Abstract
In newly diagnosed acute myeloid leukemia (AML), immediate initiation of treatment is standard of care. However, deferral of antileukemic therapy may be indicated to assess comorbidities or pretherapeutic risk factors. We explored the impact of time from diagnosis to treatment on outcomes in newly diagnosed AML undergoing venetoclax-based therapy in two distinct cohorts. By querying the Study Alliance Leukemia database and the global health network TriNetX, we identified 138 and 717 patients respectively with an average age of 76 and 72 years who received venetoclax-based first-line therapy. When comparing patients who started treatment earlier or later than 10 days after initial diagnosis, no significant difference in median overall survival was observed - neither in the SAL cohort (7.7 vs. 9.6 months; P=0.42) nor in the TriNetX cohort (7.5 vs. 7.2 months; P=0.41). Similarly, severe infections, bleeding, and thromboembolic events were equally observed between early and later treatments, both in the overall patient groups and specific subgroups (age ≥75 years or leukocytes ≥20x109/L). This retrospective analysis indicates that delaying the start of venetoclax-based therapy in newly diagnosed AML might be a safe option for selected patients, provided that close clinical monitoring is performed.
Introduction
The diagnosis of acute myeloid leukemia (AML) is deemed a medical emergency, given that untreated cases result in dismal outcomes.1 Immediate initiation of treatment has therefore been the standard of care for newly diagnosed patients with AML since the early days of leukemia therapy.2 Deferral of treatment was an exception and may only have occurred when assessment or treatment of comorbidities was necessary. Researchers have confirmed the paradigm of immediate treatment initiation when in 2009 Sekeres et al. showed that response rates and overall survival (OS) worsened if a treatment delay of more than 5 days occurred in patients under the age of 60 years.3 However, as more and more targeted treatment approaches become available, deferral of treatment in newly diagnosed AML may be indicated to treat according to molecular analysis.4 -7 A study conducted in 2013 showed comparable treatment outcomes in patients with curative intent receiving intensive chemotherapy even when treatment is postponed.8 Likewise, in a comprehensive analysis published by the Study Alliance Leukemia (SAL) in 2020 involving 2,263 patients undergoing intensive chemotherapy induction, no disparity in overall survival or other clinical outcomes was observed based on the initiation of treatment.9 Conversely, a separate Swedish study with 2,374 patients and a study from the National Cancer Database of the US with 55,985 patients noted a survival impact with treatment delays.10,11 However, results of these population-based studies may be dominated by selection of induction regimens and their relapse rates rather than by treatment delay. It is noteworthy that patients who underwent early treatment were skewed towards a younger age. A meta-analysis conducted in 2023 including these studies and others but excluding the extensive US study, found a correlation between prolonged time from diagnosis to treatment initiation (TDT) and a decreased likelihood of achieving complete remission (CR).12 Overall, the described differences in these studies were marginal and the clinical relevance of these results may be limited. We argue that the prognosis of younger or fit patients is only minimally affected, if at all, by the TDT. But as all available data was derived exclusively from AML patients who are eligible for intensive chemotherapy, we sought to expand this clinical question to elderly patients unfit for intensive therapy.
The approval of venetoclax in combination with hypomethylating agents (HMA) in newly diagnosed AML in 2018 by the Food and Drug Administration represented a significant advance in AML therapy, offering improved outcomes for elderly or frail patients not eligible for intensive chemotherapy. Although there are subtype-specific differences in the antileukemic efficacy of venetoclax, venetoclax-based therapies are indicated regardless of Wolrd Health Organization/European LekemiaNet (ELN)/International Consensus Classification.13 With the advent of targeted therapies, there are new opportunities to address genetic alterations in AML also in patients ineligible for intensive treatment, and thus increase the number of treatment options.14,15 Since a comprehensive genetic diagnosis or addressing pre-existing conditions and associated complications can take more than 7-10 days, we asked whether a prolonged TDT with venetoclax-based therapies impairs outcome in patients with newly diagnosed AML.
