Abstract
Highly conserved among primate lentiviruses, the human immunodeficiency virus type 1 (HIV-1) Nef protein enhances viral infectivity by an unknown mechanism. Nef-defective virions are blocked at a stage of the HIV-1 life cycle between entry and reverse transcription, possibly virus uncoating. Nef is present in purified HIV-1 particles; however, it has not been determined whether Nef is specifically recruited into HIV-1 particles or whether virion-associated Nef plays a functional role in HIV-1 replication. To address the specificity and potential functionality of virion-associated Nef, we determined the subviral localization of Nef. HIV-1 cores were isolated by detergent treatment of concentrated virions followed by equilibrium density gradient sedimentation. Relative to HIV-1 virions, HIV-1 cores contained equivalent amounts of reverse transcriptase and integrase, decreased amounts of the viral matrix protein, and trace quantities of the viral transmembrane glycoprotein gp41. Examination of the particles by electron microscopy revealed cone-shaped structures characteristic of lentiviral cores. Similar quantities of proteolytically processed Nef protein were detected in gradient fractions of HIV-1 cores and intact virions. In addition, detergent-resistant subviral complexes isolated from immature HIV-1 particles contained similar quantities of Nef as untreated virions. These results demonstrate that Nef stably associates with the HIV-1 core and suggest that virion-associated Nef plays a functional role in accelerating HIV-1 replication.
The accessory protein Nef is essential for efficient replication and pathogenesis of primate lentiviruses. Disruption of the nef open reading frame results in attenuation of simian immunodeficiency virus in rhesus macaques, and the rapid reversion of point mutations is strong evidence for a requirement for Nef in virus replication (20). In human immunodeficiency virus type 1 (HIV-1), the presence of a functional nef gene stimulates virus replication in culture by a poorly defined mechanism (11, 30, 35). Nef stimulates HIV-1 infectivity by 5- to 50-fold in single-cycle infection assays, and this stimulation has been attributed to enhancement of reverse transcription in target cells (6, 34). Although Nef also downregulates cell surface expression of the HIV-1 receptor CD4 (14), this effect appears to be insufficient to account for HIV-1 infectivity enhancement by Nef (6, 30). A 5- to 10-fold enhancement of HIV-1 infectivity by Nef (Nef phenotype) is observed when HIV-1 is produced from CD4-negative cells. Furthermore, pseudotyping HIV-1 particles by the envelope proteins of amphotropic murine leukemia virus fails to rescue the Nef phenotype when target cells either express or lack CD4 (6, 31). Additionally, CD4 downregulation and infectivity enhancement are genetically separable activities of Nef (17). Collectively, these results suggest a functional independence of CD4 downregulation and Nef-mediated HIV-1 infectivity enhancement. Nef is required for efficient formation of the earliest reverse transcription products; this suggests that Nef acts at an early step in the virus life cycle prior to reverse transcription (6, 34). The recent observation that pseudotyping HIV-1 particles by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic route and suppresses the Nef phenotype is also consistent with a role of Nef in postentry events such as uncoating or intracellular transport (3).
Despite the finding that Nef-defective HIV-1 particles resemble wild-type virions both structurally and biochemically (31), Nef has been detected in HIV-1 particles (8, 32, 38). The presence of Nef within virions was confirmed by the observation that Nef is a substrate for the viral protease and is cleaved into a small amino-terminal fragment and a larger carboxy-terminal domain. These results suggest that Nef may function directly in the HIV-1 particle or after entry into the target cell. Nevertheless, it remains unclear whether Nef is specifically recruited into virions and whether virion-associated Nef plays a functional role in HIV-1 replication. A key to understanding whether Nef plays a functional role in HIV-1 particles is to determine the localization of Nef within the virion. Nef is a myristoylated protein and is expected to associate with the inner face of the viral lipid envelope; alternatively, Nef may associate with the viral core. To address these issues, we isolated HIV-1 cores and tested for the presence of Nef.
Isolation of HIV-1 core structures.
