Abstract
A human recombinant monoclonal antibody to herpes simplex virus (HSV) glycoprotein D labeled with the fluorescent dye Cy5 was administered to mice infected in the cornea with HSV type 1 (HSV-1). The distribution of such antibody in the corneas and trigeminal ganglia of the mice was then investigated by confocal microscopy. The antibody was detected on HSV-infected nerve fibers in the cornea—identified by colocalization with HSV antigens and the neuritic markers neurofilament, GAP-43, synapsin-1, and CNPase—and on the perikarya of sensory neurons in the HSV-1-infected neurons in ipsilateral trigeminal ganglia. Antibodies have been shown to be effective against many neurotropic viruses, often in the absence of obvious cell damage. Observations from experimental HSV infections suggest that antibodies could act in part by interfering with virus expression in the ganglia and/or with axonal spread. The present results provide morphological evidence of the localization of antiviral antibodies at anatomical sites relevant to such putative antibody-mediated protective actions and suggest that viral glycoproteins are accessible to antibodies on infected nerve fibers and sensory neurons.
The herpes simplex viruses (HSVs) are transmitted by contact with infected skin, mucous membranes, and secretions (44). Following mucosal or cutaneous primary infections, they spread axonally to the host dorsal root ganglia (DRG), where they establish latent infections and undergo periodic reactivations (38). Upon reactivation, HSV is transported axonally centrifugally to the originally infected or adjacent dermatomes, resulting in either recurrent clinical lesions or asymptomatic viral shedding (42, 44). The viral and host factors that control the establishment and the maintenance of HSV latency and the eventual recurrences are still only partially understood (33). The role of cellular immunity in HSV infection is unquestionable, as is the role of local cytokine responses (22, 24, 30, 37). However, several observations also suggest that antibodies could interfere with HSV expression and possibly with axonal spread in vivo. These include evidence both from experimental infections and in vitro studies. In fact, passive immunization with either murine or human monoclonals can effect protection or delay clinical progression in the mouse after the virus is already in the peripheral nervous system (6, 17, 35), and specific antibodies reduce HSV yields in infected cells in vitro (25). Lastly, it was recently shown that certain antibodies, including the one used for this study, can interfere with the axonal spread of HSV type 1 (HSV-1) in vitro in a model in which axons from explanted sensory ganglia are allowed to grow through an agarose diffusion barrier and innervate skin explants cultured in a separate chamber (21).
In the present study, we sought to investigate the anatomical basis for putative antibody-mediated nonlytic antiherpetic activities which could limit virus expression and spread in vivo. To this end, we investigated whether a parenterally administered antibody could interact with HSV-infected nerve fibers and neurons. The human recombinant antibody used in this study, termed HSV8, is a group Ib human monoclonal immunoglobulin G1 to glycoprotein D (gD) (5). This antibody was highly protective both systemically in the flank and corneal models of HSV infection and topically in the vaginal model (35, 46). In systemic passive immunization, it was effective even when administered 24 h postinfection, a time when the virus is already in the peripheral nervous system (35). The cornea was selected for the study because experimental corneal infection of the mouse is relevant to human eye infections, which can lead to herpetic stromal keratitis (HSK). HSK has an incidence of approximately 300,000 cases per year and is second only to trauma as a cause of corneal blindness (39, 44). Furthermore, passive immunization with monoclonal antibodies has proven effective in animal models of HSK, suggesting that antibody-mediated activities may affect this herpetic manifestation (20, 31, 40). Lastly, the cornea is highly innervated and nerve fibers in the cornea are easily visualized by laser scanning confocal microscopy (LSCM) in whole-mount preparations.
