Abstract
Efanesoctocog alfa (Altuviiio,TM Sanofi-SOBI) is a B domain-deleted single-chain Factor VIII (FVIII) connected to D’D3 domain of von Willebrand Factor (vWF). Its ingenious design allows efanesoctocog alfa to operate independently of endogenous vWF and results in an outstanding 3-4 times longer half-life compared to standard and extended half-life (EHL) FVIII products. The prolonged half-life ensures sustained high levels of factor activity, maintaining normal to near-normal ranges for the majority of the week, facilitating the convenience of once-weekly administration. Efanesoctocog alfa received regulatory approval in 2023 for application in both adults and children with inherited hemophilia A in the United States and Japan. Its sanctioned use encompasses both prophylaxis and ‘on demand’ treatment for bleeding episodes. The European Medicines Agency (EMA) is currently undertaking a comprehensive review of Altuviiio.TM This comprehensive review focuses on the immunological profile of efanesoctocog alfa, a highly sophisticated new class of EHL FVIII molecule. The integration of the vWF D’D3 domain, XTEN polypeptides, and potential regulatory T-cell epitopes within various segments of efanesoctocog alfa collectively serves as a mitigating factor against the development of a neutralizing T-cell-mediated immune response. We hypothesize that such distinctive attribute may significantly reduce the risk of neutralizing antibodies, particularly in previously untreated patients. The discussion extends beyond regulatory approval to encompass the preclinical and clinical development of efanesoctocog alfa, including considerations for laboratory monitoring. The review also highlights areas that warrant further investigation to deepen our understanding of this groundbreaking therapeutic agent.
Introduction
Hemophilia A (HA) is an X-linked genetic disorder in which blood clotting ability is impaired due to a deficiency in Factor VIII (FVIII), resulting in prolonged bleeding.1 Affected individuals exhibit severe, moderate, or mild forms, categorized by circulating FVIII plasma levels.1 Bleeding severity correlates with FVIII deficiency. Joints are the primary site of bleeding, resulting in hemophilic arthropathy, characterized by irreversible joint damage, physical disability, and chronic pain.2 Prophylaxis is the sole proven method significantly reducing the risk of hemophilic arthropathy and is considered the gold standard for managing patients with frequent bleeding.3 Historical challenges, such as frequent injections and limited venous access, impeded widespread prophylaxis use in high-income countries and posed cost barriers in middle- and low-income countries. In the past 15 years, advances in HA treatment include extended halflife FVIII (EHL-FVIII), FVIII mimetics, non-factor therapies, and gene therapy.4 Recent progress has improved patient quality of life (QoL) by reducing prophylactic injection frequency and transitioning from intravenous to subcutaneous injections. However, despite these advancements, hemophilic arthropathy remains a significant complication of hemophilia in 2023.5
Efanesoctocog alfa (Altuviiio,® BIVV001, Swedish Orphan Biovitrum AB ([SOBI]-Sanofi) is a new class of rFVIII molecule with EHL. It has been specifically designed and developed to address the limitations associated with currently available FVIII concentrates.
From standard to first-generation extended-half-life Factor VIII molecules
A decade ago, with only plasma-derived or standard half-life (SHL) recombinant FVIII (rFVIII) concentrates available for treating HA, the primary clinical concern was the risk of developing inhibitors.6 Hematologists, especially when dealing with previously untreated patients (PUP), based treatment choices on the inhibitor risk associated with the available products. Conflicting study reports on inhibitor development risk for recombinant and plasma-derived FVIII products,6-8 observations of young adults discontinuing prophylaxis, treatment burden with venous access challenges, painful injections, and the time required for intravenous infusion9 collectively contributed to adherence issues and accelerated the development of novel EHL-rFVIII molecules to improve patient compliance with long-term prophylaxis.
Strategies used to prolong the half-life of rFVIII molecules include - i) covalent attachment of rFVIII to polyethylene glycol (PEGylation) which reduces interactions with clearance receptors, thereby prolonging half-life; ii) fusing rFVIII to the crystallizable fragment (Fc) part of IgG1 immunoglobulins, delaying rFVIII degradation through recycling in FcRn-bearing cells.
