Kidney injury is a common complication of multiple myeloma (MM),1 with light chain cast nephropathy (LCCN) being a well described MM-defining event correlated with poor outcomes.2 LCCN results from the precipitation of monoclonal free light chains (FLC) with Tamm-Horsfall protein in distal tubules.2 Kidney involvement in other mature B-cell neoplasms with plasmacytic differentiation is much rarer than in MM.1 When present it results mainly from monoclonal heavy or light chain deposition in the glomerular basement membrane, such as monoclonal immunoglobulin deposition disease (MIDD), interstitial infiltration of neoplastic lymphoplasmacytic cells, light chain amyloidosis or LCCN.3,4 In the light of the work by Royal et al.,2 who described the clinicopathological predictors of renal outcomes in MM-associated LCCN, we have set out to describe the clinical, biological, and pathological presentation of LCCN in non-MM mature B-cell neoplasms. We also aim to compare the clinicopathological presentations of MM and non-MM-associated LCCN and understand whether both diseases have similar renal manifestations and outcomes.
Patients were selected from the renal biopsy databases of the Pathology Departments of five hospitals. Research ethics board approval was granted by the local Ethics Committee of the Assistance Publique Hôpitaux de Paris. Patients were informed about the purpose of the study and gave their consent to participation. Renal biopsy samples for light microscopy and immunofluorescence were processed as standard and sections were independently reviewed by two pathologists. Pathology variables and scoring definitions were categorized in the same manner as used by Royal and collaborators2 to enable the comparison between MM and non-MM LCCN. Continuous variables were described using mean, median, and interquartile range (IQR) values and categorical variables were described by frequencies and percentages. A Mann-Whitney test was used to compare the medians of continuous variables, a Fisher exact test was performed to compare groups and the Kaplan-Meier method was implemented to analyze overall survival. The Pearson correlation coefficient was used to measure the statistical relationship between two continuous variables.
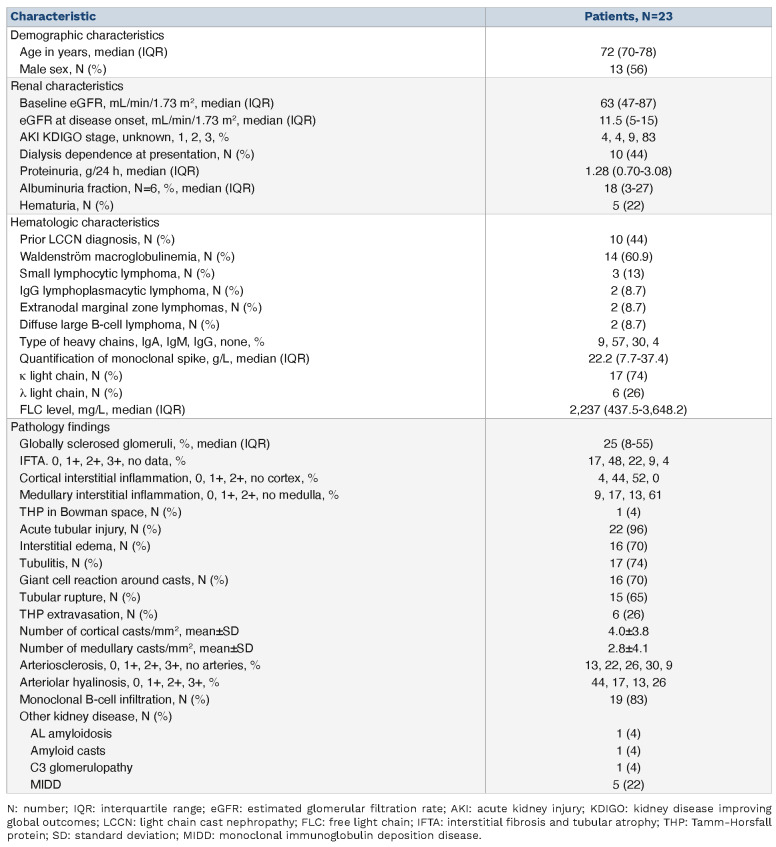
A total of 23 patients with biopsy-proven non-MM-associated LCCN were included. Their demographic, clinical, and histopathological characteristics are shown in Table 1. The median age of the patients was 72 (70-78) years and 56% (n=13) of the patients were male. Fourteen patients were diagnosed with IgM lymphoplasmacytic lymphoma/Waldenström macroglobulinemia, two with IgG lymphoplasmacytic lymphoma, two with extranodal marginal zone lymphomas with plasmacytic differentiation, three with small lymphocytic lymphoma and two with diffuse large B-cell lymphoma. Baseline estimated glomerular filtration rate (eGFR), defined as renal function prior to LCCN and calculated using the Chronic Kidney Disease Epidemiological Collaboration equation (CKD-EPI), was 63 (47-87) mL/min/1.73 m2 and eGFR at kidney disease onset was 11.5 (5-15) mL/min/1.73 m2.5 Median proteinuria was 1.28 (0.70-3.08) g/g and 22% (n=5) of the patients had hematuria at presentation. Eighty-three percent (n=19) of the patients presented with Kidney Disease Improving Global Outcomes (KDIGO) stage 3 acute kidney injury, and 44% (n=10) needed dialysis at disease onset. The mean level of FLC at diagnosis was 2,237 (437.5-3,648.2) mg/L and none of the patients underwent extracorporeal removal of FLC. Our results show that non-MM neoplasms have a similar clinical presentation as that of MM.2 Acute kidney injury KDIGO stage 3 was the most common clinical presentation in both entities (82% in MM and 83% in non-MM), with almost half of the patients needing dialysis at disease onset (47% in the MM group and 44% in the non-MM group).2 It is noteworthy that FLC levels at diagnosis in non-MM LCCN appear to be lower than those in LCCN associated with MM (2,237 vs. 5,010 mg/L).2
