Abstract
Integrase (IN) is the only retroviral enzyme necessary for the integration of retroviral cDNA into the host cell’s chromosomes. The structure and function of IN is highly conserved. The human immunodeficiency virus type 2 (HIV-2) IN has been shown to efficiently support 3′ processing and strand transfer of HIV-1 DNA substrate in vitro. To determine whether HIV-2 IN protein (IN2) could substitute for HIV-1 IN function in vivo, we used HIV-1 Vpr to deliver the IN2 into IN mutant HIV-1 virions by expression in trans as a Vpr-IN fusion protein. Trans-complementation with IN2 markedly increased the infectivity of IN-minus HIV-1. Compared with the homologous trans-IN protein, infectivity was increased to a level of 16%. Since IN has been found to play a role in reverse transcription (Wu et al., J. Virol. 73:2126–2135, 1999), cells infected with IN2-complemented HIV-1 were analyzed for DNA products of reverse transcription. DNA levels of approximately 18% of that of wild type were detected. The homologous trans-IN protein restored the synthesis of viral cDNA to approximately 86% of that of wild-type virus. By complementing integration-defective HIV-1 IN mutant viruses, which were not impaired in cDNA synthesis, the trans-IN2 protein was shown to support integration up to a level of 55% compared with that of the homologous trans-IN protein. The delivery of heterologous IN protein into HIV-1 particles in trans offers a novel approach to understand IN protein function in vivo.
Like all other retroviruses, human immunodeficiency virus type 1 (HIV-1) and HIV-2 integrate a DNA copy of their RNA genome into the host cell’s chromosomes. Integration of the viral cDNA is catalyzed by the integrase (IN) protein (3). Sequence analysis has shown that many features of the primary structure of IN are highly conserved among retroviruses and retrotransposons. The amino-terminal domain contains an array of histidine and cysteine residues that form a zinc finger motif (5, 6, 18). The central domain contains the catalytic core, which is defined by three acidic residues with stereotyped spacing. This D,D-35-E motif is a universal feature of integrase proteins and is essential for catalytic activity (9, 10, 22, 25, 36). The carboxy-terminal domain is the least conserved region of IN. It binds to the viral DNA ends and also exhibits nonspecific DNA binding properties (19, 24, 30, 31, 37). The sequence conservation of integrase suggests strong structure-function relationships. IN function has been studied extensively in vitro by using purified enzyme and short oligonucleotide substrate that mimics the ends of the viral DNA molecule (8, 21, 32). Such analysis has shown that IN proteins can utilize different retroviral DNA substrates in the in vitro integration reaction. The HIV-2 IN (IN2) has been shown to efficiently support 3′ processing and strand transfer of HIV-1 substrate in vitro (37). Similarly, the feline immunodeficiency virus (FIV) IN can cleave and integrate HIV and Moloney murine leukemia virus (MoMLV) DNA ends (38). HIV-1 IN is active on FIV substrate but is barely active on MoMLV substrate (35, 38). While the analysis of the integration reaction in vitro has provided a great deal of information on IN function and has helped to elucidate the molecular mechanism of viral DNA integration, the conditions used to study IN in vitro do not fully duplicate those in vivo.
In the context of a replicating virus, HIV-1 IN is expressed and assembled into virions as the C-terminal component of a larger 160-kDa Gag-Pol polyprotein (Pr160Gag-Pol). After proteolytic processing of Pr160Gag-Pol and entry of the virus core into the host cell, IN exists as a 32-kDa protein together with other viral and cellular proteins that make up the viral nucleoprotein complex (34). Several in vivo studies have suggested that the IN protein may be involved in other step of the virus life cycle. Host cell proteins that bind to IN or the HIV-1 preintegration complex (PIC) and promote integration have been identified (13, 20). Through analysis in nondividing cells, mutations in the C terminus of IN have been shown to disrupt the movement of the viral PIC into the nucleus (15), suggesting a role of IN in the nuclear import machinery pathway (15, 16). In other studies, some IN mutations, including those in highly conserved amino acids residues, were found to have no apparent effect on IN activity in vitro while dramatically reducing the formation of the provirus in infected cells (11, 12, 26, 29). These results were initially explained in part by changes in the structure of the Pr160Gag-Pol precursor protein, resulting in aberrant virus assembly and maturation. By incorporating IN protein into virions in trans, independently of Pr160Gag-Pol, we recently demonstrated that the mature IN protein itself promotes the initiation of viral DNA synthesis (40). These results indicate that IN may play important roles in the virus life cycle at several different levels.
