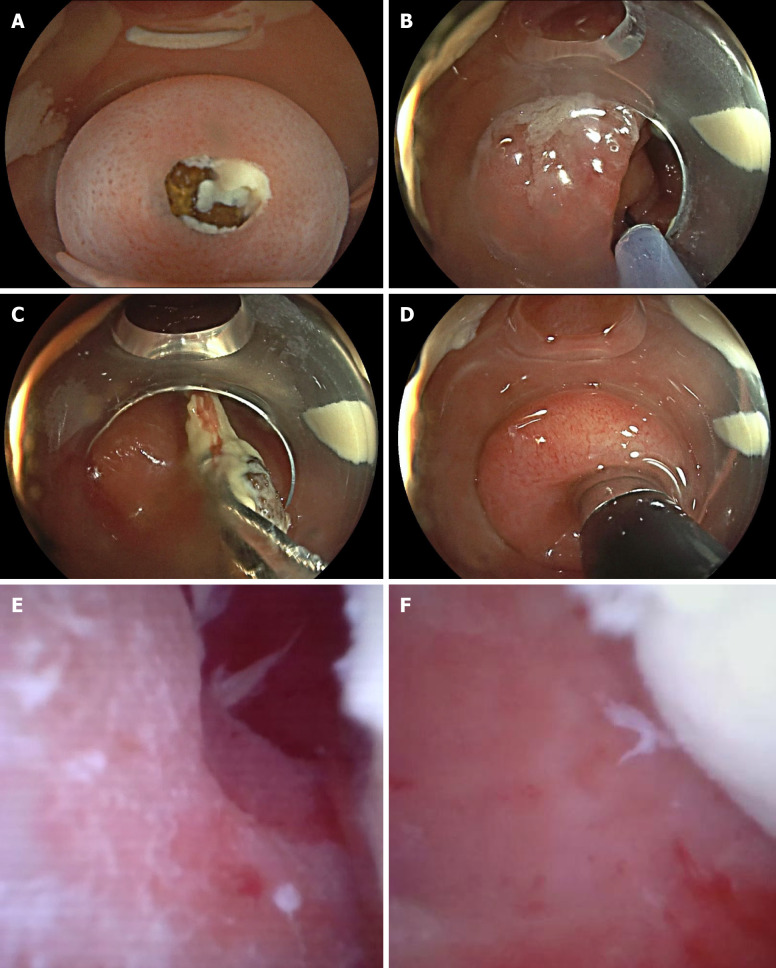
Appendoscope Procedure: Patients undergo bowel preparation 6 hours before the procedure with 3.0 L of polyethylene glycol electrolyte solution or 1.5 L of lactulose solution to cleanse the bowel. For patients with clinical symptoms of nausea and vomiting or unable to cooperate with oral laxatives, five 500 mL saline enemas are administered 30 minutes before the procedure. All patients receive intravenous general anesthesia and are positioned supine. A colonoscope (Olympus 290 or Fuji 7000) with a transparent cap is inserted into the cecum, and the appendoscope is advanced through the biopsy channel of the colonoscope into the appendiceal lumen to observe the interior, perform irrigation, stone retrieval, and other treatments as needed. If a fecalith is seen obstructing the appendiceal orifice, stone retrieval treatment can be performed first (Figure 1).
Figure 1.
Appendoscope treatment. A: Fecalith obstructing the appendiceal orifice; B: A retrieval basket enters the appendiceal lumen; C: Removal of fecalith with the retrieval basket; D: Insertion of the appendoscope into the appendiceal lumen; E: Images of the appendiceal lumen; F: Pus and localized mucosal congestion and edema.
The first clinical application of the appendoscope was reported in June 2022 and published in the top international endoscopy journal, Endoscopy. A 73-year-old female patient was diagnosed with chronic appendicitis. We observed a fecalith obstructing the appendiceal orifice via colonoscopy. After removing the fecalith with a retrieval basket, we inserted the appendoscope to observe the appendiceal lumen, where mucosal congestion and edema were visible, with no residual fecalith. The patient experienced significant relief of abdominal pain postoperatively, with no related complications occurring. She was discharged one week later and has had no recurrence to date[23]. The clinical application of the appendoscope for acute uncomplicated appendicitis has been completed in two cases. Both young patients were admitted with abdominal pain and underwent appendoscopic examination after CT imaging suggested appendicitis. One patient had a fecalith removed, and both were discharged within two days postoperatively without complications and had no recurrence during follow-up (Table 1).
Table 1.