For 13 patients (57%), the diagnoses of both the LCCN and the hematologic malignancy were concomitant and in the other ten patients (43%) the LCCN diagnosis was made 9.7 (4.4-14.3) years after the diagnosis of the hematologic malignancy when this latter relapsed or progressed. In contrast to MM, in which cast nephropathy is most often revealed at the time of the hematologic diagnosis (92% of the cases before first-line therapy),2 in other B-cell neoplasms in half the cases cast nephropathy may be an event occurring during follow-up and even several years after the initial diagnosis of the hematologic malignancy. This result highlights the need for regular and longitudinal assessment of renal function of patients with mature B-cell neoplasms with plasmacytic differentiation.
The main kidney pathological findings are summarized in Table 1 and illustrated in Figure 1. The median number of casts per square millimeter was 2.50 (1.18-4.70) in the cortex, 1.25 (0.04-5.17) in the medulla, and 2.35 (1.24-4.70) in the entire kidney biopsy, which was close to the findings of Royal et al. (3.2/mm2 in the cortex).2 The median percentage of globally sclerosed glomeruli was 25% (8-55) and almost half of the patients (48%, n=11) had mild interstitial fibrosis and tubular atrophy (IFTA). All except one patient had acute tubular injury. Most of the patients had interstitial edema (70%, n=16), tubulitis (74%, n=17), giant cell reaction around the casts (70%, n=16) and tubular rupture (65%, n=15), as previously described in MM LCCN.2 Only 26% (n=6) of the patients displayed extravasation of Tamm-Horsfall protein. As myeloma casts are known to be formed through binding to uromodulin, we performed an immunohistochemistry analysis targeting uromodulin.6 In our patients, the observed monotypic light chain casts were also associated with uromudulin as in MM LCCN (Online Supplementary Figure S1). Ninety-six percent (n=22) of the patients had a cortical interstitial lymphoid infiltrate and 30% (n=7) had medullary interstitial lymphoid infiltrate. Importantly, this infiltration was in most cases due to a monoclonal B-cell infiltration, observed in 83% (n=19) of the patients. In contrast, in the cohort of 178 patients with MM, only 1.7% (n=3) had interstitial infiltration by neoplastic cells.2 It should be noted that the extent of the tumor infiltration may be such that it overrides LCCN, especially considering the presence of other conditions secondary to the circulating paraprotein, potentially reducing the detection of hematologic malignancy-related LCCN in these patients. The most frequent concomitant kidney pathology was MIDD, which was diagnosed in 22% (n=5) of the patients. Other kidney pathologies were AL amyloidosis, amyloid cast nephropathy and C3 glomerulopathy. In the cohort of 178 patients with MM, 10.6% (n=19) had other kidney diseases with, as in our cohort, MIDD being most frequently associated disease (n=11, 6.2%).2
Table 1.
Demographic, baseline renal and hematologic characteristics and histological findings in the study cohort.
Figure 1.