To examine whether IN2 could function in place of HIV-1 IN during virus infection, two experimental approaches were undertaken. In the first, we replaced the HIV-1 IN coding region with that of the IN2, generating a virus that contained an HIV-1–HIV-2 chimeric pol gene (Fig. 1). In the second approach, the IN2 protein was incorporated into HIV-1 virions in trans by expression as a fusion partner of Vpr (Vpr-IN). This strategy is based on our previously findings and those of others, which have shown that Vpr can be used as a vehicle to deliver enzymatically active IN into HIV-1 virions in trans, independently of Pr160Gag-Pol (14, 28, 40, 41).
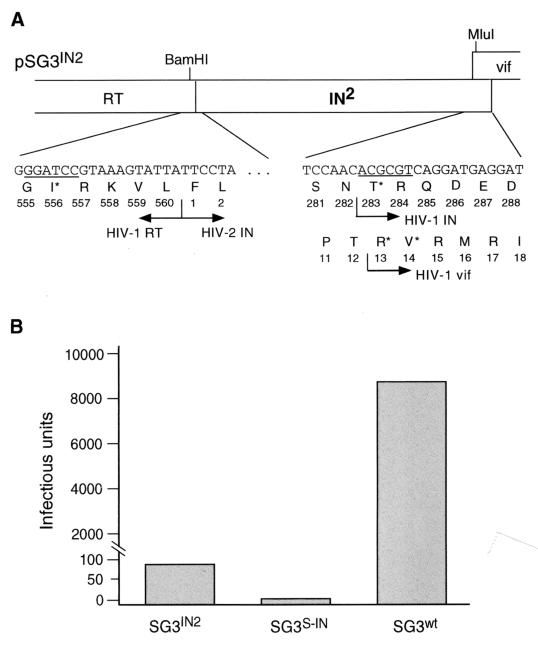
FIG. 1.
Analysis of IN2 protein function when expressed in cis with the HIV-1 genome. (A) Insertion of IN2 into the pol gene of HIV-1. The HIV-1 SG3wt DNA (41) was cut with the BamHI and SalI endonucleases to remove IN, vif, and the 5′ half of vpr from the DNA genome. A fragment of pSG3wt DNA encompassing 16 bp of 3′ IN sequence, vif and the 5′ half of vpr was amplified by PCR, wherein the sense primer contained a MluI restriction site. In a separate reaction, an IN-containing DNA fragment was PCR amplified from the HIV-2 ST clone, wherein the sense and antisense primers contained BamHI and MluI restriction sites, respectively. The two PCR-amplified DNA fragments were ligated into the BamHI-SalI-cut pSG3wt DNA, generating the chimeric virus pSG3IN2. Sequence analysis confirmed an open chimeric pol reading frame (PR-RT-IN2). The 3′ 18 nucleotides of the HIV-1 IN are retained in the chimeric protein. (B) Infectivity of SG3IN2 virions. 293T cells were transfected by calcium phosphate DNA precipitation methods with pSG3IN2 DNA. Forty-eight hours later, the culture supernatants were collected, filtered through 0.45-μm-pore-size filters, and analyzed for HIV-1 p24 antigen by enzyme-linked immunosorbent assay (Coulter Inc.). Next, 25, 5, and 1 ng of each virus (p24 antigen equivalents) was used to infect monolayer cultures of P4 indicator cells (7). Two days later, the cells were stained, and infection-positive cells were counted as described earlier (41). The virus infectivity results represent infectious units per 25-ng equivalent of p24 antigen. These results were highly reproducible in three independent experiments. The data shown are from a single representative experiment.
Characterization of HIV-1 that encodes an HIV-1–HIV-2 chimeric Pol protein.
An IN-containing DNA fragment was PCR amplified from the HIV-2 ST proviral clone (23) and ligated into the BamHI and MluI restriction sites of the HIV-1 pSG3wt molecular clone, generating pSG3IN2 (Fig. 1A). To determine whether the SG3IN2 virus was infectious, 293T cell monolayers were transfected with pSG3IN2 DNA. For controls, HIV-1 wild-type (SG3wt) and IN-minus (pSG3S-IN; see references 28 and 41) viruses were also transfected. The supernatants of the transfected cultures were collected 72 h later and divided into three aliquots. One aliquot was ultracentrifuged to pellet virus for immunoblot analysis, the second aliquot was analyzed for reverse transcriptase (RT) activity and p24 antigen concentration (Coulter Inc.), and the third was used to infect CD4-LTR-β-galactosidase indicator cells (P4) (7). No differences between the wild-type and SG3IN2 virions with respect to RT activity (relative to p24 antigen concentration) and proteolytic processing of the Gag and Pol proteins were detected (data not shown). However, the SG3IN2 virions exhibited a marked decrease in infectivity (Fig. 1B). Compared with the IN-minus SG3IN2 virions, the SG3IN2 virions were reproducibly more infectious.