Methods
This study used real-world data from two independent cohorts to compare TDT in AML: the patient registry of the SAL and electronic health records (EHR) from TriNetX, LLC (“TriNetX”), a global federated health research network. Patients from both cohorts were stratified into two treatment groups: those treated within the first 9 days (TDT 0-9) and those treated from day 10 onwards (TDT ≥10). The SAL trial is registered under the name “Clinical AML Registry and Biomaterial Database of the SAL”. The SAL trial was conducted in accordance with the principles of the Declaration of Helsinki and the protocol has been approved by the ethics committees of all participating centers. The study is registered under the clinicaltrials gov. Identifier: NCT03188874. TriNetX utilizes aggregated de-identified patient records, therefore no ethics committee approval was required.
SAL registry query design
The SAL registry captures cases of adult AML, examining laboratory values, genomics, survival, and relapse for academic and clinical insights. We selected patients with newly diagnosed AML treated with venetoclax in combination with HMA or low-dose cytarabine (LDAC) between January 1, 2018, and April 15, 2023. Only patients with a follow-up of at least 6 months or death within this period were included. Data query was performed on November 28, 2023. In order to enhance data validity and specificity, patients receiving treatment >50 days after diagnosis were excluded from the analysis. Patient characteristics were analyzed using descriptive statistical methods. The binary outcomes CR or complete remission with incomplete count recovery (CRi), early death (ED) and allocation to allogeneic hematopoietic stem cell transplant (HSCT) were expressed as a percentage and compared using the χ2 test, while for OS, event-free survival (EFS) and relapse-free survival (RFS) the Kaplan-Meier approach was used. EFS was defined as either primary treatment failure or relapse or death, RFS was calculated from the time of CR/CRi until relapse or death.
TriNetX query design
TriNetX is a healthcare network that facilitates access to EHR from currently more than 250 million patients worldwide providing data for clinical and retrospective studies.16 We searched for patients from the TriNetX database who were treated with newly diagnosed AML between January 1, 2015, and July 1, 2023, and met the following criteria: venetoclax treatment in combination with HMA or LDAC, follow-up of at least 1 year or death, no prior treatment of anthracycline/ mitoxantrone or venetoclax before diagnosis of AML, no prior diagnosis of AML, age ≥20 years, no intensive therapy within the first 8 weeks after diagnosis and no prior HSCT. Data query on the platform was performed on October 24, 2023. Analyses were done utilizing the statistical tools provided within the TriNetX network, as only aggregated data was accessible. The baseline characteristics age and body mass index (BMI) were therefore described by means and standard deviations (SD), while the other continuous variables were described by median and interquartile range (IQR). Age and BMI were compared using Student’s t test, dichotomous variables by the χ2 test. As no statistical analyses for aggregated data other than the Student’s t test are available on the platform, no comparison could be made for median and IQR. Further details are described in the Online Supplementary Appendix.
Results
We identified a total of 855 patients (717 TriNetX, 138 SAL registry) who received first-line treatment of AML with venetoclax based regimens. The patient selection process is illustrated as CONSORT flow diagram (Online Supplementary Figure S1).
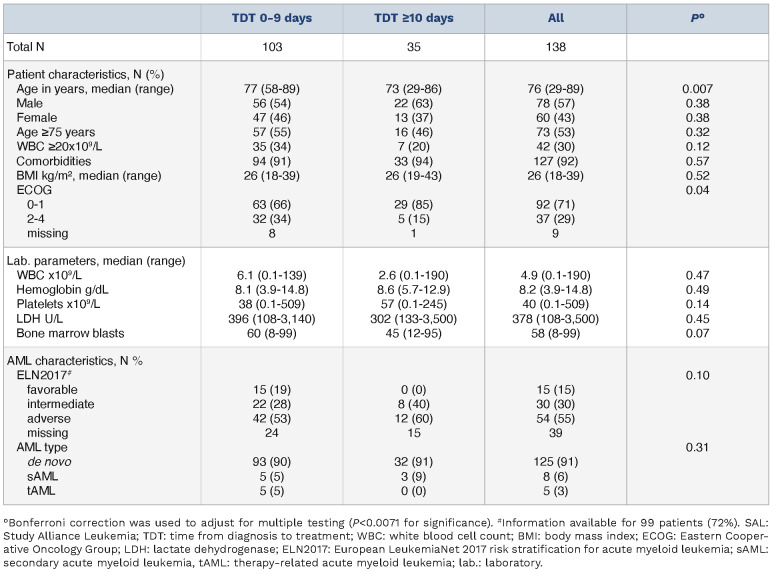
Table 1.