HSV8, the human recombinant monoclonal antibody used for this study, was expressed in CHO cells and affinity purified in accordance with standard techniques as previously reported (4, 35). Cy5 labeling of HSV8 was carried out with a kit from Amersham (Pittsburgh, Pa.) in accordance with the manufacturer’s recommendations. Antibody labeled in this fashion was effective in labeling HSV-infected Vero cells in direct immunofluorescence (not shown). HSV-1 (F), the kind gift of Bernard Roizman (University of Chicago), was used to infect homozygous athymic nude mice with a BALB/c background and aged 5 to 8 weeks. The central cornea of mice deeply anesthetized with metofane was gently scarified with a 23-gauge needle 10 times in parallel horizontal lines and 10 more times perpendicularly. Virus was then applied in a 2-μl drop of tissue culture medium containing approximately 105 PFU. Four to 5 days postinfection, animals were injected intraperitoneally with approximately 200 μg of Cy5-labeled antibody in sterile saline. The following day, mice were sacrificed by cervical dislocation following metofane anesthesia. The following controls were also included: uninfected mice injected with the Cy5-labeled antibody; infected mice injected with a Cy5-labeled aspecific rabbit serum; infected mice injected with the Cy5-labeled antibody in the presence of a 30-fold molar excess of unlabeled human Fc. Four to six animals per group were employed. Infected cornea and ipsilateral trigeminal ganglia were dissected and placed in 4% paraformaldehyde for 4 h. Corneas were then either washed in 0.1 M phosphate-buffered saline (PBS) and observed microscopically without further treatment as whole mounts or cryoprotected by incubation in 16% sucrose overnight. The trigeminal ganglia were also cryoprotected. Cryoprotected samples were cryostat sectioned (35 μM) and thaw mounted on Superfrost/plus slides (Fisher Scientific, Pittsburgh, Pa.). Corneas were sectioned coronally. Sections were then immunoreacted with a battery of antibodies, including a rabbit polyclonal to HSV-1 (Dako, Carpinteria, Calif.) used at a 1:100 dilution; a cocktail of mouse monoclonal antibodies against HSV gD (monoclonal antibody 1103) and glycoprotein B (gB) (monoclonal antibodies 1105 and 1123) from Goodwin Scientific (Plantation, Fla.) at 0.3 μg/ml each; and mouse monoclonals to neurofilament 68k (Sigma, St. Louis, Mo.), synaptophysin (Boehringer Mannheim Biochemicals, Indianapolis, Ind.), Gap43 (Sigma), and CNPase (Boehringer Mannheim), used at 1 μg/ml. Minimal cross-reactivity fluorescein isothiocyanate (FITC) or lissamine rhodamine secondary antibodies (Jackson Laboratories, West Grove, Pa.) were used for detection. Primary antibodies were incubated at 4°C overnight in PBS containing 0.3% Triton X-100 (Fisher Scientific) and 1 mg of bovine serum albumin (BSA) (Sigma)/ml; secondary antibodies were used at 1:100 in PBS–Triton X-100–BSA for 1 h at room temperature. Preparations were mounted with Fluoroguard antifade reagent (Bio-Rad, Hercules, Calif.), coverslipped, and sealed with clear nail polish. LSCM was carried out with a Bio-Rad MRC-1024 LSCM as previously described (36). Color in the figures was computer generated.
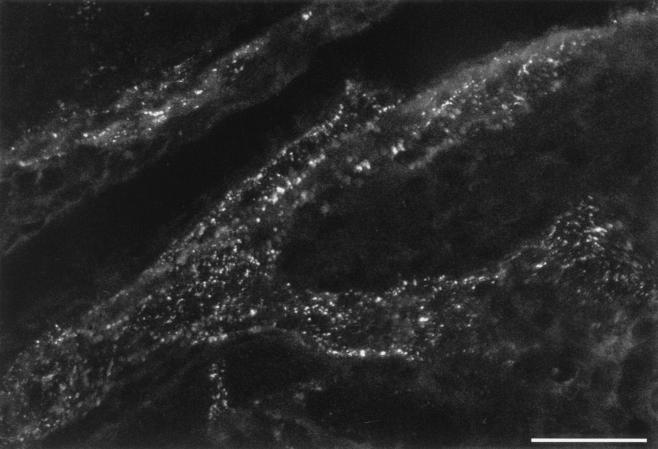
Mice infected with HSV-1 by scarification of the central cornea were injected with a Cy5-labeled human recombinant monoclonal antibody 4 to 5 days postinfection, when the virus was spreading centrifugally back to the cornea following replication in the ganglia. Twenty-four hours later, the animals were sacrificed and their corneas were initially examined by LSCM as whole mounts. In these preparations Cy5 labeled structures reminiscent of bundles of corneal nerve fibers (see reference 15 and discussion below), suggesting that the antibody was localized to HSV-infected corneal nerve fibers (Fig. 1). These fibers could be observed projecting centripetally from the limbal region of the cornea towards the central cornea. In the limbus, fiber bundles could be observed in the vicinity of vessels of the limbal microvascular system but without colocalization with them (Fig. 2). Similar results were obtained in mice injected with the same Cy5-labeled human recombinant monoclonal antibody in the presence of 30-fold excess of unlabeled human Fc (not shown) but not in uninfected animals injected with the Cy5-labeled human monoclonal antibody (Fig. 2C and D) nor in HSV-infected animals injected with a Cy5-labeled aspecific rabbit antiserum (not shown).