The HIV Gag-Pol precursor protein not only serves to incorporate the viral enzymes in the virus particle but also plays an important role in virion assembly. Several studies have shown that mutations within IN can cause defects in virus particle production, and virion composition and morphology (1, 2, 4, 11, 33). While our results show that the chimeric SG3IN2 virus was impaired in infectivity, they do not precisely show at what step(s) in the life cycle the virus was defective. Therefore, it was not possible to understand whether virus infection was blocked because the chimeric Gag-Pol protein was not properly folded and caused a defect in the late stages of the virus life cycle or whether the heterologous IN protein itself was unable to mimic the function of the HIV-1 IN protein during the early stages of the virus life cycle.
Incorporation of IN2 protein into HIV-1 virions by expression in trans.
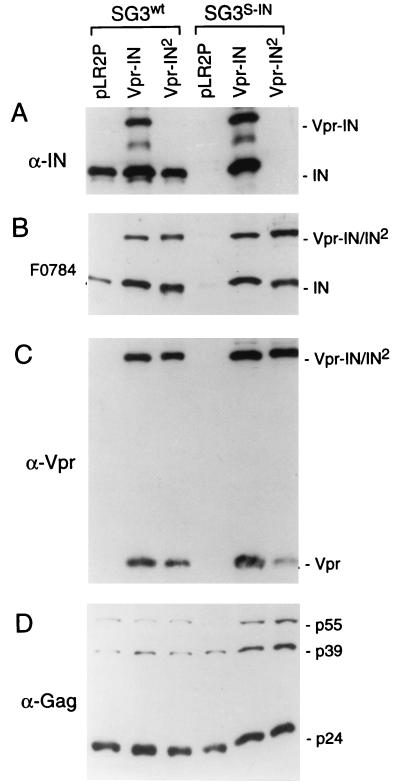
To avoid the possible dominant-negative effects of the chimeric Gag-Pol precursor protein, we fused the HIV-1 vpr gene with the HIV-2 IN gene (IN2) and placed the vpr-IN2 gene fusion into the pLR2P expression plasmid (pLR2P-vprIN2) for complementing HIV-1 IN mutant viruses in trans. The pLR2P-vprIN2 plasmid was cotransfected into 293T cells with the pSG3S-IN IN-minus clone. As controls, pSG3S-IN was cotransfected with the Vpr HIV-1 IN expression vector (pLR2P-vprIN; references 28, 40, 41) and the pLR2P vector. Immunoblot analysis of the progeny virions detected two species of anti-HIV-1 IN-reactive proteins (Fig. 2A), a 47-kDa protein, which is consistent with the combined masses of IN (32 kDa) and Vpr (15 kDa), and a 32-kDa protein. The detection of the 32-kDa protein in SG3S-IN virions complemented with Vpr-IN indicated proteolytic processing of the fusion protein and liberation of IN. The Vpr-IN2 fusion protein was not detected with the HIV-1 anti-IN antiserum. However, using antiserum (F0784) obtained from an HIV-2-infected individual as a probe, the 47- and 32-kDa proteins were detected in both SG3wt and SG3S-IN virions that were complemented with either the Vpr-IN or Vpr-IN2 fusion proteins (Fig. 2B). Using anti-Vpr antibody, the 47-kDa Vpr-IN and Vpr-IN2 fusion proteins and their respective Vpr cleavage products were detected (Fig. 2C). While SG3 contains an open vpr reading frame, the virally encoded Vpr protein was not detected under the conditions used. Anti-Gag antibody detected approximately similar amounts of viral Gag proteins (Fig. 2D). These results confirmed that the IN2 protein can be incorporated into HIV-1 virions when expressed in trans as a fusion partner of Vpr, and subsequently liberated during proteolytic maturation of the virus particle.
FIG. 2.
Incorporation of the Vpr-IN fusion protein into IN-minus virions. (A) pSG3S-IN and pSG3wt were separately cotransfected into 293T cells with pLR2P-vprIN2, pLR2P-vpr-IN, and pLR2P (vector alone), respectively. As described earlier (41), the extracellular progeny virions were concentrated from the supernatants of each culture by ultracentrifugation, lysed, and analyzed by immunoblot analysis using anti HIV-1 IN peptide antibody (A), human anti-HIV-2 antiserum (B), anti-Vpr (C), and anti-Gag (D) antibodies as probes.