Patient characteristics SAL registry.
SAL registry
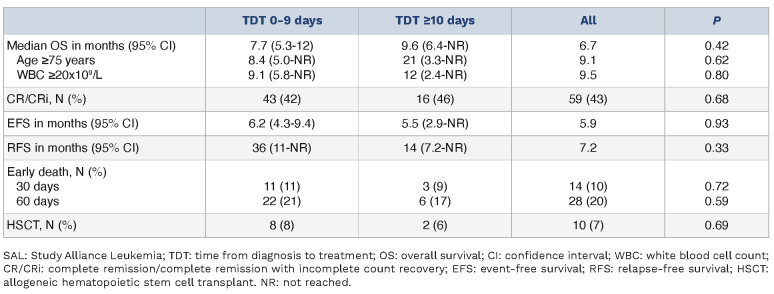
At data cutoff, a total of 8,681 newly diagnosed AML patients were registered in the SAL registry, of whom 138 received venetoclax in combination with HMA or LDAC as first line treatment and met inclusion criteria for this analysis. Of these patients, 103 received treatment within the first 9 days after diagnosis (75%, TDT 0-9), while 35 patients were treated on day 10 or later (25%, TDT ≥10) (Table 1). Median TDT was 4 days (IQR, 2-6 days) in the TDT 0-9 group and 15 days (IQR, 12-21 days) in the TDT ≥10 group, respectively (Online Supplementary Figure S2). With a median age of 77 years (range, 58-89 years), patients in the TDT 0-9 group were significantly older than in the TDT ≥10 group (median age 73 years; range, 29-86 years; P=0.007). We observed no significant difference in hemoglobin levels (8.1 g/dL vs. 8.6 g/dL; P=0.49), platelet counts (38x109/L vs. 57x109/L; P=0.14), percentage of bone marrow blasts (60% vs. 45%; P=0.07) or lactate dehydrogenase (LDH) (396 U/L vs. 302 U/L; P=0.45). The percentage of patients with leukocytosis defined as white blood cell count (WBC) ≥20x109/L was similar (34% vs. 20%; P=0.12). According to the ELN2017 risk stratification, favorable, intermediate, and unfavorable genetic risks were present in 15%, 30% and 55% of patients, respectively. Patients with favorable genetic risks were only identified in the TDT 0-9 group; however, this difference was not statistically significant. Comorbidities were present to the same extent in both cohorts (91% vs. 94%; P=0.57). CR or CRi was achieved in 43 of 103 (42%) and 16 of 35 (46%) patients, respectively. The median OS was 6.7 months with a median follow-up time of 16 months in the TDT 0-9 and 12 months in the TDT ≥10 group (Table 2). HSCT was realized in 10 of 138 patients (7%). In order to determine whether very early treatment provides a survival benefit, we divided the TDT 0-9 group along the median into a TDT 0-4 and a TDT 5-9 subgroup. No differences in OS were observed (median survival 6.1 vs. 9.5 months; P=0.2).
With an OS of 7.7 (95% confidence interval [CI]: 5.3-12) versus 9.6 (95% CI: 6.4- not reached [NR]) months, there was only a small, statistically non-significant difference between the TDT 0-9 and TDT ≥10 groups (P=0.42) (Figure 1A). Likewise, a numerical but not statistically significant difference was observed for RFS or EFS (36 vs. 14 months; P=0.33 and 6.2 vs. 5.5 months; P=0.93; Online Supplementary Figure S3). RFS at 12 months was 57% (95% CI: 42-78) and 55% (95% CI: 32-96), median EFS at 12 months was 37% (95% CI: 29-48) and 38% (95% CI: 25-59). The OS in subgroups of individuals aged ≥75 years (8.4 vs. 21 months; P=0.62) or with WBC ≥20x109/L (9.1 vs. 12 months; P=0.80) displayed no statistically significant differences between early and late treatment. The OS after 12 months was 42% (95% CI: 31-58) and 52% (95% CI: 30-88) in the patients aged ≥75 years and 46% (95% CI: 31-67) and 38% (95% CI: 14-100) in the WBC ≥20x109/L subgroup (Table 2; Online Supplementary Figure S4). Early death after 30 days occurred in 14 of 138 (10%) patients with no significant difference between the groups (11% vs. 9%; P=0.72). Hydroxyurea administration lacked consistent documentation and thus is not included in the analysis.