FIG. 1.
LSCM of a whole-mount cornea preparation from a mouse ocularly infected with HSV-1 and injected 4 days later with a Cy5-labeled human recombinant monoclonal antibody. A punctate Cy5 labeling is evident and was interpreted as HSV-infected nerve fibers projecting centripetally towards the central cornea on the basis of corneal nerve fiber morphology (15) and double-labeling experiments (see Fig. 3 and 4). Scale bar = 40 μm.
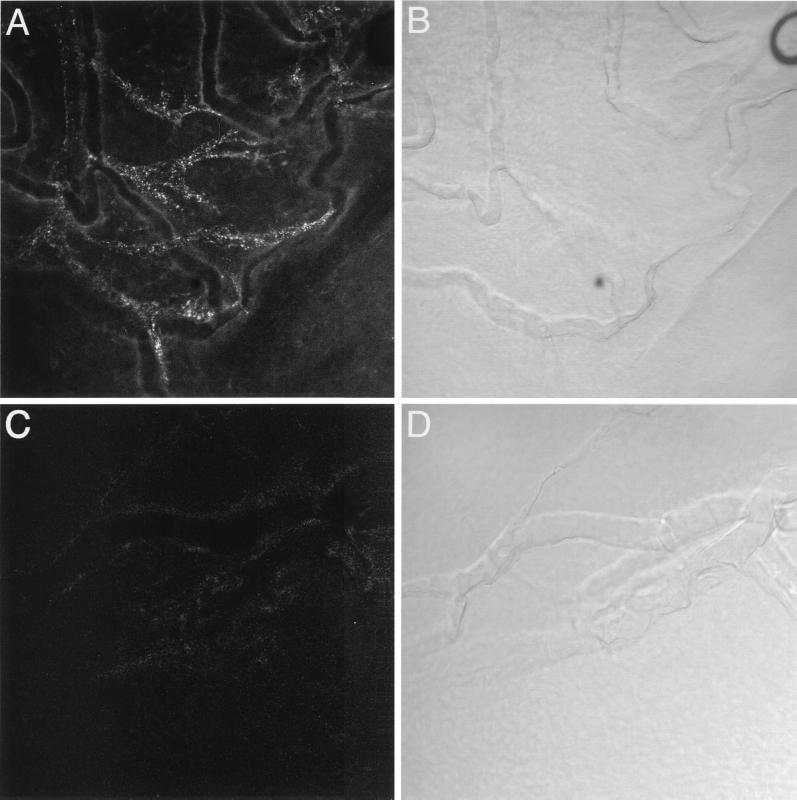
FIG. 2.
LSCM of whole-mount cornea preparations from a mouse ocularly infected with HSV-1 and injected with a Cy5-labeled human recombinant monoclonal antibody (A) and an uninfected control (C). Bright-field images of the same fields are displayed in panels B and D, respectively. In the limbus, Cy5-labeled fiber bundles (A) could be observed in the vicinity of vessels of the limbal microvascular system (B) but without colocalizing with them. No Cy5-labeled fibers were seen in uninfected animals injected with the Cy5-labeled human monoclonal antibody (C). Scale bar = 40 μm.
To further characterize the nature of the Cy5-labeled structures, we carried out immunohistochemical labeling for HSV antigens and with antibodies to neuritic markers. Corneal cross sections were employed since whole-mount preparations proved unsuitable because of lack of penetration of the antibodies used for immunohistochemistry in the excised fixed cornea. Cross sections of corneas from HSV-1-infected mice injected with the Cy5-labeled human monoclonal antibody revealed dotted Cy5 labeling. The signal was predominantly but not exclusively located in the subepithelial plexus, consistent with impressions from optical sectioning of whole-mount preparations by LSCM. Similar results could be obtained by immunoreacting cross sections of corneas from animals injected with the human monoclonal antibody with a FITC-labeled anti-human secondary antibody (not shown). Double labeling experiments using cross sections of infected corneas revealed that Cy5 labeling colocalized with immunoreactivity for HSV gD and gB, as revealed by a cocktail of murine monoclonal antibodies (Fig. 3). Cy5 labeling also colocalized with the axonal markers neurofilament, Gap43, and synaptophysin, as well as the Schwann cell marker CNPase (Fig. 4). These observations suggest that the passively transferred human monoclonal antibody localized to HSV-infected corneal nerve fibers.