By contrast with EHL recombinant Factor IX molecules, which have a half-life 4 to 5 times longer than that of standard Factor IX, the application of these approaches has only been able to extend the half-life of FVIII by approximately 1.5 to 1.8 times. A comprehensive understanding of interactions between FVIII and von Willebrand Factor (vWF) is critical to appreciate the challenges posed by first-generation EHL rFVIII. Factor VIII is produced by vascular endothelial cells and hepatic sinusoidal cells.10 Most of the produced FVIII circulates bound to its chaperone protein, the large multimeric vWF11 which is synthesised by endothelial cells and megakaryo-cytes. Plasma vWF levels are in a 30 to 50-fold molar excess over FVIII. In the absence of vWF, FVIII is rapidly cleared from the circulation (with a half-life of 2 hours), as seen in type 3 vWD. By contrast, vWF-bound FVIII remains in the blood for 12 hours, vWF itself having a half-life of 12 to 15 hours.10 Recombinant FVIII molecules synthesized using PEGylation or fusion approaches retain their ability to bind vWF, and follow the degradation pathways of the transporter protein. As a result, their half-life remains driven by that of vWF. Therefore, PEGylated and fusion FVIII products exhibit nearly identical pharmacokinetic (PK) profiles in terms of half-life, clearance, area under the curve (AUC). They all fail to maintain high trough levels (>5%) with considerable inter-subject variation in all PK parameters, primarily influenced by blood group, particularly its impact on vWF levels.12
Receptors and cell types involved in the clearance of FVIII and vWF are not fully understood. Scavenger receptors involved in the clearance of FVIII, vWF or the FVIII/vWF complex include low-density lipoprotein receptor-related protein-1 (LRP1), low-density lipoprotein receptor (LDLR), asialoglycoprotein receptor (ASGPR), macrophage mannose receptor type 1 (MMR/CD106), heparan sulphate protease type 1 (HSPT1), macrophage mannose receptor type 1 (MMR/ CD206), heparan sulphate proteoglycans, sialic acid-binding IgG-like lectin 5 (Siglec5), scavenger receptor class A member 5 (SCARA5), stabilin-2 (STAB2) and C-type lectin domain family 4 member M (CLEC4M).13
Structure and synthesis of efanesoctocog alfa
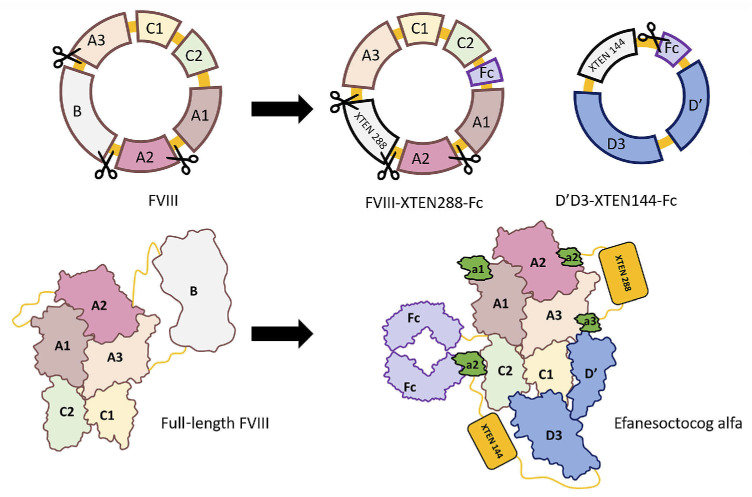
Type 2N vWD, characterized by normal plasma vWF but reduced FVIII levels, is associated with mutations in the D’D3 domains of vWF that interfere with FVIII binding.14 The vWF D’D3 domains alone are sufficient to stabilize FVIII in vivo.15 The development of efanesoctocog alfa (Efa) was based on the hypothesis that the half-life limitation imposed by vWF could be overcome by designing a rFVIII molecule unable to bind endogenous vWF. Efa is produced in HEK293 cells after transfection with three expression vectors:16 i) one encoding human BDD FVIII-XTEN-Fc; ii) one encoding vWF D1D2D’D3C1099A/C1142A domains-XTEN-Fc; and iii) one encoding PACE/furin (Figure 1). PACE/furin mediates the intracellular cleavage of the vWF D1D2 propeptide, while cis-addition of the vWF propeptide D1D2 ensures optimal folding of vWF D’D3 and highest affinity for FVIII. The resulting heterodimeric protein is purified by affinity chromatography on a FVIII-Select resin, followed by additional chromatographic steps.
XTEN, an unstructured hydrophilic recombinant polypeptide, can be customized to any molecular weight. Fusion of XTEN to therapeutic proteins enhances their PK properties.17 Efa contains 2 XTEN polypeptides: a 288 amino acid-long XTEN is located between the A2 and a3 domains of FVIII, and a 144 amino acid-long XTEN is located between the D’D3 and Fc domains. This configuration helps reduce the in vivo clearance of the molecule. In addition, a thrombin cleavage site, the FVIII acidic region a2, was inserted between the D’D3 and Fc domains of Efa. This site allows rFVIII to be released from the D’D3 domains as soon as the first traces of thrombin are generated after coagulation activation. Finally, removal of the PPVLKRHQR sequence in the N-terminal end of FVIII a3 domain eliminates the furin cleavage site between the heavy and light chains of FVIII, favoring the production of FVIII as a single polypeptide.
Preclinical development of efanesoctocog alfa
The PK profile of Efa was evaluated in FVIII knockout (KO), double FVIII and vWF KO (DKO) mice and in non-human primates. PK studies in DKO mice showed that fusion with D’D3 domains stabilizes FVIII in the absence of endogenous vWF. Observation of the same half-life in FVIII KO and DKO mice further demonstrated the absence of interaction between Efa and endogenous vWF. The calculated half-life of Efa in mice and primates showed a 3- to 4-fold increase compared to wild-type FVIII.18 The same studies in mice reported reduced blood loss similar to that of the tail clip experiment, reflecting comparable in vivo hemostatic activity between FVIII and Efa. These preclinical experiments were complemented by an in vitro study evaluating the hemostatic properties of Efa and its in vivo hemostatic capacity in the saphenous vein bleeding model.19 No difference in efficacy was observed between wild-type FVIII and Efa in terms of fibrin polymerization and fibrin resistance to fibrinolysis in the presence of tPA. The kinetics of platelet thrombus formation in the saphenous vein model was also comparable.18
Figure 1.