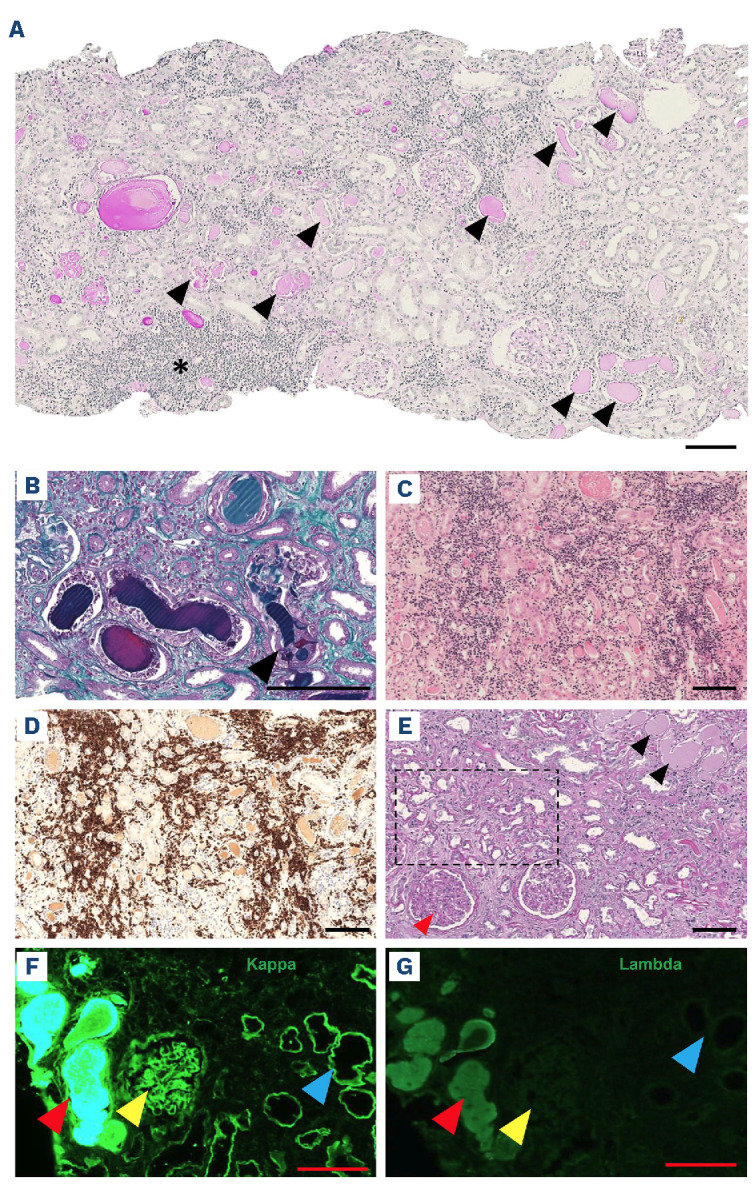
Pathology illustrations. (A) Representative image of a patient’s kidney biopsy viewed by light microscopy after staining with periodic acid-Schiff (PAS), showing multiple pale tubular casts with PAS staining (black arrows) and areas of interstitial infiltration (black asterisk). (B) Light microscopy after staining with Masson trichrome showing typical polychromatophilic casts with a giant cell reaction around a fractured cast (black arrow). (C) Light microscopy after hematoxylin & eosin staining showing diffuse interstitial lymphoma infiltration. (D) Immunohistochemistry analysis targeting CD79A showing diffuse interstitial B-cell lymphoma infiltration. (E ) Light microscopy after PAS staining of a biopsy from a patient with monoclonal immunoglobulin deposition disease (MIDD) and associated cast nephropathy showing PAS-positive mesangial expansion (red arrow) and PAS-positive tubular basement membrane thickening (black square) together with the presence of PAS-negative tubular casts (black arrows). (F, G) Immunofluorescence analyses of a patient with MIDD and associated cast nephropathy using anti-k and anti-A. antibodies showing monotypic k light chain staining within a tubular cast (red arrows), glomerular mesangium (yellow arrows) and tubular basement membrane (blue arrows). Scale bar 100 [im.