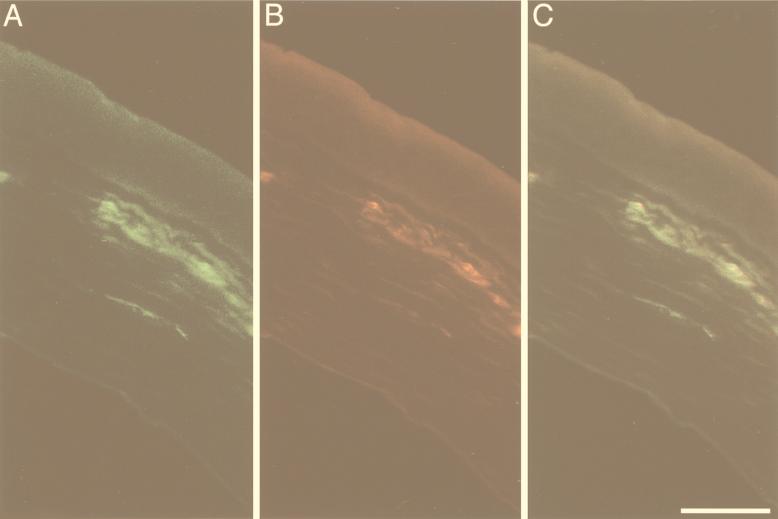
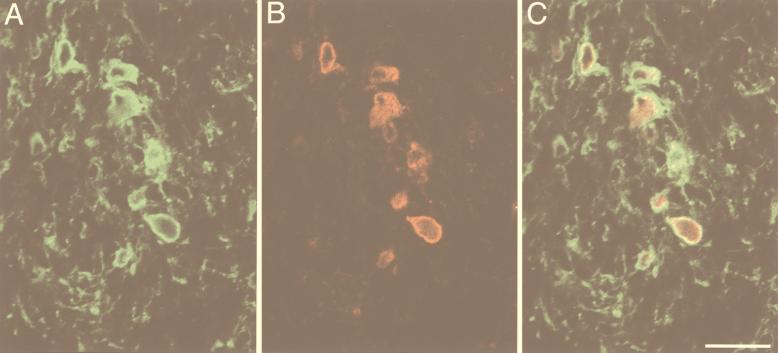
FIG. 3.
Double labeling of HSV antigens in a cornea cross section from an HSV-infected mouse injected with a Cy5-labeled human recombinant monoclonal antibody, LSCM (pseudocolored digital image). (A) Immunofluorescence for HSV gD and gB appeared to be mostly but not exclusively located in the subepithelial plexus. (B) Punctate Cy5 labeling colocalized with immunoreactivity for the HSV glycoproteins. (C) Superimposition of the images in panels A and B. Scale bar = 40 μm.
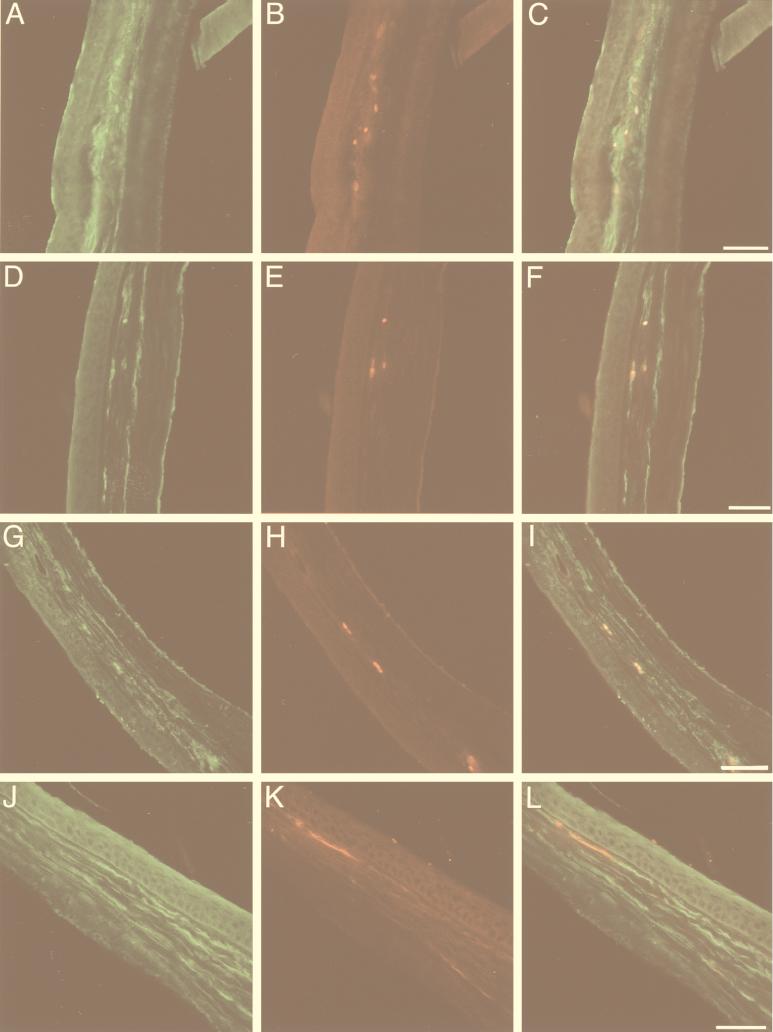
FIG. 4.