The IN2 protein restores the infectivity of HIV-1 IN-minus virus.
To analyze whether the IN2 protein was functional, SG3S-IN virions complemented with the trans-IN2 protein were analyzed on P4 indicator cells. Our results indicated that virus infectivity was rescued to a level of 16% compared with virions complemented with the homologous HIV-1 trans-IN protein (Table 1). This result represents an increase of approximately 100-fold over noncomplemented SG3S-IN virions. Immunoblot analysis performed on the viral stocks that were used for infection confirmed that the trans-IN- and trans-IN2-complemented virions contained approximately equal amounts of the respective fusion protein (data not shown). These results suggested that the IN2 protein can function in place of the HIV-1 IN in vivo, albeit with significantly reduced efficiency.
TABLE 1.
Infectivity of IN-minus HIV-1 complemented with HIV-2 IN protein in trans
| Virus | Result of cotransfection witha:
|
||
|---|---|---|---|
| Control | Vpr-IN2 | Vpr-IN | |
| SG3wt | 28.7 × 103 | 22.4 × 103 | 23.1 × 103 |
| SG3S-IN | 3 | 0.75 × 103 (16) | 4.8 × 103 (100) |
Four micrograms each of the SG3wt and SG3S-IN clones was separately cotransfected into 293T cells with 2 μg of the pLR2P (control), Vpr-IN2, or Vpr-IN expression plasmids. Numbers indicate the number of infectious units per 50-ng equivalent (p24 antigen) of input virus, as determined using P4 indicator cells. The numbers in parentheses indicate infectious units relative to the number of Vpr-IN-complemented SG3S-IN virions, which was arbitrarily set to 100. These results are representative of three independent experiments.
IN2 supports integration of the HIV-1 provirus.
To directly examine whether the heterologous trans-IN2 protein could complement the defect in integration of IN-minus HIV-1, a single-cycle integration assay was used. This assay utilized VSV-G-pseudotyped virus, which contains the hygromycin resistance gene within the HIV-1 genome in place of env. In cells infected with pseudotyped virus, where viral DNA is synthesized, integrated, and expressed, the hygromycin resistance gene allows for the outgrowth of cells in the presence of selection medium. VSV-G-pseudotyped, hygromycin-resistant, IN-minus virus (hy-SG3S-IN) was complemented by DNA cotransfection with Vpr-IN2 and Vpr-IN, respectively. Transfection-derived viral stocks were filtered through 0.45-μm-pore-size filters, and divided into two aliquot sets. One aliquot set was subjected to ultracentrifugation to pellet virions and was examined by immunoblot analysis. Similar levels (relative to CA protein) of virion associated Vpr-IN and Vpr-IN2 were detected (data not shown). The second aliquot set was normalized for p24 antigen concentration and was used to infect HeLa CD4 cells in hygromycin selection medium as described earlier (40). Complementation with the IN2 protein produced 22% of the number of resistant colonies produced by complementation with the homologous trans-IN protein (Table 2). Complementation of wild-type virions (hy-SG3wt) with either Vpr-IN or Vpr-IN2 resulted in a slight reduction (approximately twofold) in CFU (data not shown). These result are consistent with the infectivity results (Table 1).
TABLE 2.
Integration of IN mutant HIV-1 complemented with HIV-2 IN protein in trans
| Hygromycinresistant virus | Result of cotransfection witha:
|
||
|---|---|---|---|
| Control | Vpr-IN2 | Vpr-IN | |
| hy-SG3wt | 24.3 × 104 | 18.9 × 104 | 20.6 × 104 |
| hy-SG3S-IN | 17 | 0.65 × 104 (22) | 3.0 × 104 (100) |
| hy-SG3D116A | 53 | 1.0 × 104 (28) | 3.6 × 104 (100) |
| hy-SG3AA35A | 23 | 2.1 × 104 (55) | 3.8 × 104 (100) |
Four micrograms of each of the hy-SG3wt, hy-SG3S-IN, hy-SG3D116A, and hy-SG3AA35A clones (40) were separately cotransfected into 293T cells with 2 μg of the VSV-G env plasmid and 2 μg of either the pLR2P (control), Vpr-IN2, or Vpr-IN expression plasmids. Numbers indicate the mean number of hygromycin-resistant colonies per 100-ng equivalent (p24 antigen) of input virus. The numbers in parentheses indicate the number of hygromycin-resistant colonies relative to the number produced by Vpr-IN-complemented SG3S-IN virions, which was arbitrarily set to 100. These results are representative of three independent experiments.