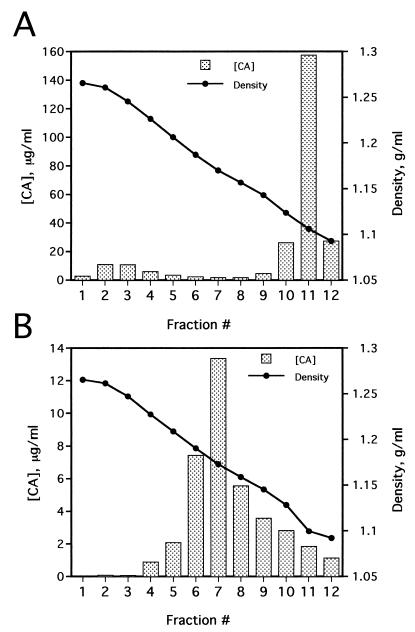
HIV-1 cores were isolated from virions by a modification of the “spin-thru” method previously described for the purification of HIV-2 cores (21). Filtered supernatants (30 ml) from 293T cells transfected with the proviral DNA construct R9 (12) were pelleted through a 4-ml cushion of 20% sucrose (120,000 × g, 3 h), and the pellet was allowed to dissolve in 0.25 ml of STE buffer (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA) for 2 h at 4°C. Linear density gradients, prepared by mixing equal volumes of 30 and 70% (wt/vol) sucrose in STE buffer in a gradient former, were overlaid with 0.25 ml of 15% sucrose containing 1% Triton X-100. This layer was covered with a 0.25-ml cushion of STE containing 7.5% sucrose, which served as a barrier to minimize mixing of the virus and detergent interfaces before centrifugation. Concentrated HIV-1 particles in STE buffer (0.25 ml) were carefully layered on top of the barrier layer, and tubes were centrifuged in a Beckman SW-41 rotor (100,000 × g, 16 h, 4°C). Twelve 1-ml fractions were collected from the bottom of each tube and assayed for reverse transcriptase (RT) activity and capsid protein (CA) content by an enzyme-linked immunosorbent assay (ELISA) (36). The density of each fraction was determined by measurements of refractive index and comparison with a standard series of sucrose concentrations of predetermined density. Fractions were collected from the bottom of the tubes and assayed for CA concentration by ELISA. Detergent treatment of HIV-1 particles resulted in sedimentation of significant quantities of CA near the bottom of the gradient (Fig. 1A). The peak of this material corresponded to the density expected for retroviral cores (1.26 g/ml). In this gradient, a large quantity of CA, corresponding to free protein released from solubilized virions, remained in the detergent-containing fractions at the top of the gradient (Fig. 1A, see fraction 11). In contrast to the detergent-containing gradient, virions sedimented through a control sucrose gradient lacking detergent to an equilibrium density of 1.16 g/ml, which corresponds to the density of intact retroviral particles (Fig. 1B).
FIG. 1.
Isolation of HIV-1 core structures by equilibrium sedimentation through a detergent layer. HIV-1 supernatants were harvested from 293T cells transiently transfected with the R9 wild-type HIV-1 proviral plasmid and filtered to remove cellular debris. Virions were concentrated by ultracentrifugation, resuspended in STE buffer, and layered onto 30 to 70% linear sucrose gradients containing a layer of 1% Triton X-100 at the top. The virions were sedimented through the detergent layer and into the sucrose gradient. Fractions (1 ml) were collected from the bottom of the gradient and assayed for CA concentration by ELISA and density by refractometry. The lane numbers correspond to gradient fractions. (A) Detergent-treated virions; (B) virions subjected to ultracentrifugation on a control gradient lacking detergent.
Biochemical analysis of HIV-1 cores.