TriNetX cohort
At the time of analysis, there were 32,058 patients with newly diagnosed AML in the TriNetX EHR library. Of these, 717 AML patients with sufficient documentation were identified who received combination treatment with venetoclax and met the inclusion criteria. Among them, 491 patients received treatment within the first 9 days after diagnosis (68%, TDT 0-9), while in 226 patients, treatment was initiated on day 10 or later (32%, TDT ≥10). The patient population comprised 80% Whites, 6% African Americans, 2% Asians and 12% other US citizens of unknown ethnicity from 41 US-based healthcare organizations.
Table 2.
Treatment outcomes SAL registry.
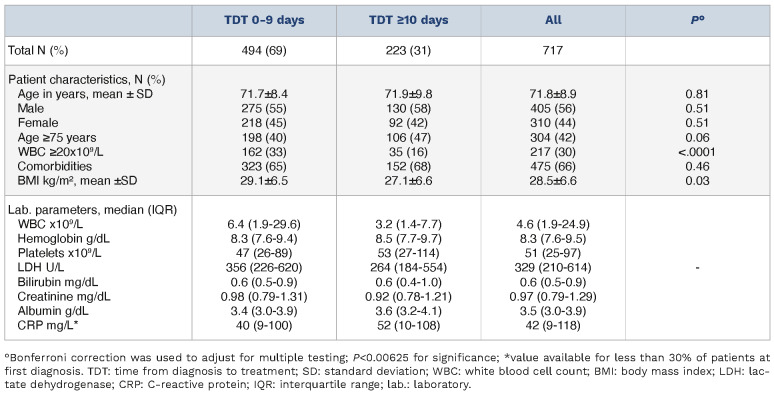
Mean age was 71.8±8.9 years, with very little variance between both groups (Table 3). We observed more patients with a WBC ≥20x109/L in the TDT 0-9 group (36% vs. 18%; P<0.0001) compared to TDT ≥10. Regarding sex or comorbidities, no significant differences between the two groups were present. Median TDT was 3 days (IQR, 1-5 days) in the TDT 0-9 group and 17 days (IQR, 13-25) in the TDT ≥10 group, respectively (Online Supplementary Figure S2). Median follow-up, defined as time from diagnosis to last documented visit or death was 11.0 months.
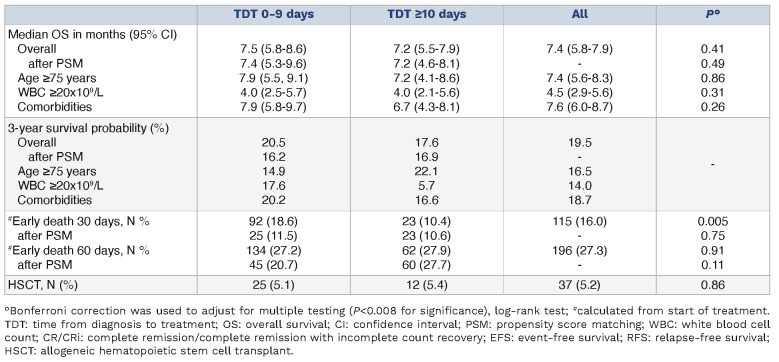
Median OS was 7.5 months in the TDT 0-9 group and 7.2 months in the TDT ≥10 group (P=0.41; Table 4; Figure 1B). This parity persisted even after using PSM to match patients for WBC and age, effectively controlling for the increased prevalence of leukocytosis in the TDT 0-9 group (7.4 vs. 7.2 months; P=0.49). Balanced across the two groups, 37 of 717 patients (5%) received HSCT for consolidation.
