Venetoclax (Ven) in combination with a hypomethylating agent (HMA) is the standard first-line treatment for elderly and unfit patients with newly-diagnosed acute myeloid leukemia (AML), after the phase III VIALE-A trial demonstrated superior complete remission (CR) with or without count recovery (CRi) rates and overall survival (OS) with azacitidine-Ven in comparison to azacitidine monotherapy. Despite superior CR/CRi rates observed in the VIALE-A study, primary refractory disease or relapse was documented in 42% of patients receiving azacitidine-Ven.1 In this regard, we have recently underlined the dismal outcome of patients with newly-diagnosed AML following failure of Ven-HMA therapy, and reported a median survival of ~3 months, which was significantly inferior in the presence of TP53, K/NRAS, or ASXL1 mutations. Importantly, in this particular study, only 11 of 71 (15%) patients received subsequent salvage therapy.2 Similarly, another study of 41 patients with relapsed/refractory AML following frontline Ven-HMA also demonstrated a median OS of 2.3 months, with 24 (59%) patients receiving salvage therapy.3 The aforementioned studies highlight the dire need for additional salvage regimens to extend survival and ensure optimal transplant eligibility, particularly for patients without targetable mutations. A recent phase II study, with cladribine, low-dose cytarabine (Ara-C) and venetoclax (CAV), alternating with azacitidine, in older patients with newly-diagnosed AML, demonstrated a CR/CRi rate of 93%.4 In routine practice, CAV is used as salvage therapy in patients with relapsed/ refractory AML lacking targetable mutations and ineligible for intensive therapy, although efficacy data with this regimen following Ven-HMA is unknown. Accordingly, we sought to determine the efficacy of CAV as a salvage regimen in AML patients following failure of Ven-HMA, including clinical and molecular predictors associated with treatment response and survival.
Under an institutional review board-approved minimum risk protocol, the Mayo Clinic (MN, AZ, FL) database was searched to identify patients with AML who progressed after treatment with Ven-HMA and subsequently received at least one cycle of CAV, outside of clinical trials, between April 2020 and April 2023. Patients received cladribine 5 mg/m2 on days 1-5, low-dose Ara-C, 20 mg/m2 twice daily on days 1-10, and Ven 100-400 mg daily, dose-adjusted based on anti-fungal prophylaxis, on days 1-21, according to treating physician discretion. CAV was administered in the inpatient setting in 17 patients, inpatient followed by outpatient after day 5 in ten, after day 6 in five, after day 7 in four, after day 4 and day 2 in one patient each, and all outpatient in one patient. All patients received anti-bacterial and anti-viral prophylaxis, on the other hand, azole and pneumocystis prophylaxis was administered in 38 (97%) and 21 (54%) of patients, respectively. Bone marrow biopsy, conventional karyotyping, and next-generation sequencing via a 4-, 11-, or 48-gene panel were collected at the discretion of the treating physician, and in most cases occurred at the time of diagnosis. The 2022 European LeukemiaNet (ELN) criteria5 were applied to define disease risk and response to treatment. Significance testing for covariates associated with response was performed via χ2 or Fischer’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Follow-up was updated in December 2023 and survival calculated from the time of CAV treatment to last follow-up or death, and group differences were assessed with the log-rank test. JMP Pro 16.0.0 software package, SAS Institute, Cary, NC was used for statistical analysis.