Structure of efanesoctocog alfa. Efanesoctocog alfa (Efa) is a heterodimeric glycoprotein produced in HEK293 cells after transfection with 3 expression vectors. These vectors include a plasmid encoding a human BDD Factor VIII (FVIII)-XTEN288-crystallizable fragment (Fc) construct, a plasmid encoding von Willebrand Factor (vWF) D1D2D’D3C1099A/C1142A domains-XTEN144-Fc, and a plasmid allowing the expression of PACE/furin. The structure of Efa includes the parental recombinant Factor VIII Fc (rFVIIIFc) with a 288 aa-long XTEN (XTEN288) integrated between the FVIII A2 and a3 domains, and the vWF D’D3 domains fused to a 114 aa-long XTEN (XTEN144) and to a Fc fragment. The C1099A and C1142A mutations in D’D3 prevent dimerization of the domain. The D1D2 and D’D3 domains are separated by a furing cleavage site. The interaction between the FVIII- XTEN288-Fc and the D’D3-XTEN144-Fc is maintained by the pairing of the 2 Fc fragments and the low affinity between D’D3 and the FVIII light chain. The heterodimers, thus, present with an avidity that exceeds the affinity of FVIII for endognous vWF in the patients’ blood, and the in vivo half-life of Efa is independent from that of the endogenous vWF.
Clinical trials: establishing the efficacy and safety of efanesoctocog alfa
The 3- to 4-fold increase in half-life observed in animal models was confirmed in an open-label phase I-IIa study, in 16 adult patients with severe HA. The geometric mean half-life of Efa was 37.6 hours for a dose of 25 IU/kg compared with 9.1 hours for standard half-life FVIII (SHL-FVIII) and 42.5 hours for a dose of 65 IU/kg compared with 13.2 hours for SHL-FVIII.20 The 8 patients who received a 65 IU/kg dose maintained a circulating FVIII level above 50% for five days. Their level of circulating FVIII was 17% at seven days and 1% 14 days after a single injection of Efa. At these time points, there was no detectable FVIII in the plasma of patients receiving the same dose of SHL-FVIII.20 A phase I repeat-dose study confirmed a mean half-life of 37-41 hours for Efa. This study also reported minimal accumulation of Efa after 4 weekly injections of 50 or 65 IU/kg. Mean AUC, obtained with doses of 65 IU/kg, was up to 7 times higher than with rFVIII (mean AUCτ 11,500 IU.h/dL in adults/adolescents).21
In phase I and II studies, none of the patients developed anti-FVIII inhibitors nor thrombotic complications. No patient treated with Efa experienced bleeding in the seven days following injection of the drug.21
Based on these results, the 50 IU/kg dose was selected for the XTEND-1 pivotal phase III study.22 This trial involved 2 arms: a group of 133 previously treated severe HA patients aged >12 years, receiving prophylaxis with a weekly dose of Efa 50 IU/kg for one year; a group of 26 similar patients receiving on demand treatment. Patients in the prophylaxis arm were on long-term prophylaxis before the study, while those in the ‘on demand’ arm had experienced at least 6 bleeds in the previous six months. The primary endpoint was the annual bleeding rate (ABR) in the prophylaxis arm, with secondary endpoints including ABR comparison on Efa prophylaxis and during the observation period, along with safety, PK, pain intensity, joint health, and QoL. The mean ABR and one-sided 97.5% Confidence Interval (CI) was estimated for prophylaxis treatment in patients who had at least six months of available efficacy data from both the prestudy and XTEND-1. Once-weekly doses of Efa 50 IU/ kg were well tolerated and no accumulation was observed after repeated infusions.
In the ‘prophylaxis’ group, plasma FVIII levels were maintained above 40 IU/dL for at least four days and at 15 IU/ dL at day 7 after the injection of Efa at steady state. Altogether, 65% of patients had zero bleeding episodes. The median ABR with Efa prophylaxis was 0 (range 0-1.04). The study demonstrated the superiority of Efa prophylaxis over SHL and EHL-FVIII products, with ABR decreasing from 2.96 (95% CI: 2.00-4.37) to 0.69 (95% CI: 0.43-1.11) (P<0.001). Patients on prophylaxis reported a reduction in chronic pain (P=0.03) and improvement in joint health as assessed by the Hemophilia Joint Health Score (HJHS) (P=0.01). All 45 target joints resolved in the 14 patients with target joints identified at baseline and at least 12 months of study prophylaxis. In the ‘on demand’ group B, 97% of bleeding events were successfully stopped with a single injection of Efa 50 IU/kg.