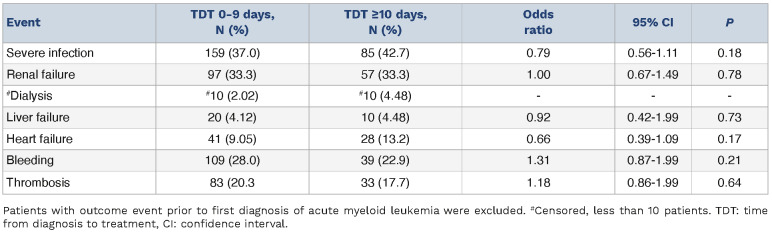
When we queried for adverse events by selected International Classification of Disease codes (Online Supplementary Table S1) no differences between TDT 0-9 and TDT ≥10 were identified. Severe infections occurred in 244 of 717 patients (34%). We found acute kidney injury in 154 of 717 patients (21%) again without significant association to TDT (Table 5). We noted a higher 30-day death rate in the TDT 0-9 group (19%) compared to the TDT ≥10 group (10%; P=0.005). The difference was not present after matching patients for WBC and age or if 60-day early mortality was analysed (Table 4). Subgroup analyses revealed no differences in OS between the TDT 0-9 versus TDT ≥10 group in patients ≥75 years old (7.9 vs. 7.2 months; P=0.86), with WBC ≥20x109/L (4.0 vs. 4.0 months; P=0.31) or any versus no comorbidities (7.9 vs. 6.7 months; P=0.26) (Online Supplementary Figures S5-S7). We observed no significant differences in clinical outcomes of these subgroups such as severe infections, renal failure, heart or liver failure, bleeding, or thromboembolic events either (Online Supplementary Tables S2-S4). As with the SAL-cohort, we divided the TDT 0-9 group along the median into a TDT 0-3 and a TDT 4-9 subgroup. Again, no differences in OS or other clinical outcomes were observed (median survival 6.9 vs. 7.9 months; P=0.32). During the initial 30 days post-diagnosis, hydroxyurea was prescribed to 32% of TDT 0-9 group patients, and 13% in the TDT ≥10 group (P<0.001). In patients with leukocytosis, hydroxyurea was administered in 77% in the TDT 0-9 group and in 54% in the TDT ≥10 group (P=0.006).
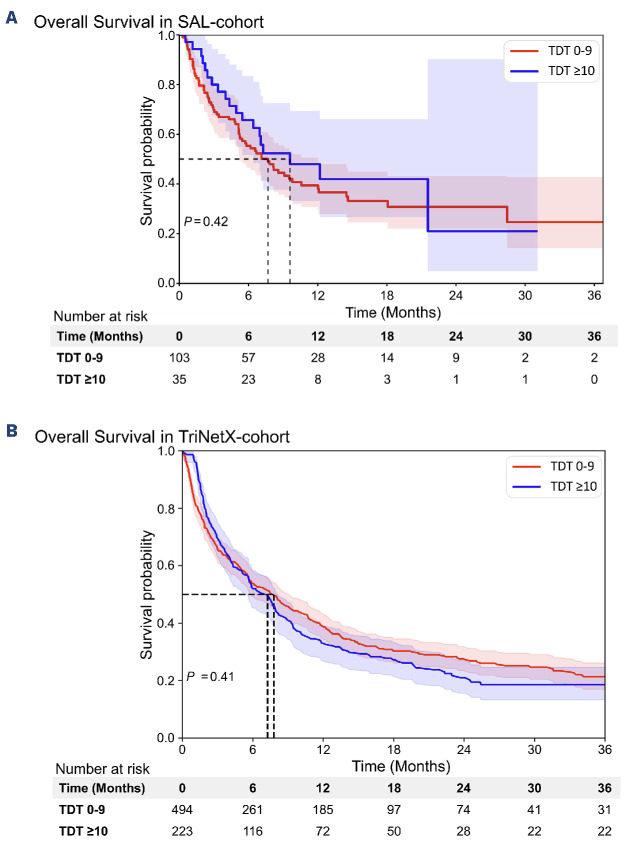
Figure 1.