We have recently reported that in addition to catalyzing integration, the HIV-1 IN protein plays a nonenzymatic role in the initiation of RT (40). Therefore, to directly analyze the ability of the heterologous IN to catalyze proviral DNA integration, independently of its role in viral DNA synthesis, the Vpr-IN and Vpr-IN2 fusion proteins were used to complement the hy-SG3D116A and the hy-SG3AA35A mutant viruses (40). The hy-SG3AA35A mutant contains alanine substitutions in each of the three amino acid residues that make up the catalytic triad of IN. While defective in integration, these mutants produce near wild-type levels of viral DNA following infection. Transfection-derived virions were normalized for p24 antigen and used to infect HeLa-CD4 cells. The Vpr-IN2-complemented hy-SG3D116A and hy-SG3AA35A viruses produced resistant colonies at levels of 28 and 55%, respectively, compared with those complemented with the homologous trans-IN protein (Table 2). Taken together, these results clearly show that heterologous IN2 protein can catalyze integration of the HIV-1 provirus. Moreover, these data confirm reports that the IN2 protein can efficiently support the integration of HIV-1 DNA substrate in vitro (36–38).
IN2 protein does not efficiently promote HIV-1 DNA synthesis.
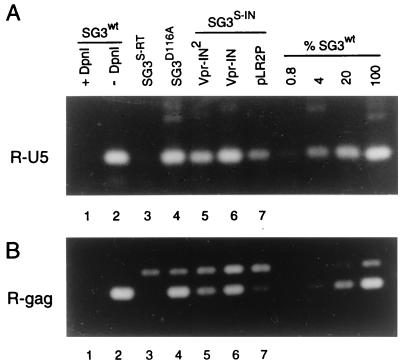
Several reports have shown that viruses containing certain mutations in IN, including IN deletion mutants, are defective in the synthesis of their viral DNA. We reported that this is predominately due to a role that the IN protein plays in the initiation step of RT. Our published data demonstrated that IN-minus virions (SG3S-IN) synthesize reduced levels of viral cDNA (5 to 10% of that of wild-type virus) and that this defect can be overcome by providing IN in trans (40). Therefore, the above results (Tables 1 and 2) would suggest that the defect in the infectivity of IN2-complemented SG3S-IN virus was largely due to a block in viral DNA synthesis after infection. To test this directly, we analyzed the synthesis of the nascent viral cDNA in infected cells. SG3S-IN virions containing Vpr-IN2 were derived by DNA cotransfection and used to infect HeLa-CD4 cells. Eighteen hours later, the cell monolayers were trypsinized, washed extensively, and divided into two aliquot. One aliquot set was lysed and analyzed for intracellular p24 antigen concentration as described earlier (28, 39). DNA was extracted from the other aliquot set, normalized for intercellular p24 antigen concentration, and analyzed for early (R-U5) and late (R-Gag) viral DNA products as described earlier (40). Serial fivefold dilutions of the SG3wt DNA were prepared and analyzed in parallel as a reference to assess the relative amounts of DNA synthesized by the mutant viruses. The IN-minus SG3S-IN virus (lane 7) produced significantly less R-U5 DNA compared with wild-type SG3wt virions (Fig. 3A). When complemented with the trans-IN2 protein the SG3S-IN virions produced 18% of the wild-type levels of R-U5 DNA (lane 5). This represents an increase in DNA synthesis of approximately four- to fivefold. Complementation of SG3S-IN with the homologous trans-IN proteins generated 86% the levels of R-U5 DNA compared with SG3wt. For control, an equivalent (p24 antigen) amount of the SG3D116A mutant virus was analyzed and found to contain near wild-type levels (89%) of R-U5 DNA. No R-U5 DNA was detected in cells infected with the RT-IN-minus SG3S-RT virions (40), confirming that the R-U5 DNA detected by PCR was the product of reverse transcription. The absence of this DNA product in cells infected with SG3S-RT also indicates that the band migrating slightly slower than the R-Gag band is not a product of viral DNA contamination. Nearly identical results were obtained using the R-Gag primer pair to detect late products of viral DNA synthesis (Fig. 3B). These results show that the IN2 protein can support the synthesis of HIV-1 DNA, but with significantly reduced efficiency compared with HIV-1 IN. They are also in strong agreement with our results on virus infectivity (Table 1) and together suggest that the factor limiting infectivity was primarily the defect in the synthesis of viral cDNA.
FIG. 3.