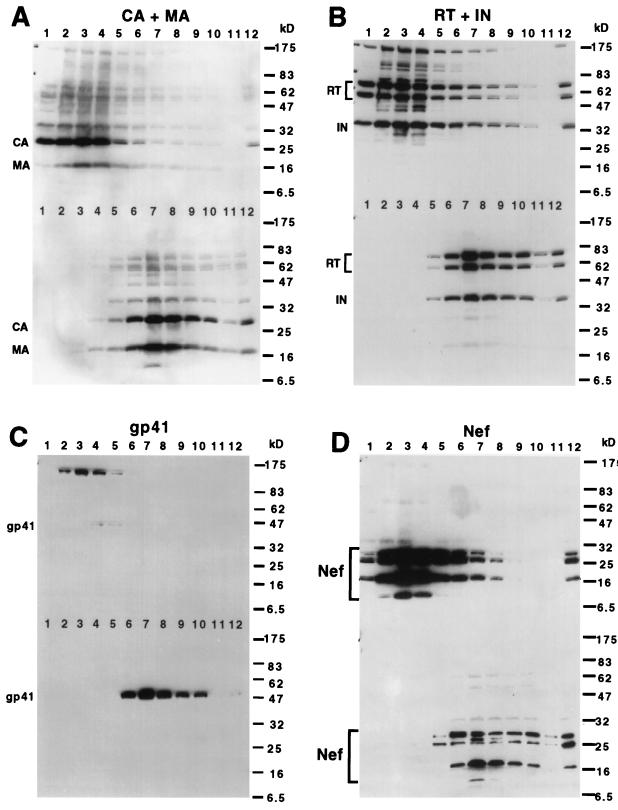
To analyze the viral protein composition of the putative HIV-1 core structures, gradient fractions of detergent-treated and untreated HIV-1 particles were subjected to immunoblot analyses after dilution with STE buffer to reduce the density of the fractions and pelleting by ultracentrifugation at 100,000 × g. As predicted, the dense fractions from the detergent-containing gradient contained CA, which forms the shell of the core (Fig. 2A, upper blot, lanes 1 to 4). In contrast, the pelletable CA in the fractions of untreated virions was present only in fractions of lower density (Fig. 2A, lower blot, lanes 6 to 9). To determine whether the dense fractions from the detergent-containing gradients contained additional HIV-1 proteins expected to be present in the HIV-1 core, the blot was reprobed with antisera specific for several HIV-1 structural proteins. In Fig. 2A, the samples from fraction 3 of the detergent-containing gradient and fraction 7 of the gradient lacking detergent contained similar quantities of CA as determined by ELISA prior to loading, and this is consistent with the CA band intensities on the immunoblots. Upon reprobing the blot with antisera specific for additional HIV-1 structural proteins, we observed that the densely sedimenting material from detergent-exposed virions contained a small fraction of the viral matrix protein (MA) present in intact virions (Fig. 2A). Although MA was previously reported as a component of the HIV-2 core (13), our results represent the first demonstration of the association of MA with the core of HIV-1. MA has been observed in viral ribonucleoprotein complexes (preintegration complexes) isolated from cells acutely infected with HIV-1 (9, 28), and it was therefore anticipated that some MA would be associated with the HIV-1 core. Association of MA with the HIV-1 core may facilitate HIV-1 uncoating or nuclear import of the preintegration complex. HIV-1 maturation is an intricate process, and MA might also regulate the proper assembly of the HIV-1 core.
FIG. 2.
Immunoblot analysis of isolated HIV-1 cores. Fractions (1 ml) from sucrose gradients were diluted to 1.5 ml with STE buffer to reduce the density of the solutions, and the particulate material was pelleted by ultracentrifugation. Pellets were dissolved in sample buffer and subjected to immunoblot analysis using various HIV-1 protein-specific antisera. Separate gels containing fractions from detergent-treated virions and untreated virions were transferred onto a single polyvinylidene difluoride filter. Blots were developed by chemiluminescence after probing with the appropriate horseradish peroxidase-conjugated secondary antiserum. In each panel, the upper half of the image shows the material isolated from detergent-treated virions, and the lower part shows control virions isolated in the absence of detergent. (A) Anti-CA plus anti-MA; (B) anti-RT plus anti-IN; (C) anti-gp41; (D) anti-Nef. The molecular masses of protein standards run on the same gel are shown in kilodaltons to the right of each panel.
Detergent-treated virions contained similar amounts of reverse transcriptase (RT) and integrase (IN) as intact virions (Fig. 2B). Only trace quantities of gp41 were detected in fractions of HIV-1 cores, while a strong gp41 signal was observed in fractions containing intact virions (Fig. 2C). Furthermore, the gp41 signal was present in fractions 4 and 5 of the cores, while the peak of core enzymes was in fraction 3. Since MA and gp41 are associated with the viral envelope (15), this result is consistent with removal of the viral envelope and with the isolation of HIV-1 core structures. In these experiments, we also observed large quantities of cellular proteins present in fractions of intermediate density (22). These may correspond to cell-derived microvesicles that frequently copurify with HIV-1 particles (7, 16). In support of this interpretation, we observed vesicular structures when intermediate fractions were subjected to ultracentrifugation and the pellets were examined by electron microscopy (23). These structures were apparently resistant to treatment with 1% Triton X-100. We suspect that the weak gp41 signals in fractions 4 and 5 of the detergent-containing gradient represent envelope protein that is associated with cell-derived microvesicles.