One patient had a fecalith removed, and both were discharged within two days postoperatively without complications and had no recurrence during follow-up
|
Patient ID
|
Gender
|
Age (years)
|
Chief complaint
|
Operation time (minutes)
|
Appendiceal lumen fecalith
|
Abdominal pain relief time (days)
|
Hospital stays
|
Follow-up time (months)
|
Complications
|
| 1 | Female | 17 | Abdominal pain for 3 days | 35 | Yes | 1 | 2 | 13 | None |
| 2 | Male | 24 | Abdominal pain for 2 days | 32 | No | 1 | 1 | 12 | None |
The appendoscope has achieved minimally invasive treatment of appendicitis, shortened hospital stay, and saved medical costs, with favorable patient outcomes. Future large-scale clinical studies are still needed to verify the feasibility, safety, and efficacy of appendoscopic treatment for acute appendicitis, providing clinical experience on how to improve the success rate of treatment for acute appendicitis and reduce the incidence of complications.
CONCLUSION
With the booming development of endoscopic minimally invasive technology, the application of ERAT and related new technologies has provided us with a new perspective on the treatment of acute appendicitis. Based on the use of biliary endoscopy systems from ERCP and considering endoscopic costs, we have developed the appendoscope and successfully applied it in the treatment of appendicitis. The appendoscope has the following advantages: (1) Direct insertion into the appendiceal lumen allows for more precise and intuitive operation under direct vision; (2) Avoidance of X-ray and contrast agent hazards; (3) Short operation time, short hospital stay, and fewer postoperative complications; and (4) Low cost, reducing the economic burden on patients. However, due to the small sample size, the study has certain limitations. We will increase the sample size in the later stage for further validation. The application of the appendoscope is not limited to the diagnosis and treatment of appendicitis. Currently, we are developing specialized biopsy forceps, stents, and other accessories for the appendoscope, which is expected to become an important tool in the diagnosis and treatment of benign and malignant appendiceal diseases.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade B
P-Reviewer: Elpek GO S-Editor: Chen YL L-Editor: A P-Editor: Zheng XM
Contributor Information
Shu-Jiong Feng, Department of Gastroenterology, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Key Laboratory of Integrated Traditional Chinese and Western Medicine for Biliary and Pancreatic Diseases of Zhejiang Province, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Hangzhou Institute of Digestive Diseases, Hangzhou 310000, Zhejiang Province, China.
Yi-Feng Zhou, Department of Gastroenterology, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Key Laboratory of Integrated Traditional Chinese and Western Medicine for Biliary and Pancreatic Diseases of Zhejiang Province, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Hangzhou Institute of Digestive Diseases, Hangzhou 310000, Zhejiang Province, China.
Jian-Feng Yang, Department of Gastroenterology, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Key Laboratory of Integrated Traditional Chinese and Western Medicine for Biliary and Pancreatic Diseases of Zhejiang Province, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Hangzhou Institute of Digestive Diseases, Hangzhou 310000, Zhejiang Province, China.
Hong-Zhang Shen, Department of Gastroenterology, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Key Laboratory of Integrated Traditional Chinese and Western Medicine for Biliary and Pancreatic Diseases of Zhejiang Province, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Hangzhou Institute of Digestive Diseases, Hangzhou 310000, Zhejiang Province, China.
Guang-Xing Cui, Department of Gastroenterology, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Key Laboratory of Integrated Traditional Chinese and Western Medicine for Biliary and Pancreatic Diseases of Zhejiang Province, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Hangzhou Institute of Digestive Diseases, Hangzhou 310000, Zhejiang Province, China.
Xiao-Feng Zhang, Department of Gastroenterology, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Key Laboratory of Integrated Traditional Chinese and Western Medicine for Biliary and Pancreatic Diseases of Zhejiang Province, Hangzhou 310000, Zhejiang Province, China; Department of Gastroenterology, Hangzhou Institute of Digestive Diseases, Hangzhou 310000, Zhejiang Province, China. zxf837@tom.com.