Overall survival of patients in the SAL and TriNetX cohort. (A) Overall survival (OS) of patients in the Study Alliance Leukemia (SAL) cohort calculated from diagnosis of acute myeloid leukemia (AML). Median OS was 7.7 (95% confidence interval [CI]: 5.3-12) months in the time from diagnosis to treatment (TDT) 0-9 and 9.6 (95% CI: 6.4- not reached) months in the TDT ≥10 group (P=0.42). Median follow-up time was 16 and 12 months, respectively. (B) OS of patients in the TriNetX-cohort calculated from diagnosis of AML. Median OS was 7.5 (95% CI: 5.8-8.6) months in the TDT 0-9 group and 7.2 (95% CI: 5.5-7.9) months in the TDT ≥10 group (P=0.41). Median follow-up time was 11 months in the whole cohort.
Table 3.
Patient characteristics TriNetX.
Table 4.
Overall survival and early death TriNetX.
Table 5.
Rates of adverse events across the TriNetX cohort stratified according to time from diagnosis to treatment.
Discussion
We examined for the first time the effect of TDT on clinical outcomes in newly diagnosed AML patients treated with venetoclax-based regimens. Both SAL and TriNetX patient cohorts were overall well balanced according to clinical parameters and patient demographics, which was mostly maintained upon stratification into short and long TDT. Although the median age was higher in the SAL TDT 0-9 group, the proportion of patients aged ≥75 years was similar across both SAL TDT groups. In the TriNetX cohort, no relevant age difference was observed, suggesting that the age disparity in the SAL cohort between TDT 0-9 and TDT ≥10 groups may be attributed to the relatively small sample size. In the SAL cohort, TDT 0-9 displayed a trend for improved RFS and EFS, with slightly shorter OS compared to the TDT ≥10 group. This observation is likely caused by a few cases with late events and again a consequence of the small sample size, impacting RFS and EFS but not OS. In the TriNetX cohort, no significant differences in OS were observed between the TDT groups, neither in the primary cohort nor in the respective subgroups.
The greater proportion of patients with leukocytosis (WBC ≥20x109/L) in the TDT 0-9 group compared to the TDT ≥10 group in the TriNetX cohort may reflect a consensus among treating physicians not to delay treatment initiation in AML with high cell turnover. In the SAL cohort, the proportion of patients with leukocytosis (WBC ≥20x109/L) did not differ between the TDT 0-9 and TDT ≥10 groups, again probably due to the lower number of patients in this cohort. As repeated WBC counts were not available for analysis, information on leukocyte dynamics was lacking. In the TriNetX cohort, overlapping survival curves suggest potential unaddressed non-proportional hazards, likely caused by sample size limitations. However, the overall lack of outcome differences in patients with WBC ≥20x109/L suggests that carefully selected individuals may tolerate elevated WBC for a few days, possibly with additional hydroxyurea treatment.
The absence of disparities in survival or other clinically significant outcomes, such as severe infections, hemorrhage, or organ failure between TDT 0-9 and TDT ≥10 groups in both the SAL and TriNetX cohorts indicates that delaying treatment does not seem to pose an elevated risk for older patients and those with comorbidities. Consistent signals of equivalence in two international cohorts imply widespread success in clinically managing patients with delayed TDT, likely reflecting good clinical practice across different regions. At the same time, the antileukemic effect of venetoclax does not appear to be affected by treatment delay. Therefore, unfavorable outcomes in unfit AML patients treated with venetoclax are likely attributed to factors beyond TDT.
We found a higher early mortality (assessed after start of treatment) in the TDT 0-9 group compared to the TDT ≥10 group of the TriNetX cohort. In order to account for the impact of older age and high WBC as significant factors in early mortality, we employed PSM to match the TriNetX groups based on these variables.17-19 No statistically significant difference in early mortality was observed after the use of PSM. Furthermore, there were no discernible differences in specific clinical outcomes or treatment-related complications that would implicate antileukemic treatment as a significant factor in 30-day early mortality. Consequently, we conclude that TDT had no effect on early mortality.