Interestingly, we detected large quantities of the Gag-Pol precursor Pr160gag-pol and the uncleaved envelope protein gp160 in fractions from detergent-containing gradients (Fig. 2B and C). These signals coincided with those of the viral enzymes, suggesting that these proteins are associated with isolated HIV-1 cores. In contrast, Pr160gag-pol and gp160 were not detected in fractions of untreated virions (Fig. 2C, bottom half of the blot). These observations imply that the isolation procedure enriched for a small fraction of particles containing these proteins. In several independent experiments, gp160 was detected in fractions of HIV-1 cores; however, the levels varied considerably. At present, the significance of the presence of Pr160gag-pol and gp160 in the isolated complexes remains unclear. Our attempts to demonstrate that the cores are infectious upon delivery into cells by lipofection or electroporation have been uniformly negative (22). The lack of infectivity observed with isolated HIV-1 cores suggests that the structures may be derived from a subset of virions that are not infectious but whose cores are stabilized by the presence of unprocessed viral protein precursors. The relatively higher yield of complexes isolated from immature versus mature HIV-1 particles is consistent with this interpretation (see below).
Association of Nef with the HIV-1 core.
The viral protein Nef is incorporated into HIV-1 particles, where it may modify the virion to stimulate infectivity. To determine whether Nef is associated with the HIV-1 core, the immunoblot of purified HIV-1 cores and intact virions was reprobed with an anti-Nef serum. HIV-1 cores copurified with proteins immunoreactive with anti-Nef serum. Both cleaved and uncleaved Nef products were detected in these fractions, demonstrating that at least a fraction of this material was derived from HIV-1 particles (Fig. 2D). Interestingly, both of the major cleavage products of Nef were present in the samples. In this experiment, the intensities of Nef protein bands present in intact virions were somewhat reduced relative to those in fractions containing HIV-1 cores. In several additional experiments, similar quantities of Nef products were present in pelleted cores and virions, suggesting that a majority of Nef in mature virions associates with the viral core (data not shown).
Ultrastructural examination of isolated HIV-1 cores.
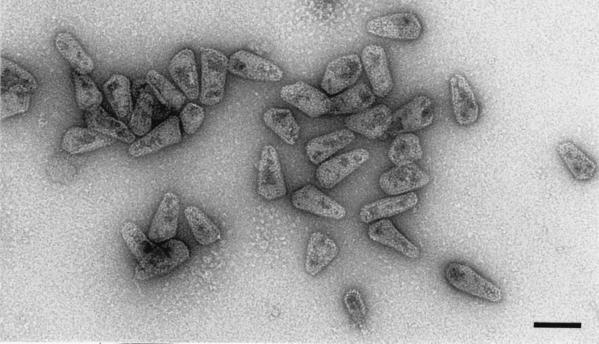
To characterize further the purified HIV-1 cores, the particles were visualized by transmission electron microscopy after negative staining (Fig. 3). The bulk of the particles were devoid of a lipid envelope and had the conical appearance of lentiviral cores observed in thin sections of intact HIV-1 virions. The size of the structures observed was also consistent with the identity of the material as isolated cores. Examination of the peak fraction of HIV-1 cores by electron microscopy revealed a virtually homogeneous preparation of HIV-1 core structures and the absence of contaminating intact virions or microvesicles. As a control, intact HIV-1 virions prepared in a parallel gradient lacking detergent were analyzed. In contrast to isolated HIV-1 cores, the majority of virions exhibited lipid envelopes surrounding an electron-dense, cone-shaped core (data not shown). These results serve to confirm the identity of the particles as HIV-1 cores. To our knowledge, this represents the first reported image of purified HIV-1 cores.
FIG. 3.
Electron microscopic analysis of HIV-1 cores. HIV-1 cores isolated from env-defective virions were pelleted, resuspended in 20 μl of STE buffer, and applied to carbon-coated grids that were glow discharged within 30 min of sample application. The grids were subsequently rinsed with 5 to 7 drops of STE buffer and stained with 1% uranyl acetate. Samples were examined in a Philips CM12 transmission electron microscope operating at 120 kV. Electron micrographs were recorded at a nominal magnification of ×35,000. Cone-shaped particles are clearly visible. The size and shape of the particles are identical to those observed in sectioned HIV-1 virions. Bar, 100 nm.