Reverse transcription of Vpr-IN2-complemented viruses. pSG3S-IN was cotransfected into 293T cells by calcium phosphate DNA transfection methods with pLR2P-vpr-IN2, pLR2P-vprIN, and pLR2P, respectively. As controls, pSG3wt, pSG3S-RT, and pSG3D116A were also transfected. Forty-eight hours later, culture supernatants were filtered through 0.45-μm-pore-size filters and analyzed by HIV-1 p24 antigen enzyme-linked immunosorbent assay (ELISA) (Coulter Inc.). The virus-containing culture supernatants were normalized to 500 ng of p24 antigen (CA), treated with RNase-free DNase H (20 U/ml for 2 h) (Promega Corporation) and placed on cultures of HeLa-CD4 cells at 37°C. After 4 h, the cell monolayers were washed, trypsinized, resuspended in fetal bovine serum, and divided into two aliquot sets. One aliquot set (which contained one-tenth of the total number of cells) was lysed in phosphate-buffered saline containing 1% Triton X-100 and analyzed by p24 antigen ELISA to quantify intracellular CA protein. The other aliquot set was placed back in culture medium at 37°C for an additional 14 h. The cells were then washed and total DNA was extracted by organic methods. Next, 250-pg equivalents (p24 antigen) of each DNA extract was analyzed by PCR methods for early (R-U5) (A) and late (R-gag) (B) viral DNA products of RT. The amplified products were resolved on 1.5% agarose gels and stained with ethidium bromide. To assess the relative amount of each of the amplified DNA products, four serial fivefold dilutions of the wild-type (SG3wt) DNA were analyzed in parallel. The undiluted 250-pg sample was arbitrarily set to 100. As a control for the efficiency of DpnI cleavage of potential carryover plasmid DNA, 6,250 copies of pSG3wt DNA were analyzed after digestion with DpnI as described previously (17, 40). The ethidium bromide staining intensity of each amplified DNA product was measured with a Lynx 5000 molecular biology workstation (Applied Imaging) as described previously (27). The data shown is from a representative experiment that was repeated three times, each time with independent transfection-derived virus preparations.
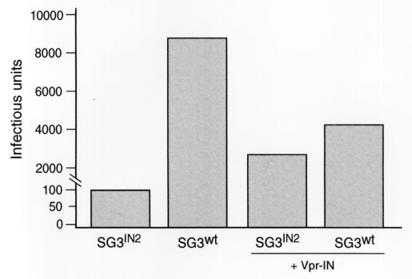
Complementation of SG3IN2 virus with trans-IN.
To better understand the defect in the infectivity of the SG3IN2 virus (Fig. 1), pSG3IN2 was cotransfected with the HIV-1 trans-IN protein (Vpr-IN) and analyzed for infectivity. Figure 4 shows a 27-fold increase in infectivity. This represented an increase that was near that of wild-type virus when complemented with Vpr-IN. This result suggested that the expression of a chimeric Gag-Pol precursor protein did not cause a severe defect in the assembly or maturation of the SG3IN2 virions. Rather, these data may support our earlier findings for a role of an RT-IN intermediate in the formation of infectious HIV-1 particles (41).
FIG. 4.
Complementation of SG3IN2 virus with homologous trans-IN protein. pSG3IN2 and pSG3wt were transfected alone and separately cotransfected into 293T cells with pLR2P-vprIN. The culture supernatants were collected 48 h later, filtered through 0.45-μm-pore-size filters, and analyzed for HIV-1 p24 antigen by enzyme-linked immunosorbent assay (Coulter Inc.). Next, 25, 5, and 1 ng of each virus (p24 antigen equivalents) was used to infect monolayer cultures of P4 indicator cells. Two days later, the cells were stained and infection-positive cells were counted. The virus infectivity results represent infectious units per 25-ng equivalent of p24 antigen. The data shown are the means from three independent experiments.
In this report, we examined whether the IN2 protein could mimic the DNA synthesis and integration activities of the HIV-1 IN at the virus replication level. Our data show that while the heterologous IN protein can support HIV-1 DNA synthesis, it is significantly less efficient than the homologous IN protein. The trans-IN2 protein causes an increase in viral DNA synthesis of IN-minus virus of four- to fivefold. However, the HIV-2 trans-IN protein appeared to support integration of the provirus with greater efficiency, the integration frequency of the integration-defective D116A mutant virus was increased by almost 200-fold. It was noteworthy that the trans-IN2 protein was consistently less efficient in rescuing the DNA synthesis defect of the SG3D116A mutant virus compared with that of the SG3AA35A mutant. It is possible that the D116A mutant IN protein has a dominant-negative effect, perhaps through the formation of nonfunctional heterodimers with the IN2 protein. The ability of the IN2 protein to support integration with relatively high efficiency indicates that it associates with the reverse transcription and preintegration complexes. Moreover, these results suggest that the mere association of the heterologous IN protein with the RT complex is not sufficient to efficiently promote viral DNA synthesis, but rather that specific interactions between the IN protein and other viral components are required. The delivery of heterologous IN protein into HIV-1 particles in trans offers a novel approach to understand IN protein function in vivo.