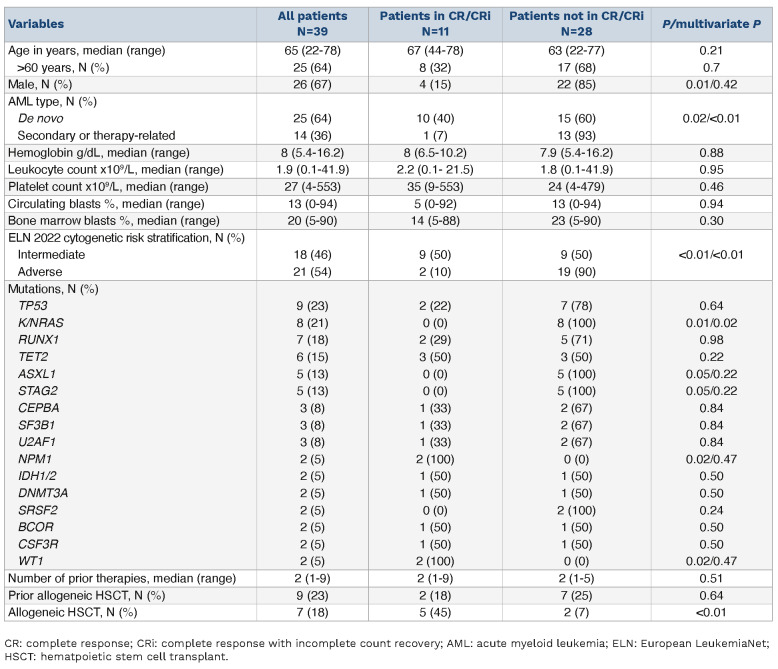
A total of 39 AML patients (median age 65 years; range, 22-78; 67% male; 64% de novo) relapsed (54%, N=21) or refractory (46%, N=18) to prior chemotherapy, which included Ven-HMA, received CAV (median of 1 cycle; range, 1-5 cycles). A majority (87%, N=34) of these patients had failed or relapsed from Ven-HMA as their most recent line of therapy. Patients had received one (N=16), two (N=14), three (N=5), four (N=2), five (N=1) or nine (N=1) prior therapies, including a median of three cycles of Ven-HMA (range, 1-14 cycles). Nine (23%) patients relapsed following allogeneic hematopoietic stem cell transplant (AHSCT). Median duration of remission in relapsed patients was 7.04 months (range, 1.2-70.1 months). ELN cytogenetic risk at diagnosis included intermediate (46%, N=18), and adverse (54%, N=21). Mutations at diagnosis involved TP53 in nine (23%), K/NRAS in eight (21%), RUNX1 in seven (18%), TET2 in six (15%), ASXL1 in five (13%), STAG2 in five (13%), and IDH1/2 in two (5%) patients, one of whom had received ivosidenib. Eleven (28%) patients achieved CR (5%, N=2) or CRi (23%, N=9); median time to response was 1 month and median response duration was 4.4 months. In addition, one patient achieved partial remission (PR) and two patients had bone marrow aplasia not fulfilling criteria for morphologic leukemia-free state (MLFS). Measurable residual disease (MRD) was negative by multiparametric flow cytometry in two of seven (29%) informative CR/CRi cases. CR/CRi rates were higher in females (54% vs. 15%; P=0.01), de novo versus secondary AML (40% vs. 7%; P=0.02), absence versus presence of adverse karyotype (50% vs. 9%; P<0.01), and absence versus presence of K/NRAS (35% vs. 0%; P=0.01), ASXL1 (32% vs. 0%; P=0.05), and STAG 2 mutations (32% vs. 0%; P=0.05) (Table 1). Multivariable analysis confirmed superior response in de novo AML (P<0.01), absence of adverse karyotype, (P<0.01), and absence of K/NRAS mutations (P=0.02). Notably, CR/ CRi rates were not impacted by relapsed versus refractory disease (29% vs. 28%; P=0.95), exposure to more than one prior line of therapy (25% vs. 33%; P=0.58), prior AHSCT (22% vs. 30%; P=0.64), or TP53 mutations (22% vs. 30%; P=0.64) (Table 1). Five (13%) of patients had monocytic leukemia, CR/CRi rates were 40% versus 26% in patients with versus without monocytic leukemia (P=0.54). The most common treatment-emergent adverse event was infection (51%, N=20), comprising bacteremia (N=11), bacterial pneumonia (N=10), and abscesses (N=6). Of these infections, 85% were grade 3 or higher, including two fatal episodes of septic shock, involving an E. faecium peri-rectal abscess and P. aeruginosa pneumonia. None of the patients developed pneumocystis pneumonia. Less frequent toxicities included liver dysfunction (18%, N=7, of which 3 were grade 3) and tumor-lysis syndrome (2%, N=1, grade 3).