Subviral complexes isolated from PR− virions contain Nef.
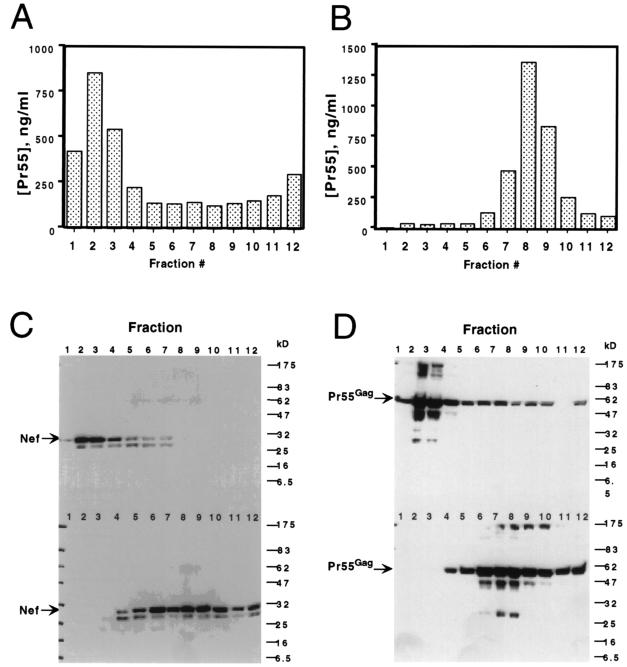
Immature HIV-1 particles have been reported previously to exhibit greater resistance to detergent than mature virions (39). To further examine the potential association of Nef with the HIV-1 core, immature virions were produced by transfection of 293T cells with an HIV-1 proviral clone encoding a triple substitution in the protease active site (R9.PR−). Virions derived from this clone are immature and noninfectious (4). Concentrated virions were subjected to ultracentrifugation through 1% Triton X-100 into a linear gradient of 30 to 70% sucrose, and gradient fractions were pelleted and subjected to immunoblot analyses. In contrast to mature HIV-1 cores, where the yield was usually 10% of the input virions, detergent treatment of immature HIV-1 virions resulted in yields of densely sedimenting complexes averaging 50% (Fig. 4A). As expected, immature virions not exposed to detergent sedimented to the upper half of the gradient (Fig. 4B). As observed with cores isolated from mature virions, immature subviral complexes contained levels of Nef similar to those in untreated immature virions (Fig. 4C). In all of the fractions containing full-length Nef, a higher-mobility band was also detected by the Nef antiserum. The ratio of the intensity of this signal to that of the full-length Nef varied across the fractions, and we suspect that this protein corresponds to a previously reported nonmyristoylated form of Nef produced by initiation of translation from an internal methionine codon (1, 19). Subsequent reprobing of the blot with anti-CA serum revealed that similar amounts of Pr55gag were present in the immature virions and cores (Fig. 4D), confirming that equal quantities of detergent-treated and untreated complexes were analyzed. The presence of Nef in subviral complexes isolated from PR− virions confirms a detergent-resistant association of Nef with an internal structural component of HIV-1 particles.
FIG. 4.
Nef is associated with immature subviral complexes isolated by detergent treatment of protease-defective virions. Concentrated supernatants from cells from the protease-defective proviral clone R9.PR− were subjected to equilibrium sedimentation through a layer of 1% Triton X-100 into a linear sucrose density gradient. Fractions (1 ml) were collected from the bottom of the tube and assayed for relative Pr55gag content by a CA-specific ELISA. (A) Fractions from the detergent-containing gradient. (B) fractions from a parallel gradient lacking detergent. Particles in each fraction were pelleted by ultracentrifugation and assayed for Nef and Pr55gag by immunoblotting. In each blot, the top panel contains samples from the detergent-containing gradient, while the bottom panel contains samples from a parallel gradient lacking detergent. (C) Analysis of the peak fractions of detergent-treated and untreated virions for Nef. (D) The blot shown in panel C reprobed with antiserum specific for HIV-1 CA.
Implications of the association of Nef with the HIV-1 core.