Acknowledgments
This research was supported by National Institutes of Health grant CA73470 and by the facilities of the AIDS Central Virus and Protein Expression Cores of the Birmingham Center for AIDS Research (P30-AI-27767). This research was also supported by a Merit Review Award funded by the Office of Research and Development, Medical Research Service, U.S. Department of Veterans Affairs.
REFERENCES
- 1.Ansari-Lari M A, Donehower L A, Gibbs R A. Analysis of human immunodeficiency virus type 1 integrase mutants. Virology. 1995;211:332–335. doi: 10.1006/viro.1995.1412. [DOI] [PubMed] [Google Scholar]
- 2.Ansari-Lari M A, Gibbs R A. Expression of human immunodeficiency virus type 1 reverse transcriptase in trans during virion release and after infection. J Virol. 1996;70:3870–3875. doi: 10.1128/jvi.70.6.3870-3875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown P. Integration. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. pp. 161–204. [PubMed] [Google Scholar]
- 4.Bukovsky A, Göttlinger H. Lack of integrase can markedly affect human immunodeficiency virus type 1 particle production in the presence of an active viral protease. J Virol. 1996;70:6820–6825. doi: 10.1128/jvi.70.10.6820-6825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke C J, Sanyal G, Bruner M W, Ryan J A, LaFemina R L, Robbins H L, Zeft A S, Middaugh C R, Cordingley M G. Structural implication of spectroscopic characterization of a putative zinc-finger peptide from HIV-1 integrase. J Biol Chem. 1992;267:9639–9644. [PubMed] [Google Scholar]
- 6.Bushman F D, Engelman A, Palmer I, Wingfield P, Craigie R. Domains of the integrase protein of human immunodeficiency virus type 1 responsible for polynucleotidyl transfer and zinc binding. Proc Natl Acad Sci USA. 1993;90:3428–3432. doi: 10.1073/pnas.90.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craigie R, Fujiwara T, Bushman F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell. 1990;62:829–837. doi: 10.1016/0092-8674(90)90126-y. [DOI] [PubMed] [Google Scholar]
- 9.Drelich M, Wilhelm R, Mous J. Identification of amino acid residues critical for endonuclease and integration activities of HIV-1 IN in vitro. Virology. 1992;188:459–468. doi: 10.1016/0042-6822(92)90499-f. [DOI] [PubMed] [Google Scholar]
- 10.Engelman A, Craigie R. Identification of conserved amino acid residues critical for human immunodeficiency virus type 1 integrase function in vitro. J Virol. 1992;66:6361–6369. doi: 10.1128/jvi.66.11.6361-6369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman A, Englund G, Orenstein J M, Martin M A, Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J Virol. 1995;69:2729–2736. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman A, Liu Y, Chen H, Farzan M, Dyda F. Structure-based mutagenesis of the catalytic domain of human immunodeficiency virus type 1 integrase. J Virol. 1997;71:3507–3514. doi: 10.1128/jvi.71.5.3507-3514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farnet C M, Bushman F D. HIV-1 cDNA integration: requirement of HMGI(Y) protein for function of preintegration complexes in vitro. Cell. 1997;88:483–492. doi: 10.1016/s0092-8674(00)81888-7. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher T M, III, Soares M A, McPhearson S, Hui H, Wiskerchen M, Muesing M A, Shaw G M, Leavitt A D, Boeke J D, Hahn B H. Complementation of integrase function in HIV-1 virions. EMBO J. 1997;16:5123–5138. doi: 10.1093/emboj/16.16.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 17.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson M S, McClure M A, Feng D-F, Gray J, Doolittle R F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci USA. 1986;83:7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn E, Mack J P G, Katz R A, Kulkosky J, Skalka A M. Retroviral integrase domains: DNA binding and the recognition of LTR sequences. Nucleic Acids Res. 1991;19:851–860. doi: 10.1093/nar/19.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalpana G V, Marmon S, Wang W, Crabtree G R, Goff S P. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science. 1994;266:2002–2006. doi: 10.1126/science.7801128. [DOI] [PubMed] [Google Scholar]