After 52 weeks of Efa prophylaxis, health-related QoL was evaluated using the Haem-A-QoL questionnaire and compared to baseline. Significant QoL improvements were noted in 7 of the 10 domains, including self-perception (P<0.0001), physical health (P=0.0001), work and school life (P=0.0038), sports and leisure (P=0.0006), treatment (P<0.0001), feeling (P=0.0078), and partnership & sexuality (P=0.0148).23 Post-treatment interviews with 29 patients indicated a significant improvement in physical activities and pain.24
The XTEND-Kids clinical trial (clinicaltrials.gov 04759131) demonstrated that once-weekly Efa administration was well tolerated and provided highly effective bleeding control and treatment in children with severe HA. Seventy-four previously treated boys participated to the study (<6 years old: N=38; 6-<12 years old: N=36). The mean half-life of Efa in children was 40.2 hours with a mean FVIII activity >40 IU/dL for three days, >15 IU/dL for approximately five days and >10 IU/dL for approximately seven days. The median (interquartile range) and mean (95% CI) ABR were 0.00 (0.00-1.02) and 0.89 (0.56-1.42), respectively. Most hemorrhages resolved with a single dose of 50 IU/kg. No FVIII inhibitor was detected (0%, 95% CI: 0-4.9%).25 The mean (95% CI) ABR, spontaneous ABR and joint ABR were respectively 0.61 (0.42-0.90), 0.16 (0.08-0.31), and 0.30 (0.16-0.57). Overall, 88% of the patients had no spontaneous bleed and 84% had no joint bleed. A single dose of Efa 50 IU/kg resolved 95% of bleeds in children.26
The PK characteristics of Efa and its clinical efficacy suggest its potential use in combination with emicizumab for surgery or major breakthrough bleeding episodes, aiming to maintain elevated FVIII levels with fewer injections. Given the higher affinity of FVIII for FIX and FX compared to emicizumab, the simultaneous use of Efa with emicizumab should not pose a safety issue.
Efanesoctocog alfa also has the potential to improve the QoL for girls and women with hemophilia and symptomatic carriers. This can be achieved by effectively addressing menorrhagia through a probable single monthly injection. Efa may also be considered to sustain tolerance under emicizumab prophylaxis or induce immune tolerance in immune tolerance induction (ITI) protocols.
Efanesoctocog alfa efficacy and safety for perioperative management were assessed in the XTEND-1 phase III study, involving 11 patients (10 on prophylaxis and 1 ‘on demand’) undergoing 12 procedures, including orthopedic and other major surgeries. Eleven of the procedures involved a preoperative Efa infusion at 50 IU/kg; one procedure had no reported prophylactic preoperative dose. The median (range) number of injections of Efa was 1.0 (1-2) for days 1-3 and 2.0 (2-4) for days 4-14. After the preoperative dose, follow-up infusions were of 30-50 IU/kg every 2-3 days. The hemostatic response was considered excellent in all 12 surgeries. No patient required blood transfusion and no serious adverse event was reported.27 The clinical efficacy of Efa suggests that disconnection from endogenous vWF does not have a major impact on the hemostatic potential of the molecule. A long-term safety and efficacy study of Efa (XTEND-ed trial) in HA as well as a phase I study in adults with type 2N and 3 vWD (clinicaltrials.gov 04770935) are ongoing.
Laboratory testing
Factor VIII activity can be measured by the one-step coagulation assay (OSA) and the chromogenic assay. Discrepant results between OSA and chromogenic assays have been reported with several first-generation EHL rFVIII products. Recommendations have been published to guide laboratories on the choice of appropriate assays, as laboratory results have a direct impact on dosing in clinical manage-ment.28
During Efa development, including the pivotal phase III trial, FVIII activity was measured using an OSA with Actin FSL reagent (Siemens Healthcare). A field study comparing OSA and chromogenic assays in assessing Efa FVIII activity reported varied results with different reagents. Actin FSL emerged as the optimal OSA reagent, while Actin FS showed a significant 2.5-fold overestimation and SynthASil exhibited underestimation. Most chromogenic kits demonstrated a 2-3-fold overestimation across all activity levels making reagents unsuitable for monitoring Efa.29 The study concluded that the activity of Efa can be reliably measured by the OSA using the majority of standard activated pro-thrombin time (aPTT) reagents.
Adverse events
No major safety concern or serious adverse event related to Efa were reported. Headache (20%), arthralgia (16%), falls (6%), and back pain (6%) were the most common adverse events reported in clinical trials.20-22 No serious anaphylactic or allergic reactions were observed. No inhibitor was recorded after the use of Efa in PTP. Three out of 206 subjects with pre-existing risk factors developed thrombosis during the XTEND extension study. The FDA requested increased pharmacovigilance for thromboembolic events for three years after approval.