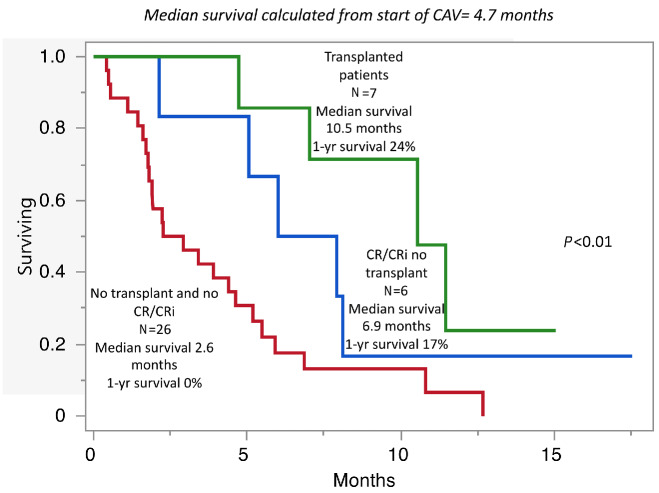
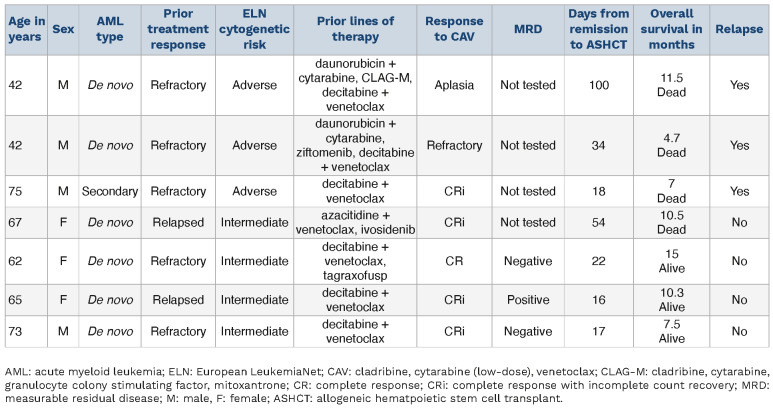
At a median follow-up of 4.7 months (range, 0.4-17.5 months) from the initiation of CAV, 34 deaths (87%), seven relapses (18%), and seven AHSCT (18%) were documented. Five patients remain alive and are disease-free at the time of this writing, three of whom underwent AHSCT, while one patient each are in CR/CRi and in MLFS for 17.5 and 8.3 months, respectively. Median survival following CAV was 4.7 months, and superior in patients achieving CR/CRi (8.1 vs. 3.2 months; P=0.01) and in patients receiving AHSCT (10.5 vs. 3.7 months; P=0.01). Figure 1 illustrates survival differences in patients that underwent AHSCT, patients achieving CR/CRi but not transplanted, and patients not achieving CR/CRi, with respective median survival of 10.5, 6.9, and 2.6 months (P<0.01). Absence of adverse cytogenetic risk was also associated with superior survival (6 vs. 3.4 months; P=0.05). Multivariable analysis confirmed the favorable survival impact of CR/CRi (P=0.03) and AHSCT (P=0.05); whereas survival impact was not apparent for secondary AML (P=0.29), K/NRAS (P= 0 .7 0) or TP53 (P=0.10) mutations. On the other hand, there was a trend towards inferior survival in nine patients with prior AHSCT; median survival 1.9 versus 4.9 months (P=0.10). Of the seven patients (18%) receiving AHSCT following CAV, five patients were in CR/CRi before proceeding to transplant, one achieved bone marrow aplasia, and one case was refractory to CAV but achieved a CRi with mitoxantrone + etoposide + cytarabine (MEC) (Table 2).
Table 1.
Clinical characteristics at time of treatment with cladribine, Ara-C (low-dose), venetoclax for 39 patients with acute myeloid leukemia, relapsed/refractory to venetoclax and hypomethylating agent stratified by achievement of complete response or complete response with incomplete count recovery.
Similar results were obtained when response and survival analysis was restricted to 34 patients who had received VenHMA as the most recent line of therapy. CR/CRi was documented in eight (24%) patients, death in 30 (88%), AHSCT in five (15%) and relapse in six (18%) cases; median survival was 4.7 months. Moreover, in 16 patients who received CAV as first salvage therapy, CR/CRi was noted in five (31%) patients, with death in 12 (75%), AHSCT in three (19%) and relapse in two (13%) patients. Median survival was 6.4 months.
The current study demonstrates that the CAV regimen can induce remissions in Ven-HMA- relapsed/refractory AML, albeit at expectedly lower CR/CRi rates in comparison to its efficacy in treatment-naïve AML (CR/CRi rate of 28% vs. 93%).4 Prior studies examining outcomes of AML patients relapsed/refractory to Ven-HMA reported CR/CRi rates of 21%3 and 27%2 following a number of salvage regimens including intensive chemotherapy and FLT3/IDH1/2 targeted agents. In the current study, only two patients harbored IDH1/2 mutations, one of whom had previously received ivosidenib. A phase II trial (clinicaltrials gov. Identifier: NCT05190549) investigating the use of CAV in relapsed/ refractory AML (N=30) reported CR/CRi rate of 27% and 1-year OS of 60%.6 In contrast to the present study which featured elderly patients with heavily treated, high-risk disease, the clinical trial enrolled a younger patient population (median age, 39.5 years) with the majority (63%) of patients with ELN 2017 favorable/intermediate risk.