Comparable to several other real-world analyses, we observed lower remission rates and OS (6.7 to 7.4 months) and a higher early death rate (10% to 16%) in both patient cohorts compared to the venetoclax pivotal study ‘VIALE-A (median OS 14.7 months, early death rate 7%).13 VIALE-A targeted an elderly and frail patient population, using specific inclusion and exclusion criteria to characterize the study population. These criteria are presumably applied less strictly in practice, which broadens the indication (e.g., patients with advanced kidney, liver or heart problems, chronic lung disease or advanced diabetes). Consequently, our data is in line with reported median overall survival rates of between 9 and 13 months in several real-world analyses and a meta-analysis including 1,134 patients that showed a pooled median survival time of 9.4 months.20-24 Outside of prospective, controlled, randomized clinical trials (RCT), it is suspected that reduced safety monitoring and bone marrow assessments as well as decreased treatment adherence may contribute to impaired prognosis as well as distortion of efficacy parameters, such as response rates, and RFS.20-23,25 While an RCT may be more useful to estimate true clinical potential of a therapeutic regimen, real-world-analyses such as our report are required to assess clinical questions for which a RCT will likely never emerge. One such clinical issue is the optimal timing of treatment initiation in patients with newly diagnosed AML.
Like all analyses based on real-world data, there are several limitations to consider. While we made efforts to accommodate known risk factors, non-randomized data inherently cannot be adjusted for unknown risk factors. For example, our results suggest that patients with evidence of rapid proliferation were treated earlier. However, there is little information on other patient or disease characteristics that may have influenced physicians’ decision to start treatment at a particular time. We cannot account for instances where patients scheduled for intensive treatment received venetoclax-based therapy or vice versa, as this information is not documented in the EHR or the SAL registry. Furthermore, both SAL and TriNetX cohorts exhibit selection bias and EHR may include misdiagnoses and lack information on potential confounders. The TriNetX cohort faces sampling bias due to non-random selection, missing individuals with limited healthcare access or patients receiving treatment outside facilities of the network. We lack information on facility size, which could affect molecular diagnostics waiting times and resource limitations for severe complications. Similar to other analyses conducted on TDT, the retrospective nature of this analysis fails to account for patients that succumb to leukemia before treatment initiation.3,8-12 But as untreated AML leads to a dismal outcome, a general assumption can be made: patients untreated for an extended period after diagnosis encounter a higher before-treatment mortality rate compared to those promptly treated. However, by focusing on time from diagnosis to treatment, the analysis addresses the question whether patients receiving treatment after a specific time frame have the same benefits from this therapy as patients who are treated immediately. Based on our results, we conclude that a delay in treatment does not result in an accumulation of risk factors or diminishes therapy efficacy and, consequently, does not influence outcome. In summary, our results show for the first time that TDT has no clinically relevant impact on OS in patients with newly diagnosed AML treated with venetoclax-based regimen as first line therapy. Furthermore, we found no differences in terms of treatment safety, EFS or RFS. Therefore, we assume that the antileukemic activity of venetoclax-based therapies is independent of the TDT. While our data do not suggest an imperative for extensive genetic testing prior to initiating AML therapy, delaying treatment, when clinically suitable and promising, may enhance outcomes for selected patients, e.g., those harboring targetable lesions and reversible comorbidities.
Supplementary Material
Acknowledgements
The authors thank all patients and caretakers for their support of the trial, and all SAL centers for their commitment in the registry.
Funding Statement
Funding: DB is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - project number 413490537 within the Kiel Clinician Scientist Program in Evolutionary Medicine (CSEM). The SAL-Trial is registered under the name “Clinical AML Registry and Biomaterial Database of the Study Alliance Leukemia” and is funded by the Technical University of Dresden.
Data-sharing statement
The data sets analyzed in this study were provided by the German SAL and TriNetX. The SAL data can be obtained from the SAL on reasoned request, the TriNetX data is available to members of the global health network.