References
- 1.Humes DJ, Simpson J. Acute appendicitis. BMJ. 2006;333:530–534. doi: 10.1136/bmj.38940.664363.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krzyzak M, Mulrooney SM. Acute Appendicitis Review: Background, Epidemiology, Diagnosis, and Treatment. Cureus. 2020;12:e8562. doi: 10.7759/cureus.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohle R, O’Reilly F, O’Brien KK, Fahey T, Dimitrov BD. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139. doi: 10.1186/1741-7015-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng KA, Abadeh A, Ligocki C, Lee YK, Moineddin R, Adams-Webber T, Schuh S, Doria AS. Acute Appendicitis: A Meta-Analysis of the Diagnostic Accuracy of US, CT, and MRI as Second-Line Imaging Tests after an Initial US. Radiology. 2018;288:717–727. doi: 10.1148/radiol.2018180318. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann JC, Trimborn CP, Hoffmann M, Schröder R, Förster S, Dirks K, Tannapfel A, Anthuber M, Hollerweger A. Classification of acute appendicitis (CAA): treatment directed new classification based on imaging (ultrasound, computed tomography) and pathology. Int J Colorectal Dis. 2021;36:2347–2360. doi: 10.1007/s00384-021-03940-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu BR. Top tips for endoscopic retrograde appendicitis therapy (with video) Gastrointest Endosc. 2024;99:625–628. doi: 10.1016/j.gie.2023.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Mi C, Li W, She J. Diagnosis of Acute Appendicitis by Endoscopic Retrograde Appendicitis Therapy (ERAT): Combination of Colonoscopy and Endoscopic Retrograde Appendicography. Dig Dis Sci. 2016;61:3285–3291. doi: 10.1007/s10620-016-4245-8. [DOI] [PubMed] [Google Scholar]
- 8.Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT. Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet. 2015;386:1278–1287. doi: 10.1016/S0140-6736(15)00275-5. [DOI] [PubMed] [Google Scholar]
- 9.Harris CW. Abraham GROVES of Fergus: the first elective appendectomy? Can J Surg. 1961;4:405–410. [PubMed] [Google Scholar]
- 10.Semm K. Endoscopic appendectomy. Endoscopy. 1983;15:59–64. doi: 10.1055/s-2007-1021466. [DOI] [PubMed] [Google Scholar]
- 11.Kotaluoto S, Pauniaho SL, Helminen MT, Sand JA, Rantanen TK. Severe Complications of Laparoscopic and Conventional Appendectomy Reported to the Finnish Patient Insurance Centre. World J Surg. 2016;40:277–283. doi: 10.1007/s00268-015-3282-3. [DOI] [PubMed] [Google Scholar]
- 12.Margenthaler JA, Longo WE, Virgo KS, Johnson FE, Oprian CA, Henderson WG, Daley J, Khuri SF. Risk factors for adverse outcomes after the surgical treatment of appendicitis in adults. Ann Surg. 2003;238:59–66. doi: 10.1097/01.SLA.0000074961.50020.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tingstedt B, Johansson J, Nehez L, Andersson R. Late abdominal complaints after appendectomy--readmissions during long-term follow-up. Dig Surg. 2004;21:23–27. doi: 10.1159/000075378. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen SR, Christophersen C, Rosenberg J, Fonnes S. Varying negative appendectomy rates after laparoscopic appendectomy: a systematic review and meta-analysis. Langenbecks Arch Surg. 2023;408:205. doi: 10.1007/s00423-023-02935-z. [DOI] [PubMed] [Google Scholar]
- 15.Chaochankit W, Boocha A, Samphao S. Negative appendectomy rate in patients diagnosed with acute appendicitis. BMC Surg. 2022;22:404. doi: 10.1186/s12893-022-01852-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu HT, Shen QY, Xie D, Zhao QZ, Xu YM. Lack of association between appendectomy and Parkinson’s disease: a systematic review and meta-analysis. Aging Clin Exp Res. 2020;32:2201–2209. doi: 10.1007/s40520-019-01354-9. [DOI] [PubMed] [Google Scholar]
- 17.Song MY, Ullah S, Yang HY, Ahmed MR, Saleh AA, Liu BR. Long-term effects of appendectomy in humans: is it the optimal management of appendicitis? Expert Rev Gastroenterol Hepatol. 2021;15:657–664. doi: 10.1080/17474124.2021.1868298. [DOI] [PubMed] [Google Scholar]
- 18.Meng W, Cai SR, Zhou L, Dong Q, Zheng S, Zhang SZ. Performance value of high risk factors in colorectal cancer screening in China. World J Gastroenterol. 2009;15:6111–6116. doi: 10.3748/wjg.15.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Ma X, Zhu C, Fang JY. Risk of colorectal cancer after appendectomy: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2023;38:350–358. doi: 10.1111/jgh.16108. [DOI] [PubMed] [Google Scholar]