Immunogenicity
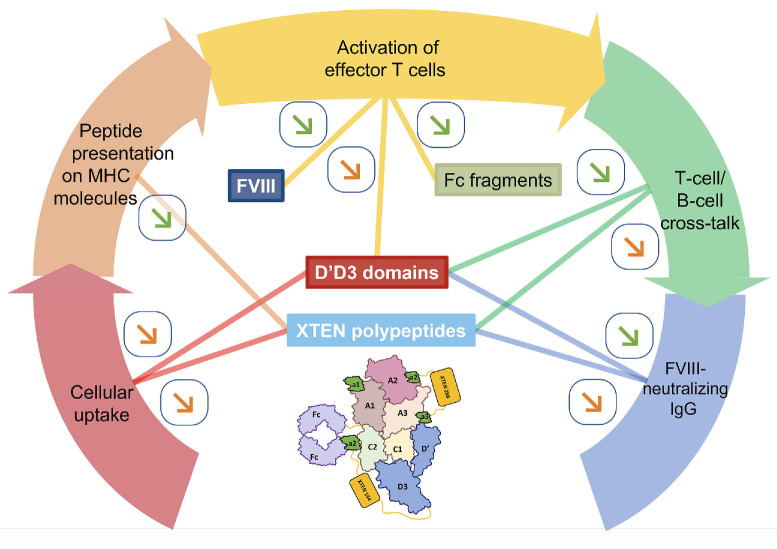
The immunogenic profile of Eloctate,® the parental Fcfused FVIII for Efa, was validated in clinical trials.30 No inhibitor development was observed in previously treated patients with severe HA:31 in PUP treated with Eloctate,® a 31.1% incidence of FVIII inhibitors was reported, consistent with other FVIII products.32 The hypothesis that Efa displays a favorable immunogenic profile is suggested by the absence of inhibitor development after the use of this highly bioengineered molecule in a large number of PTP.22 The development of the anti-FVIII immune response implicates sequential events that include the capture and endocytosis of FVIII by antigen-presenting cells (APC), the processing and presentation on MHC molecules of FVIII-derived peptides to naïve FVIII-specific T cells, the activation of effector T cells, the cross-talk between activated T cells and FVIII-specific B cells leading to B-cell maturation and differentiation into plasma cells, and the production of FVIII-neutralizing IgG. We discuss here whether the peculiar structural features of Efa confer it with a favorable immunogenic profile (Figure 2).
D’D3 domains
vWF, the chaperon for FVIII in the circulation, reduces FVIII uptake by APC. Notably, vWF interferes with the binding of FVIII with the macrophage mannose receptor (CD206) and shields charged residues in the C1 and C2 domains of FVIII, thus reducing FVIII uptake in vitro33-35 and immunogenicity in preclinical models.36 However, it is unknown whether the D’D3 domains of vWF alone can resume the protective action of the entire vWF. Conversely, the lack of the whole vWF molecule may prevent the vWF-mediated endocytosis of the vWF-FVIII complex by the scavenger receptor stabilin-2 and reduce the risk for the onset of an anti-FVIII immune response, as shown in FVIII-KO mice.37
XTEN polypeptides
XTEN polypeptides are added to therapeutic proteins to increase their hydrodynamic range, and limit their renal clearance. Increasing the hydrodynamic radius of proteins or particles may reduce their cellular uptake.17 For Efa, this could reduce the risks of endocytosis by APC and of activating T cells and initiating neutralizing immune responses. Importantly, XTEN polypeptides lack the hydrophobic residues necessary for peptide binding to MHC molecules, making them devoid of T-cell epitopes. Additionally, in Efa, neo T-cell epitopes resulting from the fusion of XTEN with the FVIII A2 and a3 domains were eliminated through mutations that do not impact FVIII function.18 The XTEN-288 is built of repeated ‘blocks’ of amino-acids: GTSESATPESGPG, SEPATSGSETP, STEPSEGSAPG, and SPAGSPTSTEEG. Repeated motifs may trigger T-cell independent immune responses, resulting in the production of low-affinity antibodies. Further investigation is required to determine if this occurs with Efa and whether the putatively induced low-affinity antibodies may affect PK or promote immune tolerance.
T-cell activation
Tregitopes are defined as epitopes that preferentially activate regulatory T cells.38 Tregitopes exhibit promiscuous HLA-DR binding, and display TCR-facing residue homology with epitopes found in other human proteins.39 Potent Tregitopes have been identified in the Fc fragment of the human IgG and in FVIII38-40 One epitope cluster with regulatory potential is predicted in the vWF D’D3 domains (personal communication, A De Groot, EpiVax, 2023). The combination of Tregitopes present in FVIII and Fc fragments is not sufficient to confer immune tolerance to rFVIIIFc, as shown by the 31% incidence of FVIII inhibitors in naïve patients treated with Eloctate®.31 Further investigation is needed to determine if adding the Tregitopes in D’D3 to those in rFVIIIFc can improve the tolerogenic profile of Efa.
Shielding of B-cell epitopes
The XTEN polypeptide between FVIII A2 and a3 domains, along with the D’D3 domains, might shield FVIII from interaction with antibodies. Similar to recent findings with vWF in FVIII-KO mice,41 this shielding could prevent the endocytosis of FVIII by naïve or memory FVIII-specific B cells, thereby mitigating the antigen-specific crosstalk between activated FVIII-specific T and B cells and reducing the risk for naïve or recall anti-FVIII immune responses.
Figure 2.
Immunological profile of efanesoctocog alfa. A classical immune response to a T-cell-dependent extracellular antigen, such as therapeutic Factor VIII (FVIII), involves: i) the uptake of the antigen by antigen-presenting cells; ii) the processing of the antigen and presentation of antigen-derived peptides on MHC class II molecules to antigen-specific naïve CD4+ T cells; iii) the activation of the T cells; iv) a cross-talk between the activated T cells and naïve B cells that express antigen-specific B-cell receptors; and v) the differentiation of the B cells into antibody-secreting cells. The figure depicts the proposed interactions of the different moieties of efanesoctocog alfa (Efa) (i.e., FVIII, crystallizable fragments [Fc], XTEN polypeptides, and von Willebrand Factor [vWF] D’D3 domains) with the different phases of the immune response. Green arrow symbols indicate a probable effect in mellowing down immunogenicity; orange arrows indicate a possible effect on immunogenicity. Thus, cellular uptake may be reduced by the virtue of the presence of the XTEN polypeptides and the vWF D’D3 domains. XTEN polypeptides are not presented on MHCII molecules to T cells. FVIII and Fc fragments contain Tregitopes that may reduce, at least in part, the immunogenicity of the molecule, particularly if D’D3 were found to also contain Tregitopes. The XTEN polypeptides and the D’D3 domains may also reduce the binding of FVIII to the B-cell receptor of FVIII-specific naïve and memory B cells, thus reducing the T/B-cell cross-talk. IgG: immunoglobulin G.