References
- 1.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114-1124. [DOI] [PubMed] [Google Scholar]
- 2.Burchenal JH, Murphy ML, Tan CTC. Treatment of acute leukemia. Pediatrics. 1956;18(4):643-660. [PubMed] [Google Scholar]
- 3.Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 Mutation. N Engl J Med. 2017;377(5):454-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. [DOI] [PubMed] [Google Scholar]
- 6.Arber DA, Orazi A, Hasserjian RP, et al. International Consensus Classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013;121(14):2618-2626. [DOI] [PubMed] [Google Scholar]
- 9.Röllig C, Kramer M, Schliemann C, et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood. 2020;136(7):823-830. [DOI] [PubMed] [Google Scholar]
- 10.Juliusson G, Hagberg O, Lazarevic VL, Lehmann S, Höglund M. Impact of treatment delay in acute myeloid leukemia revisited. Blood Adv. 2021;5(3):787-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsouqi A, Rothenberger SD, Boyiadzis M, Lontos K. Time from diagnosis to treatment is associated with survival in patients with acute myeloid leukemia: an analysis of 55,985 patients from the National Cancer Database. Br J Haematol. 2022;199(2):256-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco S, Geng X, Korostyshevskiy V, Karp JE, Lai C. Systematic review and meta-analysis: Prognostic impact of time from diagnosis to treatment in patients with acute myeloid leukemia. Cancer. 2023;129(19):2975-2985. [DOI] [PubMed] [Google Scholar]
- 13.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. [DOI] [PubMed] [Google Scholar]
- 14.Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531. [DOI] [PubMed] [Google Scholar]
- 15.Short NJ, Nguyen D, Ravandi F. Treatment of older adults with FLT3-mutated AML: emerging paradigms and the role of frontline FLT3 inhibitors. Blood Cancer J. 2023;13(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palchuk MB, London JW, Perez-Rey D, et al. A global federated real-world data and analytics platform for research. JAMIA Open. 2023;6(2):ooad035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C-J, Hong Y-C, Kuan AS, et al. The risk of early mortality in elderly patients with newly diagnosed acute myeloid leukemia. Cancer Med. 2020;9(4):1572-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberoi S, Lehrnbecher T, Phillips R, et al. Early mortality in hyperleukocytosis in patients with acute myeloid leukemia: a systematic review and meta-analysis. Blood. 2013;122(21):2647. [Google Scholar]
- 19.Rinaldi I, Sutandyo N, Winston K. Comparison of early mortality between leukapheresis and non-leukapheresis in adult acute myeloid leukemia patients with hyperleukocytosis: a systematic review and meta-analysis. Hematology. 2022;27(1):141-149. [DOI] [PubMed] [Google Scholar]
- 20.Vachhani P, Flahavan EM, Xu T, et al. Venetoclax and hypomethylating agents as first-line treatment in newly diagnosed patients with AML in a predominately community setting in the US. Oncologist. 2022;27(11):907-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoff FW, Patel PA, Belli AJ, et al. Real-world outcomes of frontline venetoclax-based therapy in older adults with acute myeloid leukemia: an analysis utilizing EHR data. Leuk Lymphoma. 2023;64(6):1123-1128. [DOI] [PubMed] [Google Scholar]
- 22.Ucar MA, Ozet G, Koyuncu MB, et al. Real world results of venetoclax combined with hypomethylating agents in relapsed/refractory AML. Eur Rev Med Pharmacol Sci. 2021;25(21):6557-6565. [DOI] [PubMed] [Google Scholar]
- 23.Gershon A, Ma E, Xu T, et al. Early real-world first-line treatment with venetoclax plus HMAs versus HMA monotherapy among patients with AML in a predominately US community setting. Clin Lymphoma Myeloma Leuk. 2023;23(5):e222-e231. [DOI] [PubMed] [Google Scholar]
- 24.Ucciero A, Pagnoni F, Scotti L, et al. Venetoclax with hypomethylating agents in newly diagnosed acute myeloid leukemia: a systematic review and meta-analysis of survival data from real-world studies. Cancers. 2023;15(18):4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F, Panagiotakos D. Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Med Res Methodol. 2022;22(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analyzed in this study were provided by the German SAL and TriNetX. The SAL data can be obtained from the SAL on reasoned request, the TriNetX data is available to members of the global health network.