A major finding of this study is that Nef is strongly associated with the HIV-1 core. This was demonstrated by the observation that both mature HIV-1 cores and detergent-resistant subviral complexes isolated from immature virions exhibited similar quantities of Nef as the corresponding intact virions. These results suggest that a specific association of Nef with an HIV-1 structural protein occurs during virion assembly. Nef has been detected in HIV-1 particles, where it is cleaved into two major fragments by the viral protease. Interestingly, we detected both of the major cleavage products of Nef in isolated HIV-1 cores. It seems unlikely that both Nef fragments would be capable of specifically binding to a component of the core; therefore, during virion maturation, Nef appears to be trapped inside the core, where it is subsequently targeted by the viral protease. A specific prediction of this hypothesis is that the core should contain at least a fraction of the viral protease.
If Nef is present within the HIV-1 core, how is it detached from the viral membrane? Nef is a myristoylated protein that associates with membranes in infected cells. Myristoylation is essential for both CD4 downregulation and HIV-1 infectivity enhancement by Nef (5, 6). It is therefore surprising that a majority of the virion-associated Nef is associated with the core. This suggests that the membrane-binding effect of the myristate may be suppressed by an interaction with a component of the core during HIV-1 maturation. Nef may also undergo a conformational change to sequester the myristoyl moiety. In this manner, Nef may be removed from the viral membrane by a process analogous to the myristoyl switch mechanism proposed for regulating the association between the HIV-1 MA protein and the plasma membrane of the cell (41). Like MA, Nef contains an amino-terminal basic domain that is required for its association with the plasma membrane (37).
Cleavage of Nef within virions is not required for HIV-1 infectivity enhancement by this protein (10, 29), and it remains unclear whether virion-associated Nef plays a role in HIV-1 infectivity enhancement. Nef-defective virions contain normal amounts of viral structural proteins and the cellular protein cyclophilin A (2, 31), and the only reported biochemical difference between wild-type and Nef-defective virions is the presence of Nef within the virions. Estimates of the quantities of Nef in virions range from 5 to 70 molecules per virion (32, 38), and our own measurements fall within this range (40). These quantities are presumably sufficient for mediating the approximate 10-fold HIV-1 infectivity enhancement mediated by Nef. Available evidence suggests that Nef stimulates an early step in the virus life cycle following entry but preceding reverse transcription (6, 34). Our observation that Nef is stably associated with the HIV-1 core supports the hypothesis that Nef stimulates HIV-1 infectivity by acting directly in virions. By associating with the viral core, Nef may enhance early events in HIV-1 infection by several possible mechanisms. First, Nef may prime the core for uncoating in a manner similar to that proposed for the cellular protein cyclophilin A (25). Nef may alter the structure of the viral core, thereby influencing HIV-1 core stability in a positive or negative manner so as to mediate the optimal disassembly of capsid monomers from the incoming viral core. A second possibility is that Nef may interact with cellular proteins that are required for efficient reverse transcription in target cells. By associating with the incoming core, Nef may recruit cellular proteins required for proper initiation of reverse transcription of the viral genome. We and others have reported that Nef is not required for reverse transcription of the viral genome in vitro (6, 34). However, these reactions are typically inefficient, and it is likely that cellular factors influence reverse transcription in vivo. Isolation of cores from wild-type and nef-defective virions may prove useful to determine whether these complexes exhibit detectable structural or biochemical differences.
A third mechanism by which Nef may enhance HIV-1 infectivity is by altering the intracellular transport of the incoming viral ribonucleoprotein complex. Because Nef interacts with components of the endocytic machinery to downregulate the expression of cell-surface CD4 and major histocompatibility complex class I (18, 24, 33), Nef may also tether the incoming viral core to adapter protein complexes associated with clathrin-coated pits and direct the core to a specific cellular compartment. This model would help to explain the suppression of the infectivity enhancement mediated by Nef when HIV-1 virions are pseudotyped by the glycoprotein of vesicular stomatitis virus (3, 26), a virus which infects cells through an endocytic pathway (27). Furthermore, if HIV-1 penetration is a stochastic process occurring most often by direct fusion with the plasma membrane but occasionally by internalization through endosomes and subsequent fusion with the endosomal membrane, this model would account for the residual infectivity exhibited by virions that are produced in the complete absence of Nef. These models for HIV-1 infectivity enhancement by Nef are not mutually exclusive, and Nef may act through a combination of these mechanisms. Further studies of the association of Nef with the HIV-1 core will be required to understand the functional consequences of this interaction.