As identified in MM, eGFR at disease onset was inversely correlated with the number of casts per square millimeter in the cortex and medulla combined (r=-0.423, P=0.045).2 In addition, patients with more than 2 casts/mm2 in the whole sample presented with higher serum creatinine (361±330 vs. 724±392 μmol/L; P=0.032) and lower eGFR (20±12 vs. 8±4 mL/min/1.73 m2; P=0.016). We also observed a positive correlation between proteinuria and the number of casts per square millimeter of the total sample (r= 0.558, P=0.009), and between proteinuria and light chain levels (r=0.590, P=0.016). Of interest, FLC level was higher in the group with >25% of IFTA (3,793 mg/L) than in the group with <25% of IFTA (1,101.5 mg/L) (P<0.05). This is similar to the findings of Royal et al.2 and further supports the hypothesis of a chronic profibrotic role for light-chain proteinuria, as mentioned by Ying et al.7 Of note, the percentage of globally sclerotic glomeruli did not correlate with onset eGFR or proteinuria, but it did correlate with baseline eGFR (r=-0.694, P=0.004) and serum creatinine (r=0.630, P=0.012). There was no statistically significant difference in terms of presenting eGFR or serum creatinine between the patients who did or did not have a giant cell reaction around casts, tubulitis, interstitial edema, tubular rupture, extravasation of Tamm-Horsfall protein or IFTA. Of particular importance, 12 patients (52%) died during the follow-up period, with a median survival of 15 months (3-48) which is comparable to that of patients with MM-associated LCCN (103 deaths, 58%, median survival of 13 months).2 In addition, all patients requiring dialysis at the time of diagnosis (n=10) progressed to end-stage renal failure or died (n=6). Among patients alive at last follow up (n=10), the median eGFR at presentation was 13.5 (4-21) mL/min/1.73 m2 and 40% (n=4) of the patients required hemodialysis at presentation. All these four patients remained under hemodialysis at last follow-up. None of the patients who did not require hemodialysis at disease onset (n=6) progressed to end-stage renal disease and presented a median eGFR at last follow-up of 36.5 (27-47) mL/min/1.73 m2. The difference between initial and final eGFR in these six patients was an increase of 14.5 (11-20) mL/min/1.73 m2, i.e., a median increase of 61%. Thus, although lymphoplasmacytic lymphomas are considered hematologic malignancies with a better overall prognosis than myeloma,8,9 the presence of associated LCCN considerably worsens the survival of patients, with mortality rates comparable to those of patients with myeloma-associated LCCN.2 It is therefore crucial to diagnose this renal complication early in all patients with lymphoplasmacytic lymphomas.
This multicenter study represents the most extensive investigation of its kind to examine and delineate the clinical and pathological attributes of non-MM LCCN while drawing comparisons with MM LCCN. However, the limited number of patients in our study, attributed to the rarity of the disease and inherent to the retrospective nature of the study, presented a challenge to achieving statistically significant results and identifying reliable prognostic markers. Overall, our pathological analysis and correlation with clinical and biological data at presentation were very similar to those of patients with MM, particularly with regard to the number of casts and correlation with initial renal dysfunction. More importantly, we show that non-MM LCCN is associated with poor survival as in MM.
Finally, with regard to clinicians and pathologists, this study identified three important points. First, it emphasizes the need for a close and longitudinal assessment of kidney function in patients with mature B-cell neoplasms with plasmacytic differentiation. Unlike MM LCCN, non-MM/lymphoma LCCN is often not concomitant with the diagnosis of the hematologic malignancy, and often develops later (43% of our cases). Secondly, it should raise pathologists’ awareness of the need to look for casts in lymphoma patients, as LCCN is classically associated with myeloma and not lymphomatous disorders. Lastly, it should also sensitize pathologists to systematically look for casts even in the presence of more exuberant histological features that may overlap, such as tumor cell infiltration (83% of our cases) and/or glomerular lesions (MIDD), which could cause them to miss the most important lesion in terms of outcome.
Supplementary Material
Acknowledgments
The authors would like to thank the French Nephropathology Group.
Data-sharing statement
All the data supporting the findings of this study are in the manuscript, Figure and Online Supplementary Figure.
References
- 1.Leung N, Bridoux F, Nasr SH. Monoclonal gammopathy of renal significance. N Engl J Med. 2021;384(20):1931-1941. [DOI] [PubMed] [Google Scholar]
- 2.Royal V, Leung N, Troyanov S, et al. Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: a multicenter retrospective study. Blood. 2020;135(21):1833-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos JM, Gustine J, Rennke HG, et al. Renal disease related to Waldenström macroglobulinaemia: incidence, pathology and clinical outcomes. Br J Haematol. 2016;175(4):623-630. [DOI] [PubMed] [Google Scholar]
- 4.Uppal NN, Monga D, Vernace MA, et al. Kidney diseases associated with Waldenström macroglobulinemia. Nephrol Dial Transplant. 2019;34(10):1644-1652. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders PW. Mechanisms of light chain injury along the tubular nephron. J Am Soc Nephrol. 2012;23(11):1777-1781. [DOI] [PubMed] [Google Scholar]
- 7.Ying WZ, Li X, Rangarajan S, Feng W, Curtis LM, Sanders PW. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J Clin Invest. 2019;129(7):2792-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140(11):1229-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are in the manuscript, Figure and Online Supplementary Figure.