Practical concerns and questions
Although only a single weekly infusion is required, intravenous infusion can be difficult in infants and patients with very limited venous access. In the era of subcutaneous non-factor therapies, will patients opt for intravenous treatment? In addition, several hemostatic properties of Efa require further clarification. The potential risk of thrombosis related to Efa in patients with diffuse atherosclerosis is unknown, only 3% of patients in XTEND-1 study were over 65 years of age. Questions have emerged concerning the unique PK of this molecule. Despite elevated trough levels ranging 15-17%, and an extended half-life of 43.3 hours, no accumulation was noted following repeated doses of Efa. To gain a comprehensive understanding of these findings, additional research is needed. This includes investigating FVIII antigen levels after repeated doses and assessing FVIII:C measurements using various reagents. Moreover, comprehensive pharmacodynamic studies employing global hemostasis assays may be helpful for a more thorough analysis of the observed results. Additionally, monitoring Efa in emicizumab-treated patients and investigating inhibitors in its presence present challenges that call for new studies.
How efanesoctocog alfa compares with other HA treatments?
Efanesoctocog alfa, the first rFVIII with an EHL rate 3-4 times that of existing EHL FVIII products, achieves normal FVIII levels for at least half a week. Its unique PK properties provide hemostatic activity equivalent to emicizumab (10-15 IU/dL) just before a weekly injection. This marks the beginning of a new era in FVIII replacement therapy. Recent advancements in non-factor therapies, have substantially enhanced key efficacy indicators like ABR, potentially achieving zero joint bleeding. Efa, in addition to offering efficacy comparable to non-factor therapies, stands out as a ‘true’ FVIII, suitable for both prophylaxis and effective treatment of acute hemorrhagic events. Efa activity can be easily monitored by commonly available laboratory tests.29 Another notable feature of the molecule is its low PK variability, which improves the predictability of FVIII levels over time. Consequently, the use of a standard dose of 50 IU/kg reduces the need for frequent monitoring of FVIII activity.42
Patients with hemophilia have an increased rate of bone resorption and an excess of osteoporosis. FVIII may affect bone metabolism through at least two pathways, by promoting bone formation through a thrombin-mediated mitogenic effect on osteoblasts, and by interfering with the RANK/RANKL/OPG pathway. The use of FVIII prophylaxis from early childhood can maintain normal bone mineral density in individuals with severe HA.43 However, the potential impact of non-factor therapies on bone and joint health remains uncertain. Although clinically relevant improvements in the HJHS were observed in HAVEN-3, similar to XTEND-1,22,44 long-term data on the effects on bone and joint health are still needed.
Subcutaneous non-factor therapies raise concerns about patient autonomy during acute bleeding episodes. Those accustomed to factor prophylaxis can self-administer intravenous injections, but those untrained or not using intravenous injections may face treatment delays, relying on healthcare facilities. Efa, being an intravenous rFVIII, supports patient self-treatment, preserving autonomy. A recent study indirectly compared the efficacy of hemophila B gene therapy with 3 EHL-FIX molecules and showed lower bleeding rates with gene therapy compared to EHL-FIX products. In addition, more patients achieved zero bleeding with gene therapy.45 Efa stands out as the only FVIII concentrate that provides circulating FVIII levels comparable to gene therapy, facilitated by its weekly injection schedule.
These 2 therapies represent a revolutionary leap forward in the treatment of HA, surpassing the conventional threshold of >3-5 IU/dL for trough levels. They signal a shift towards normalizing FVIII levels, granting patients the opportunity to embrace a lifestyle comparable to that of the general population, engaging in everyday activities with newfound freedom.
Limiting their assessment solely to ABR and its derivatives would be limiting, failing to capture the profound changes these therapies can make in patients’ daily lives. Beyond mere numerical metrics, their impact extends to tangible improvements like reduced school and work absenteeism, enhanced socio-professional integration, newfound freedom in daily and sports activities, diminished constraints associated with frequent injections, and a notable boost in self-esteem and self-confidence.
In this era of transformative therapies, it becomes imperative to forge and validate novel criteria capable of effectively measuring the life-altering influence of these treatments. Going beyond the numbers, it is about recognizing and embracing the holistic improvements that extend far beyond the confines of traditional evaluation metrics.
Acknowledgments
We wish to thank Annie De Groot and William Martin (Epivax; Providence, RI, USA), for performing Tregitope prediction on the D’D3 amino-acid sequence present in Efa.