Acknowledgments
We thank Dean Ballard, Terry Dermody, and Paul Spearman for helpful comments on the manuscript. The following antisera were obtained through the NIH AIDS Research and Reference Reagent Program: antiserum to HIV-1 RT (catalog no. 634) from Division of AIDS, National Institute of Allergy and Infectious Diseases, and antiserum to HIV-1 IN (catalog no. 758) from Duane Grandgenett.
This study was funded by NIH grant R01 AI 40363. C.A. was sponsored in part by an AmFAR/Genetech, Inc., Scholar Award.
REFERENCES
- 1.Ahmad N, Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988;241:1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- 2.Aiken C. Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity. Virology. 1998;248:139–147. doi: 10.1006/viro.1998.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiken, C. Unpublished data.
- 5.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 6.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bess J W, Jr, Gorelick R J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 8.Bukovsky A, Dorfman T, Weimann A, Gottlinger H G. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J Virol. 1997;71:1013–1018. doi: 10.1128/jvi.71.2.1013-1018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y-L, Trono D, Camaur D. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J Virol. 1998;72:3178–3184. doi: 10.1128/jvi.72.4.3178-3184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowers M Y, Spina C A, Kwoh T J, Fitch N J S, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 13.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 14.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 15.Gelderblom H R, Hausmann E H S, Orzel M, Pauli G, Koch M A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987;156:171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 16.Gluschankof P, Mondor I, Gelderblom H R, Sattentau Q J. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology. 1997;230:125–133. doi: 10.1006/viro.1997.8453. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith M A, Warmerdam M T, Atchison R E, Miller M D, Greene W C. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J Virol. 1995;69:4112–4121. doi: 10.1128/jvi.69.7.4112-4121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminchik J, Bashan N, Pinchasi D, Amit B, Sarver N, Johnston M I, Fischer M, Yavin Z, Gorecki M, Panet A. Expression and biochemical characterization of human immunodeficiency virus type 1 nef gene product. J Virol. 1990;65:583–588. doi: 10.1128/jvi.64.7.3447-3454.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kestler III H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 21.Kewalramani V N, Emerman M. Vpx association with mature core structures of HIV-2. Virology. 1996;218:159–168. doi: 10.1006/viro.1996.0176. [DOI] [PubMed] [Google Scholar]
- 22.Kotov, A., and C. Aiken. Unpublished data.
- 23.Kotov, A., P. Flicker, and C. Aiken. Unpublished data.
- 24.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J M, Schwartz O. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 25.Luban J. Absconding with the chaperone: essential cyclophilin-Gag interactions in HIV-1 virions. Cell. 1996;87:1157–1159. doi: 10.1016/s0092-8674(00)81811-5. [DOI] [PubMed] [Google Scholar]
- 26.Luo T, Douglas J L, Livingston R L, Garcia J V. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology. 1998;241:224–233. doi: 10.1006/viro.1997.8966. [DOI] [PubMed] [Google Scholar]
- 27.Matlin K S, Reggio H, Helenius A, Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982;156:609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- 28.Miller M D, Farnet C M, Bushman F D. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller M D, Warmerdam M T, Ferrell S S, Benitez R, Greene W C. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology. 1997;234:215–225. doi: 10.1006/viro.1997.8641. [DOI] [PubMed] [Google Scholar]
- 30.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller M D, Warmerdam M T, Page K A, Feinberg M B, Greene W C. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J Virol. 1995;69:570–584. doi: 10.1128/jvi.69.1.579-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandori M W, Fitch N J S, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piguet V, Chen Y L, Mangasarian A, Foti M, Carpentier J L, Trono D. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz O, Marechal V, Danos O, Heard J-M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehrly K, Chesebro B. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods. 1997;12:288–293. doi: 10.1006/meth.1997.0481. [DOI] [PubMed] [Google Scholar]
- 37.Welker R, Harris M, Cardel B, Krausslich H-G. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J Virol. 1998;72:8833–8840. doi: 10.1128/jvi.72.11.8833-8840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welker R, Kottler H, Kalbitzer H R, Krausslich H-G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 39.Wiegers K, Rutter G, Kottler H, Tessmer U, Hohenberg H, Krausslich H-G. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J Virol. 1998;72:2846–2854. doi: 10.1128/jvi.72.4.2846-2854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, J., and C. Aiken. Unpublished data.
- 41.Zhou W, Resh M D. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J Virol. 1996;70:8540–8548. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]