Double labeling of neuritic antigens in cornea cross sections from an HSV-infected mouse injected with a Cy5-labeled human recombinant monoclonal antibody, as seen by LSCM (pseudocolored digital image). Immunoreactivity for the axonal markers neurofilament (A), Gap43 (D), and synaptophysin (G) and for the Schwann cell marker CNPase (J) is shown. Cy5 labeling (B, E, H, K) appears to colocalize with the neural markers (C, F, I, L), suggesting that the passively transferred human monoclonal antibody localizes to corneal nerve fibers. Scale bar = 40 μm.
In the trigeminal ganglia ipsilateral to the HSV-1-infected corneas, variable numbers of Cy5-labeled cell bodies could be observed in the rostro-medial (ophthalmic) region of the ganglion (Fig. 5). They were arrayed predominantly in rostro-caudal columnary collections as expected from anatomical studies on the distribution of corneal sensory afferent neurons (15, 16). The majority of these somata were of small diameter (15 to 20 μm) and round or roughly polygonal, consistent with the size and morphology of sensory neurons innervating the cornea (15, 16). Double labeling with a rabbit polyclonal antibody to HSV-1 showed a strict colocalization between the Cy5 signal and the HSV-1 antigens, supporting the identification of these cells as HSV-1-infected neurons (Fig. 5).
FIG. 5.
Double labeling of HSV antigens in a trigeminal ganglion from an HSV-infected mouse injected with a Cy5-labeled human recombinant monoclonal antibody, LSCM (pseudocolored digital image). Neurons stained with an antiserum to HSV can be seen (A) which also display Cy5 labeling (B). (C) Superimposition of the images in panels A and B. The size and appearance of such neurons is consistent with their identification as cornea-innervating sensory neurons (15, 16). Scale bar = 40 μm.
The cornea is very densely innervated by sensory fibers from the trigeminal ganglion. Nerve bundles containing both myelinated and unmyelinated fibers penetrate into the connective stroma around the cornea circumference (15, 34). A few millimeters inside the cornea, these fibers give off collaterals that form a subepithelial plexus (34). The subepithelial plexus is formed of small diameter “preterminal” sensory axons which are mostly unmyelinated (34). These are usually separated from the extracellular environment only by the accompanying Schwann cells and their basal lamina and lack perineural sheaths (34). Neural processes devoid of Schwann cells penetrate from the subepithelial plexus into the epithelium as free nerve endings (34). In this report, we showed that a human recombinant monoclonal antibody to HSV envelope gD, when passively transferred to HSV-infected animals, localized to cornea nerve fibers (Fig. 1 and 2). Cross sections of the cornea suggested a predominant localization in the subepithelial plexus, where, as mentioned, anatomical isolation of nerve fibers from the surrounding extracellular space is minimal (34). Such a localization of the exogenous antibody to HSV-infected nerve fibers was confirmed by double labeling with both nerve and viral antigens (Fig. 3 and 4).
Deposition of the human antibody on neurons in the ipsilateral trigeminal ganglia was also seen. Double labeling with antibodies to HSV-1 showed a high degree of colocalization between the human antibody and viral antigens. Sensory neurons innervating the cornea—which are functionally and anatomically equivalent to sensory neurons of dorsal root ganglia—are located in the ophthalmic region of the trigeminal or Gasseri ganglion (15, 16). They are arrayed in a roughly cranio-caudal manner and are virtually imbedded in the medial part of the ophthalmic-maxillary branch of the ganglion, which in the rodent exits the ganglion as one (15, 16). The size, morphology, and location of the somata labeled by the passively transferred antibody in trigeminal ganglia as well as colocalization with HSV-1 immunoreactivity were consistent with their identification as HSV-1-infected sensory neurons innervating the cornea. Although labeling of ganglionic neurons for the human recombinant monoclonal antibody was usually peripheral, in a few neurons it appeared to extend to the cytoplasm (not shown). These cells are likely to be neurons that died in the course of ganglionic viral replication (33), although the possibility that, to some extent, the antibody could be taken up by infected neurons, cannot be ruled out.