Funding Statement
Funding: SLD and ARR are supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Sorbonne Université, Université de Paris Cité, and funded by the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement n. 859974 (EDUC8) and by grants from Sanofi-Genentech (Waltham, MA, USA) and Swedish Orphan Biovitrum AB (Höllviksnäs, Sweden). ARR was the recipient of a fellowship from MSCA-ITN EDUC8 (n. 859974).
References
- 1.Mannucci PM, Franchini M. Haematology clinic: haemophilia A. Hematology. 2014;19(3):181-182. [DOI] [PubMed] [Google Scholar]
- 2.Lafeber FP, Miossec P, Valentino LA. Physiopathology of haemophilic arthropathy. Haemophilia. 2008;14(Suppl 4):3-9. [DOI] [PubMed] [Google Scholar]
- 3.Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125(13):2038-2044. [DOI] [PubMed] [Google Scholar]
- 4.Mannucci PM. Hemophilia treatment innovation: 50 years of progress and more to come. J Thromb Haemost. 2023;21(3):403-412. [DOI] [PubMed] [Google Scholar]
- 5.Gualtierotti R, Solimeno LP, Peyvandi F. Hemophilic arthropathy: current knowledge and future perspectives. J Thromb Haemost. 2021;19(9):2112-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of Factor VIII and neutralizing antibodies in hemophilia A. New Engl J Med. 2016;374(21):2054-2064. [DOI] [PubMed] [Google Scholar]
- 7.Gouw SC, van der Bom JG, Ljung R, et al. Factor VIII products and inhibitor development in severe haemophilia A. N Engl J Med. 2013;368(3):231-239. [DOI] [PubMed] [Google Scholar]
- 8.Fischer K, Carcao M, Male C, et al. Different inhibitor incidence for individual factor VIII concentrates in 1076 previously untreated patients with severe hemophilia A: data from the PedNet cohort. J Thromb Haemost. 2023;21(3):700-703. [DOI] [PubMed] [Google Scholar]
- 9.Brod M, Bushnell DM, Neergaard JS, Waldman LT, Busk AK. Understanding treatment burden in hemophilia : development and validation of the Hemophilia Treatment Experience Measure (Hemo-TEM). J Patient Rep Outcomes. 2023;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pipe SW, Montgomery RR, Pratt KP, Lenting PJ, Lillicrap D. Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A. Blood. 2016;128(16):2007-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlot AJ, Koppelman SJ, van den Berg MH, Bouma BN, Sixma JJ. The affinity and stoichiometry of binding of human factor VIII to von Willebrand factor. Blood. 1995;85(11):3150-3157. [PubMed] [Google Scholar]
- 12.Carcao MD, Chelle P, Clarke E, et al. Comparative pharmacokinetics of two extended half-life FVIII concentrates (Eloctate and Adynovate) in adolescents with hemophilia A: is there a difference? J Thromb Haemost. 2019;17(7):1085-1096. [DOI] [PubMed] [Google Scholar]
- 13.van der Flier A, Liu Z, Tan S, et al. FcRn rescues recombinant Factor VIII Fc fusion protein from a VWF independent FVIII clearance pathway in mouse hepatocytes. PLoS One. 2015;10(4):e0124930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazurier C, Goudemand J, Hilbert L, Caron C, Fressinaud E, Meyer D. Type 2N von Willebrand disease: clinical manifestations, pathophysiology, laboratory diagnosis and molecular biology. Best Pract Res Clin Haematol. 2001;14(2):337-347. [DOI] [PubMed] [Google Scholar]
- 15.Yee A, Gildersleeve RD, Gu S, et al. A von Willebrand factor fragment containing the D’D3 domains is sufficient to stabilize coagulation factor VIII in mice. Blood. 2014;124(3):445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller JR, Knockenhauer KE, Leksa NC, Peters RT, Batchelor JD. Molecular determinants of the factor VIII/von Willebrand factor complex revealed by BIVV001 cryo-electron microscopy. Blood. 2021;137(21):2970-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schellenberger V, Wang CW, Geething NC, et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat Biotechnol. 2009;27(12):1186-1190. [DOI] [PubMed] [Google Scholar]
- 18.Seth Chhabra E, Liu T, Kulman J, et al. BIVV001, a new class of factor VIII replacement for hemophilia A that is independent of von Willebrand factor in primates and mice. Blood. 2020;135(17):1484-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demers M, Aleman MM, Kistanova E, Peters R, Salas J, Seth Chhabra E. Efanesoctocog alfa elicits functional clot formation that is indistinguishable to that of recombinant factor VIII. J Thromb Haemost. 2022;20(7):1674-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konkle BA, Shapiro AD, Quon DV, et al. BIVV001 fusion protein as Factor VIII replacement therapy for hemophilia A. N Engl J Med. 2020;383(11):1018-1027. [DOI] [PubMed] [Google Scholar]
- 21.Lissitchkov T, Willemze A, Katragadda S, Rice K, Poloskey S, Benson C. Efanesoctocog alfa for hemophilia A: results from a phase 1 repeat-dose study. Blood Adv. 2022;6(4):1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Drygalski A, Chowdary P, Kulkarni R, et al. Efanesoctocog alfa prophylaxis for patients with severe hemophilia A. N Engl J Med. 2023;388(4):310-318. [DOI] [PubMed] [Google Scholar]