In the current study, de novo AML, absence of adverse karyotype, and absence of K/NRAS mutation were associated with a higher likelihood of response. In that regard, detailed single-cell DNA sequencing analysis of patient samples preand post-treatment with Ven-based regimens, including Ven-HMA and Ven-LDAC, demonstrated frequent acquired mutations in kinase activating pathways, specifically FLT3 and RAS.7 The frequency of K/NRAS mutations in our cohort was 21%, slightly higher than the general prevalence of K/ NRAS mutations in newly diagnosed AML (10-15%).8 Thus, RAS pathway activating mutations may represent a form of acquired resistance to BCL2 inhibition and predict lower response to salvage therapy. The infectious complications observed in our study were comparable to those previously reported in the phase 2 study of CAV alternating with azacitidine in newly-diagnosed AML.4
Figure 1.
Median survival following treatment with cladribine, cytarabine (low-dose), venetoclax in 39 patients with acute myeloid leukemia, relapsed/refractory to venetoclax and hypomethylating agent, stratified by achievement of complete response or complete response with incomplete count recovery and allogeneic transplantation. CAV: cladribine, cytarabine (low-dose), venetoclax; CR: complete response; CRi: complete response with incomplete count recovery; yr: year.
Table 2.
Clinical characteristics and survival of seven patients with venetoclax/hypomethylating agent-relapsed/refractory acute myeloid leukemia receiving allogeneic stem cell transplant following salvage treatment with cladribine, cytarabine (low-dose), and venetoclax.
Survival following CAV was marginally superior at 4.7 months than previously reported survival rates in AML patients following Ven-HMA failure not receiving salvage therapy (median OS ~ 3 months), although there are likely confounders, such as selection of relatively fit patients to receive additional lines of therapy, which preclude comparison. Notably, survival was superior in patients achieving CR/CRi following CAV, particularly in patients bridged to AHSCT. Our findings suggest the CAV regimen, while associated with a high risk of infectious complications, offers a therapeutic option for patients without targeted treatment options after failure of Ven-HMA and has salvage value as a bridge to AHSCT. The current study underlines efficacy of the CAV regimen in the setting of Ven-HMA failure, suggesting that all such cases may not be due to Ven resistance. On the other hand, in the instance of treatment resistance, the use of Ven has unveiled a new type of leukemia stem cell designated as monocytic leukemia stem cell which is resistant to Ven-azacitidine and addition of cladribine to the Ven-azacitidine regimen has been shown to eradicate these stem cells in both in vitro and in vivo preclinical models.9 Next steps include incorporation of cladribine to Ven-HMA in the front-line setting with the goal to improve remission rates and reduce relapses.
Data-sharing statement
Please email the corresponding author.
References
- 1.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. [DOI] [PubMed] [Google Scholar]
- 2.Gangat N, Ilyas R, Johnson IM, et al. Outcome of patients with acute myeloid leukemia following failure of frontline venetoclax plus hypomethylating agent therapy. Haematologica. 2023;108(11):3170-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiti A, Rausch CR, Cortes JE, et al. Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica. 2021;106(3):894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadia TM, Reville PK, Wang X, et al. Phase II study of venetoclax added to cladribine plus low-dose cytarabine alternating with 5-azacitidine in older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2022;40(33):3848-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Ge S, Huang Y, et al. Efficacy and safety of cladribine, low-dose cytarabine and venetoclax in relapsed/refractory acute myeloid leukemia: results of a pilot study. Blood Cancer J. 2024;4(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera D, Kim K, Kanagal-Shamanna R, et al. Implications of RAS mutational status in subsets of patients with newly diagnosed acute myeloid leukemia across therapy subtypes. Am J Hematol. 2022;97(12):1599-1606. [DOI] [PubMed] [Google Scholar]
- 9.Pei S, Shelton I, Gillen A, et al. A novel type of monocytic leukemia stem cell revealed by the clinical use of venetoclax-based therapy. Cancer Discov. 2023;13(9):2032-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please email the corresponding author.