Evidence has been accumulating that antibody-mediated protective mechanisms are important against several neurotropic viruses, including enteroviruses, rabies, reoviruses, and alphaviruses, to name a few (2, 3, 9, 13, 14, 27, 29, 41, 45). Protection often does not correlate with in vitro neutralizing activity. In some cases, antibodies appear to limit virus spread to the central nervous system (CNS). Tyler and colleagues (41), for instance, showed that specific monoclonal antibodies could protect the CNS not only from reoviruses that spread through the bloodstream but also from reoviruses that spread transneuronally. The natural resistance of certain mouse strains to street rabies, which also spreads transneuronally, also appears to depend on serum antibodies (29). In other cases, specific antibodies seem to reduce or abolish virus expression in infected neurons. Evidence that antibodies can affect virus expression in in vitro paradigms has been presented for neurotropic and nonneurotropic viruses, including measles (8), vesicular stomatitis virus (26), and Friend leukemia virus (11). Passive immunization of nude mice infected intracerebrally with Theiler’s murine encephalomyelitis virus results in reduced virus yields in the brain and variable degrees of recovery from the demyelinating lesions (3). Similarly, monoclonal antibodies protect newborn Lewis rats from lethal measles encephalitis by attenuating viral gene expression (14). Lastly, the expression of Sindbis virus in the CNS of SCID mice could be virtually abolished by passive immunization, in a manner clearly independent of cellular immunity or complement and in the absence of detectable cell damage (13).
In the case of HSV, passive immunization protects experimental animals from HSV encephalitis (see for example references 1, 6, 7, 12, 18, 35). Administration of specific antibodies was shown to reduce the number of infected ganglionic sensory neurons following viral challenge (17, 18, 43). Interestingly, antibodies can be protective even if administered 24 to 48 h after HSV infection when the virus is already in the peripheral nervous system (6, 17, 35). In addition, certain monoclonal antibodies are able to reduce virus expression in neuronal cells in vitro (25). In one in vivo study, Mester et al. (18) found that 50% of the mice treated with anti-gC mouse monoclonal antibodies harbored reactivatable HSV in their ganglia, while none of the animals treated with anti-gD antibodies did. These results allowed the authors to suggest that such anti-gD antibodies conferred protection by limiting virus spread to the ganglia, while the anti-gC antibodies could have acted primarily by mechanisms leading to decreased virus expression (18). Taken together, these studies support the hypothesis that both interference with axonal spread and restriction of virus expression in sensory neurons could contribute to antibody-mediated protection.
The mode of HSV axonal transport remains to be fully elucidated. Electron microscopic studies revealed nude HSV capsids being transported centrifugally from the ganglia to the periphery, both in axons of the cornea (19, 32) and in other peripheral nerves (our unpublished data). Similar observations were made in an in vitro preparation (28). The origin of such unenveloped virions in nerve fibers remains to be determined. Current knowledge of the role of HSV surface glycoproteins in attachment and penetration (10, 23) suggests, however, that infectious virus released by nerve terminals is likely to be enveloped and suggests that the unenveloped capsids seen in axons could acquire an envelope budding from axonal and/or terminal membranes. Antibodies could interfere with virus spread by patching glycoproteins, neutralizing egressing virus, activating antibody-dependent immune effectors, or other mechanisms. The present study does not directly address the mode of axonal transport of HSV and its envelope glycoproteins; however, our results support the notion that glycoproteins are accessible to antibodies on the surface or—less likely—within infected nerve fibers as well as on infected ganglionic sensory neurons.
In the experiments reported here, we have shown that a recombinant human antibody administered to HSV-1-infected animals strongly localized to HSV-infected nerve fibers and sensory neurons. Such localizing ability could be at the basis of antibodies’ putative ability to interfere with HSV axonal transport and expression in vivo, as suggested by passive immunization experiments in the mouse model (6, 17, 18, 35). Electron microscopic studies are needed to clarify the ultrastructural aspects of such interactions.
Acknowledgments
We thank J. Lindsay Whitton and Floyd E. Bloom of TSRI for critical discussions and reviews of the manuscript. We are also especially thankful to Floyd E. Bloom for support and encouragement.