- 20.Wu SC, Chen WT, Muo CH, Ke TW, Fang CW, Sung FC. Association between appendectomy and subsequent colorectal cancer development: an Asian population study. PLoS One. 2015;10:e0118411. doi: 10.1371/journal.pone.0118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kooij IA, Sahami S, Meijer SL, Buskens CJ, Te Velde AA. The immunology of the vermiform appendix: a review of the literature. Clin Exp Immunol. 2016;186:1–9. doi: 10.1111/cei.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu BR, Song JT, Han FY, Li H, Yin JB. Endoscopic retrograde appendicitis therapy: a pilot minimally invasive technique (with videos) Gastrointest Endosc. 2012;76:862–866. doi: 10.1016/j.gie.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Feng S, Ling K, Zhang T, Zhang X, Yan X, Yang J, Zhou Y. Application of an appendoscope in chronic appendicitis. Endoscopy. 2022;54:E296–E297. doi: 10.1055/a-1519-6903. [DOI] [PubMed] [Google Scholar]
- 24.Liu BR, Ma X, Feng J, Yang Z, Qu B, Feng ZT, Ma SR, Yin JB, Sun R, Guo LL, Liu WG. Endoscopic retrograde appendicitis therapy (ERAT) : a multicenter retrospective study in China. Surg Endosc. 2015;29:905–909. doi: 10.1007/s00464-014-3750-0. [DOI] [PubMed] [Google Scholar]
- 25.Yang B, Kong L, Ullah S, Zhao L, Liu D, Li D, Shi X, Jia X, Dalal P, Liu B. Endoscopic retrograde appendicitis therapy versus laparoscopic appendectomy for uncomplicated acute appendicitis. Endoscopy. 2022;54:747–754. doi: 10.1055/a-1737-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding W, Du Z, Zhou X. Endoscopic retrograde appendicitis therapy for management of acute appendicitis. Surg Endosc. 2022;36:2480–2487. doi: 10.1007/s00464-021-08533-8. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Yang B, Liao J, Li Y, Liu D, Zhao L, Meng X, Hu H, Kong L, Podda M, Ullah S, Liu B. Endoscopic retrograde appendicitis therapy or antibiotics for uncomplicated appendicitis. Br J Surg. 2023;110:635–637. doi: 10.1093/bjs/znad023. [DOI] [PubMed] [Google Scholar]
- 28.Pata F, Nardo B, Ielpo B, Di Martino M, Murzi V, Di Saverio S, Yang B, Ortenzi M, Pisanu A, Pellino G, Podda M. Endoscopic retrograde appendicitis therapy versus appendectomy or antibiotics in the modern approach to uncomplicated acute appendicitis: A systematic review and meta-analysis. Surgery. 2023;174:1292–1301. doi: 10.1016/j.surg.2023.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Kang J, Zhang W, Zeng L, Lin Y, Wu J, Zhang N, Xie X, Zhang Y, Liu X, Wang B, Yang R, Jiang X. The modified endoscopic retrograde appendicitis therapy versus antibiotic therapy alone for acute uncomplicated appendicitis in children. Surg Endosc. 2021;35:6291–6299. doi: 10.1007/s00464-020-08129-8. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Jiang K, Bi Y. Endoscopic retrograde appendicitis therapy in a pregnant patient with acute septic appendicitis. Asian J Surg. 2022;45:2070–2071. doi: 10.1016/j.asjsur.2022.04.098. [DOI] [PubMed] [Google Scholar]
- 31.Song M, Ullah S, Liu B. Endoscopic Retrograde Appendicitis Therapy for Treating Periappendiceal Abscess: First Human Case Report. Am J Gastroenterol. 2021;116:1119. doi: 10.14309/ajg.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Liu T, Li A, Liu J, Jiang B, Yang B. Endoscopic retrograde appendicitis therapy for giant periappendiceal abscess with intestinal obstruction. Endoscopy. 2023;55:E1116–E1117. doi: 10.1055/a-2173-7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui G, Lv W, Wang J, Zhang X. Endoscopic retrograde appendicitis therapy: a novel approach for peri-appendiceal abscess. Endoscopy. 2022;54:E186–E187. doi: 10.1055/a-1471-2871. [DOI] [PubMed] [Google Scholar]
- 34.Luo Q, Zeng S, Jiang M, Zhang Q, Cheng C. Funnel-hood-assisted endoscopic retrograde appendicitis therapy for acute appendicitis. Endoscopy. 2024;56:E142–E143. doi: 10.1055/a-2241-8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong LJ, Liu D, Zhang JY, Ullah S, Zhao L, Li D, Yang H, Liu BR. Digital single-operator cholangioscope for endoscopic retrograde appendicitis therapy. Endoscopy. 2022;54:396–400. doi: 10.1055/a-1490-0434. [DOI] [PubMed] [Google Scholar]
- 36.Kong L, Zhang H, Liu K, Zhao L, Lu BR. How to manage appendicitis in pregnancy better. Gastrointest Endosc. 2022;95:1018–1019. doi: 10.1016/j.gie.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Wang F, Zhou XR, Zhang Y, Wang Y, Du ZQ, Liu WH. Digital single-operator cholangioscopy-guided appendiceal intubation for endoscopic retrograde appendicitis therapy in a pregnant woman (with video) Gastrointest Endosc. 2023;98:1034–1035. doi: 10.1016/j.gie.2023.06.066. [DOI] [PubMed] [Google Scholar]