- 21.Katzman M, Katz R A, Skalka A M, Leis J. The avian retroviral integration protein cleaves the terminal sequences of linear viral DNA at the in vivo site of integration. J Virol. 1989;63:5319–5327. doi: 10.1128/jvi.63.12.5319-5327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkosky J, Jones K S, Katz R A, Mack J P G, Skalka A M. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequences transposases. Mol Cell Biol. 1992;12:2331–2338. doi: 10.1128/mcb.12.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, Hui H, Kappes J C, Haggarty B S, Hoxie J A, Arya S K, Shaw G M, Hahn B H. Molecular characterization of an attenuated human immunodeficiency virus type 2 isolate. J Virol. 1990;64:890–891. doi: 10.1128/jvi.64.2.890-901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaFemina R L, Callahan P L, Cordingley M G. Substrate specificity of recombinant human immunodeficiency virus integrase protein. J Virol. 1991;65:5624–5630. doi: 10.1128/jvi.65.10.5624-5630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaFemina R L, Schneider C L, Robbins H L, Callahan P L, LeGrow K, Roth E, Schlief W A, Emini E A. Requirement of active human immunodeficiency virus type 1 integrase enzyme for productive infection of human T-lymphoid cells. J Virol. 1992;66:7414–7419. doi: 10.1128/jvi.66.12.7414-7419.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leavitt A D, Robles G, Alesandro N, Varmus H E. Human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J Virol. 1996;70:721–728. doi: 10.1128/jvi.70.2.721-728.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Wu X, Newman M, Shaw G M, Hahn B H, Kappes J C. The Vif protein of human and simian immunodeficiency viruses is packaged into virions and associates with viral core structures. J Virol. 1995;69:7630–7638. doi: 10.1128/jvi.69.12.7630-7638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Wu X, Xiao H, Conway J A, Kappes J C. Incorporation of functional human immunodeficiency virus type 1 integrase into virions independent of the Gag-Pol precursor protein. J Virol. 1997;71:7701–7710. doi: 10.1128/jvi.71.10.7704-7710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda T, Planelles V, Krogstad P, Chen I S Y. Genetic analysis of human immunodeficiency virus type 1 integrase and the U3 att site: unusual phenotype of mutants in the zinc finger-like domain. J Virol. 1995;69:6687–6696. doi: 10.1128/jvi.69.11.6687-6696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller M D, Farnet C M, Bushman F D. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mumm S R, Grandgenett D P. Defining nucleic acid-binding properties of avian retroviruses integrase by deletion analysis. J Virol. 1991;65:1160–1167. doi: 10.1128/jvi.65.3.1160-1167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman P A, Fyfe J A. Human immunodeficiency virus integration protein expressed in Escherichia coli processes selective DNA cleavage activity. Proc Natl Acad Sci USA. 1990;87:5119–5123. doi: 10.1073/pnas.87.13.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin C-G, Taddeo B, Haseltine W A, Farnet C M. Genetic analysis of the human immunodeficiency virus type 1 integrase protein. J Virol. 1994;68:1633–1642. doi: 10.1128/jvi.68.3.1633-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanstrom R, Wills J W. Synthesis, assembly, and processing of viral proteins. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. pp. 263–334. [PubMed] [Google Scholar]
- 35.van Gent D C, Elgersma Y, Bolk M W J, Vink C, Plasterk R H A. DNA binding properties of the integrase protein of human immunodeficiency viruses types 1 and 2. Nucleic Acids Res. 1991;19:3821–3837. doi: 10.1093/nar/19.14.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Gent D C, Oude Groeneger A A M, Plasterk R H A. Mutational analysis of the integrase protein of human immunodeficiency virus type 2. Proc Natl Acad Sci USA. 1992;89:9598–9601. doi: 10.1073/pnas.89.20.9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Gent D C, Vink C, Oude Groeneger A A M, Plasterk R H A. Complementation between HIV integrase proteins mutated in different domains. EMBO J. 1993;12:3261–3267. doi: 10.1002/j.1460-2075.1993.tb05995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vink C, van der Linden K H, Plasterk R H A. Activities of the feline immunodeficiency virus integrase protein produced in Escherichia coli. J Virol. 1994;68:1468–1474. doi: 10.1128/jvi.68.3.1468-1474.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Schwedler U, Song J, Aiken C, Trono D. Vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu W, Liu H, Xiao H, Conway J A, Hehl E, Kalpana G V, Prasad V, Kappes J C. Human immunodeficiency virus type 1 integrase protein promotes reverse transcription through specific interactions with the nucleoprotein reverse transcription complex. J Virol. 1999;73:2126–2135. doi: 10.1128/jvi.73.3.2126-2135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X, Liu H, Xiao H, Conway J A, Hunter E, Kappes J C. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. EMBO J. 1997;16:5113–5122. doi: 10.1093/emboj/16.16.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]