This work was partially supported by PHS grant AI37582 (to P.P.S.) and by a young investigator award (to P.P.S.) from the National Alliance for Research on Schizophrenia and Depression (NARSAD). Some of the work was conducted at the National Center for Microscopy and Imaging Research, which is supported by PHS grant RR04050 (to M.H.E.).
REFERENCES
- 1.Alexander T S, Rosenthal K S. Autologous antibody is protective against HSV-1 infection of the immunocompromised mouse. J Lab Clin Med. 1990;116:400–407. [PubMed] [Google Scholar]
- 2.Baldridge J R, Pearce B D, Parekh B S, Buchmeier M J. Teratogenic effects of neonatal arenavirus infection on the developing rat cerebellum are abrogated by passive immunotherapy. Virology. 1993;197:669–677. doi: 10.1006/viro.1993.1642. [DOI] [PubMed] [Google Scholar]
- 3.Buchmeier M J, Lewicki H A, Talbot P J, Knobler R L. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology. 1984;132:261–270. doi: 10.1016/0042-6822(84)90033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burioni R, Williamson R A, Sanna P P, Bloom F E, Burton D R. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc Natl Acad Sci USA. 1994;91:355–359. doi: 10.1073/pnas.91.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Logu A, Williamson R A, Rozenshteyn R, Ramiro-Ibanez F, Simpson C D, Burton D R, Sanna P P. Characterization of a type-common human recombinant monoclonal antibody to herpes simplex virus with high therapeutic potential. J Clin Microbiol. 1998;36:3198–3204. doi: 10.1128/jcm.36.11.3198-3204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dix R D, Pereira L, Baringer J R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981;34:192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eis Hubinger A, Schmidt D S, Schneweis K E. Anti-glycoprotein B monoclonal antibody protects T cell-depleted mice against herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membranes. J Gen Virol. 1993;74:379–385. doi: 10.1099/0022-1317-74-3-379. [DOI] [PubMed] [Google Scholar]
- 8.Fujinami R S, Norrby E, Oldstone M B. Antigenic modulation induced by monoclonal antibodies: antibodies to measles virus hemagglutinin alters expression of other viral polypeptides in infected cells. J Immunol. 1984;132:2618–2621. [PubMed] [Google Scholar]
- 9.Fujinami R S, Rosenthal A, Lampert P W, Zurbriggen A, Yamada M. Survival of athymic (nu/nu) mice after Theiler’s murine encephalomyelitis virus infection by passive administration of neutralizing monoclonal antibody. J Virol. 1989;63:2081–2087. doi: 10.1128/jvi.63.5.2081-2087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller A O, Lee W C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genovesi E V, Collins J J. In vitro growth inhibition of murine leukemia cells by antibody specific for the major envelope glycoprotein (gp71) of Friend leukemia virus. J Cell Physiol. 1983;117:215–229. doi: 10.1002/jcp.1041170213. [DOI] [PubMed] [Google Scholar]
- 12.Lausch R N, Staats H, Oakes J E, Cohen G H, Eisenberg R J. Prevention of herpes keratitis by monoclonal antibodies specific for discontinuous and continuous epitopes on glycoprotein D. Investig Ophthalmol Vis Sci. 1991;32:2735–2740. [PubMed] [Google Scholar]
- 13.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 14.Liebert U G, Schneider S S, Baczko K, Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990;64:706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marfurt C F, Del Toro D R. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol. 1987;261:450–459. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- 16.Marfurt C F, Kingsley R E, Echtenkamp S E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig Ophthalmol Vis Sci. 1989;30:461–472. [PubMed] [Google Scholar]
- 17.McKendall R R, Klassen T, Baringer J R. Host defenses in herpes simplex infections of the nervous system: effect of antibody on disease and viral spread. Infect Immun. 1979;23:305–311. doi: 10.1128/iai.23.2.305-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mester J C, Glorioso J C, Rouse B T. Protection against zosteriform spread of herpes simplex virus by monoclonal antibodies. J Infect Dis. 1991;163:263–269. doi: 10.1093/infdis/163.2.263. [DOI] [PubMed] [Google Scholar]
- 19.Metcalf J F, Hamilton D S, Reichert R W. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979;26:1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metcalf J F, Koga J, Chatterjee S, Whitley R J. Passive immunization with monoclonal antibodies against herpes simplex virus glycoproteins protects mice against herpetic ocular disease. Curr Eye Res. 1987;6:173–177. doi: 10.3109/02713688709020086. [DOI] [PubMed] [Google Scholar]