- 23.Wilson A, Nemes L, Quon DV, et al. Efanesoctocog alfa prophylaxis improves health-related quality of life in patients with hemophilia A: results from the XTEND-1 phase 3 study. Haemophilia. 2023;29(Suppl 1):105. [Google Scholar]
- 24.Wilson A, Kragh N, DiBenedetti D, et al. Patient experience with efanesoctoocg alfa: results from the xtend-1 phase 3 clinical trial exit interviews in patients with severe haemophilia A. Haemophilia. 2023;29 (Suppl 1):137. [Google Scholar]
- 25.Malec L, Peyvandi F, Chan A, et al. Efanesoctocog alfa prophylaxis for previously treated patients <12 years of age with severe hemophilia A. Res Pract Thromb Haemost. 2023;7(Suppl 2):1-2. [Google Scholar]
- 26.Malec L, Dunn A, Carcao M, et al. Treatment of bleeding episodes with efanesoctoctocog alfa in children with severe hemophilia A in XTEND-Kids phase 3 Study. Blood. 2023;142(Suppl 1):3993. [Google Scholar]
- 27.Klamroth R, von Drygalski A, Hermans C, et al. Perioperative management with efanesoctocog alfa in patients with haemophilia A in the phase 3 XTEND-1 study. Haemophilia 2023;29(Suppl 1):87-88. [Google Scholar]
- 28.Kitchen S, Tiefenbacher S, Gosselin R. Factor activity assays for monitoring extended half-life FVIII and FIX replacement therapies. Sem Thromb Haemost. 2017;43(3):331-337. [DOI] [PubMed] [Google Scholar]
- 29.Pipe S, Sadeghi-Khomami A, Konkle BA, et al. A global comparative field study to evaluate the factor VIII activity of efanesoctocog alfa by one-stage clotting and chromogenic substrate assays at clinical haemostasis laboratories. Haemophilia. 2024;30(1):214-223 [DOI] [PubMed] [Google Scholar]
- 30.Hermans C, Mancuso ME, Nolan B, Pasi KJ. Recombinant factor VIII Fc for the treatment of haemophilia A. Eur J Haematol. 2021;106(6):745-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahlangu J, Powell jS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Königs C, Ozelo MC, Dunn A, et al. First study of extended half-life rFVIIIFc in previously untreated patients with hemophilia A: PUPs A-LONG final results. Blood. 2022;139(26):3699-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasgupta S, Navarrete AM, Bayry J, et al. A role for exposed mannosylations in presentation of human therapeutic self-proteins to CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2007;104(21):8965-8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangadharan B, Ing M, Delignat S, et al. The C1 and C2 domains of blood coagulation factor VIII mediate its endocytosis by dendritic cells. Haematologica. 2017;102(2):271-281.27758819 [Google Scholar]
- 35.Delignat S, Rayes J, Dasgupta S, et al. Removal of mannoseending glycan at Asn2118 abrogates FVIII presentation by human monocyte-derived dendritic cells. Front Immunol. 2020;11:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delignat S, Dasgupta S, Andre S, et al. Comparison of the immunogenicity of different therapeutic preparations of human factor VIII in the murine model of hemophilia A. Haematologica. 2007;92(10):1423-1426. [DOI] [PubMed] [Google Scholar]
- 37.Swystun LL, Lai JD, Notley C, et al. The endothelial cell receptor stabilin-2 regulates VWF-FVIII complex half-life and immunogenicity. J Clin Invest. 2018;128(9):4057-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Groot AS, Moise L, McMurry JA, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes.” Blood. 2008;112(8):3303-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moise L, Gutierrez AH, Bailey-Kellogg C, et al. The two-faced T cell epitope: examining the host-microbe interface with JanusMatrix. Hum Vaccines Immunother. 2013;9(7):1577-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Groot AS, Rosenberg AS, Miah SMS, et al. Identification of a potent regulatory T cell epitope in factor V that modulates CD4+ and CD8+ memory T cell responses. Clin Immunol. 2021;224:108661. [DOI] [PubMed] [Google Scholar]
- 41.Oleshko O, Vollack-Hesse N, Tiede A, Hegermann J, Curth U, Werwitzke S. von Willebrand factor modulates immune complexes and the recall response against factor VIII in a murine hemophilia A model. Blood Adv. 2023;7(21):6771-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bioverative Therapeutics Inc. Efanesoctocog alfa US prescribing information. 2023; https://www.altuviiio.com Accessed 7 March 2023. [Google Scholar]
- 43.Khawaji M, Akesson K, Berntorp E. Long-term prophylaxis in severe haemophilia seems to preserve bone mineral density. Haemophilia. 2009;15(1):261-266. [DOI] [PubMed] [Google Scholar]
- 44.Kiialainen A, Niggli M, Kempton CL, et al. Effect of emicizumab prophylaxis on bone and joint health markers in people with haemophilia A without factor VIII inhibitors in the HAVEN 3 study. Haemophilia. 2022;28(6):1033-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klamroth R, Bonner A, Gomez K, et al. Indirect treatment comparisons of the gene therapy etranacogene dezaparvovec versus extended half-life factor IX therapies for severe or moderately severe haemophilia B. Haemophilia. 2024;30(1):75-86. [DOI] [PubMed] [Google Scholar]