- 21.Mikloska Z, Sanna P P, Cunningham A L. Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J Virol. 1999;73:5934–5944. doi: 10.1128/jvi.73.7.5934-5944.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milligan G N, Bernstein D I, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- 23.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 24.Nash A A, Jayasuriya A, Phelan J, Cobbold S P, Waldmann H, Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 25.Oakes J E, Lausch R N. Monoclonal antibodies suppress replication of herpes simplex virus type 1 in trigeminal ganglia. J Virol. 1984;51:656–661. doi: 10.1128/jvi.51.3.656-661.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Rourke E J, Guo W H, Huang A S. Antibody-induced modulation of proteins in vesicular stomatitis virus-infected fibroblasts. Mol Cell Biol. 1983;3:1580–1588. doi: 10.1128/mcb.3.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pena Rossi C, Cash E, Aubert C, Coutinho A. Role of the humoral immune response in resistance to Theiler’s virus infection. J Virol. 1991;65:3895–3899. doi: 10.1128/jvi.65.7.3895-3899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penfold M E, Armati P, Cunningham A L. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA. 1994;91:6529–6533. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry L L, Lodmell D L. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J Virol. 1991;65:3429–3434. doi: 10.1128/jvi.65.7.3429-3434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posavad C M, Koelle D M, Corey L. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]
- 31.Ritchie M H, Oakes J E, Lausch R N. Passive transfer of anti-herpes simplex virus type 2 monoclonal and polyclonal antibodies protect against herpes simplex virus type 1-induced but not herpes simplex virus type 2-induced stromal keratitis. Investig Ophthalmol Vis Sci. 1993;34:2460–2468. [PubMed] [Google Scholar]
- 32.Rivera L, Beuerman R W, Hill J M. Corneal nerves contain intra-axonal HSV-1 after virus reactivation by epinephrine iontophoresis. Curr Eye Res. 1988;7:1001–1008. doi: 10.3109/02713688809015146. [DOI] [PubMed] [Google Scholar]
- 33.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. New York, N.Y: Raven Press; 1996. pp. 2231–2395. [Google Scholar]
- 34.Rozsa A J, Beuerman R W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 35.Sanna P P, De L A, Williamson R A, Hom Y L, Straus S E, Bloom F E, Burton D R. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology. 1996;215:101–106. doi: 10.1006/viro.1996.0011. [DOI] [PubMed] [Google Scholar]
- 36.Sanna P P, Keyser K T, Deerinck T J, Ellisman M H, Karten H J, Bloom F E. Distribution and ontogeny of parvalbumin immunoreactivity in the chicken retina. Neuroscience. 1992;47:745–751. doi: 10.1016/0306-4522(92)90182-2. [DOI] [PubMed] [Google Scholar]
- 37.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens J G, Cook M L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971;173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 39.Streilein J W, Dana M R, Ksander B R. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 40.Su Y H, Yan X T, Oakes J E, Lausch R N. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J Virol. 1996;70:1277–1281. doi: 10.1128/jvi.70.2.1277-1281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyler K L, Mann M A, Fields B N, Virgin H W., IV Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J Virol. 1993;67:3446–3453. doi: 10.1128/jvi.67.6.3446-3453.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wald A, Zeh J, Selke S, Ashley R L, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 43.Walz M A, Yamamoto H, Notkins A L. Immunological response restricts number of cells in sensory ganglia infected with herpes simplex virus. Nature. 1976;264:554–556. doi: 10.1038/264554a0. [DOI] [PubMed] [Google Scholar]
- 44.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. New York, N.Y: Raven Press; 1996. pp. 2297–2342. [Google Scholar]
- 45.Wilfert C M, Buckley R H, Mohanakumar T, Griffith J F, Katz S L, Whisnant J K, Eggleston P A, Moore M, Treadwell E, Oxman M N, Rosen F S. Persistent and fatal central-nervous-system ECHOvirus infections in patients with agammaglobulinemia. N Engl J Med. 1977;296:1485–1489. doi: 10.1056/NEJM197706302962601. [DOI] [PubMed] [Google Scholar]
- 46.Zeitlin L, Whaley K J, Sanna P P, Moench T R, Bastidas R, De Logu A, Williamson R A, Burton D R, Cone R A. Topically applied human recombinant monoclonal IgG1 antibody and its Fab and F(ab′)2 fragments protect mice from vaginal transmission of HSV-2. Virology. 1996;225:213–215. doi: 10.1006/viro.1996.0589. [DOI] [PubMed] [Google Scholar]