Abstract
Background
Anthracycline‐containing regimens (ACR) are the most prevalent regimens in the management of patients with advanced follicular lymphoma (FL). However, there is no proof that they are superior to non‐anthracycline‐containing regimens (non‐ACR).
Objectives
To compare the efficacy of ACRs to other chemotherapy regimens, in the treatment of FL.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 3), MEDLINE (January 1966 to April 2013), smaller databases, relevant conference proceedings (2004 to 2012) and the National Medical Library (April 2013).
Selection criteria
We included randomized controlled trials (RCTs) comparing ACR with non‐ACR for adult patients with FL. We excluded trials in which immunotherapy, radiotherapy alone or stem‐cell transplantation were used in one arm alone. Our primary outcome was overall survival (OS). Secondary outcomes included disease control, as measured by progression‐free survival (PFS) or remission duration (RD).
Data collection and analysis
Two review authors assessed the quality of trials and extracted data. We contacted study authors for additional information. We analyzed trials separately according to resemblance of the chemotherapeutic regimens in study arms, other than the addition of anthracyclines ('same' versus 'different' chemotherapy). Hazard ratios (HR) and risk ratios (RR) with 95% confidence intervals (CI) were estimated and pooled using the fixed‐effect model.
Main results
Eight RCTs, conducted between 1974 and 2011, and involving 2636 patients were included in this meta‐analysis. All trials included therapy‐naive patients. Rituximab was used in one trial only. Follow‐up was between three and five years in most trials (range three to 18 years). All trials were published in peer‐reviewed journals.
Five trials compared similar chemotherapeutic regimens, except for the anthracycline. In three studies reporting overall survival specifically in FL patients, there was no statistically significant difference between ACR and non‐ACR arms (HR 0.99; 95% CI 0.77 to 1.29; I2 = 0%). ACR significantly improved disease control (HR 0.65; 95% CI 0.52 to 0.81; four trials). Progression or relapse at three years were reduced (RR 0.73; 95% CI 0.63 to 0.85). Anthracyclines did not significantly increase rates of complete response (RR 1.05; 95% CI 0.94 to 1.18) or overall response (RR 1.06; 95% CI 1.00 to 1.12), but heterogeneity was substantial.
Overall, ACR were more often associated with cytopenias, but not with serious infections or death related to chemotherapy. Cardiotoxicity, albeit rare, was associated with anthracycline use (RR 4.55; 95% CI 0.92 to 22.49; four trials).
Three trials added anthracycline to one arm of two different regimens. None showed benefit to ACR regarding OS, yet there was a trend in favor of anthracyclines for disease control. Results were heterogeneous.
We judged the overall quality of these trials as moderate as all are unblinded, some are outdated and are not uniform in outcome definitions.
Authors' conclusions
The use of anthracyclines in patients with FL has no demonstrable benefit on overall survival, although it may have been mitigated by the more intense regimens given in the control arms of three of five trials. ACR improved disease control, as measured by PFS and RD with an increased risk for side effects, notably cardiotoxicity. The current evidence on the added value of ACR in the management of FL is limited. Further studies involving immunotherapy during induction and maintenance may change conclusion.
Keywords: Adult; Humans; Anthracyclines; Anthracyclines/therapeutic use; Antibiotics, Antineoplastic; Antibiotics, Antineoplastic/therapeutic use; Antibodies, Monoclonal, Murine‐Derived; Antibodies, Monoclonal, Murine‐Derived/therapeutic use; Antineoplastic Combined Chemotherapy Protocols; Antineoplastic Combined Chemotherapy Protocols/therapeutic use; Induction Chemotherapy; Induction Chemotherapy/methods; Lymphoma, Follicular; Lymphoma, Follicular/drug therapy; Lymphoma, Follicular/mortality; Lymphoma, Follicular/pathology; Maintenance Chemotherapy; Maintenance Chemotherapy/methods; Randomized Controlled Trials as Topic; Rituximab
Plain language summary
Anthracyclines in the treatment of follicular lymphoma (FL) in adults
FL is the most common indolent non‐Hodgkin's lymphoma (NHL). It is a slowly progressive disease, with a risk of transformation to a more aggressive lymphoma. Advanced disease (stage III and IV) has been considered incurable. However, in recent years, the use of combination therapy and treatment with the monoclonal anti‐CD20 antibody rituximab, have prolonged survival and decreased transformation rate, and is now considered standard of care in FL patients. One of the most common chemotherapies used in the treatment of FL is the combination of rituximab with cyclophosphamide, vincristine, adriamycin, prednisone (R‐CHOP), which contains an anthracycline (adriamycin). Other anthracycline‐containing regimens (ACR) have also been used to treat FL patients. However, there is no proof that ACRs are better than non‐anthracycline‐containing regimens (non‐ACRs), and there are no standard guidelines for the initial treatment of advanced FL. Importantly, anthracyclines have serious side effects, notably a decrease in blood counts and potential damage to the heart, depending on the dose.
The aim of this systematic review and meta‐analysis was to examine if there is a benefit in the use of anthracyclines for patients with FL. We looked at overall survival (OS); measures of disease control; response rates; and side effects.
We found eight randomized controlled trials, of which five compared similar chemotherapy regimens in both trial arms, except for the anthracycline. Even among these five trials, three included more intense chemotherapy in the control arm. Most trials were conducted in the 1980s and 1990s. Only one of them included rituximab as part of the chemotherapy regimen. Almost all patients were treatment‐naive with advanced disease. Follow‐up ranged between three and five years in most trials. The main results from this set of trials are.
1. There is no evidence that OS is prolonged with the use of anthracyclines, although it may have been hampered by the more intense regimens given in the control arms of three of five trials.
2. Anthracyclines improved disease control. Concordantly, less patients progressed or relapsed within three years of treatment with ACR.
3. There is no statistically significant difference in complete or overall response rates.
4. Qualitatively, more side effects were reported with ACR, myelotoxicity and cardiotoxicity included.
This evidence is limited, mainly due to disparities in regimens between control and study arms, but also since most included trials were conducted over one to two decades ago, and only one employed rituximab. Importantly, results from this study were in agreement with pooled‐outcomes from trials of the pre‐rituximab era.
It is essential to find the optimal chemotherapeutic regimen in conjunction with rituximab and other novel agents, and understand the role of anthracyclines in this combination, especially with current methods that are able to reduce their toxicity. With longer follow‐up periods we may better understand whether improved disease control will eventually translate to an increase in survival.
Summary of findings
Summary of findings for the main comparison. ACR compared to non‐ACR for treatment of follicular lymphoma in adults.
| ACR compared to non‐ACR for treatment of follicular lymphoma in adults | ||||||
| Patient or population: adults receiving treatment for follicular lymphoma Settings: Intervention: Aanthracycline Comparison: no anthracycline same chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No anthracycline same chemotherapy | Anthracycline | |||||
| Overall survival number of dead patients Follow‐up: median 50 months | 538 per 1000 | 535 per 1000 (449 to 631) | HR 0.99 (0.77 to 1.29) | 464 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Mortality at 3 years | 260 per 1000 | 239 per 1000 (174 to 327) | RR 0.92 (0.67 to 1.26) | 465 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Overall response | 839 per 1000 | 889 per 1000 (839 to 940) | RR 1.06 (1 to 1.12) | 622 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| Disease control number of patients with progression Follow‐up: median 30 months | 492 per 1000 | 356 per 1000 (297 to 423) | HR 0.65 (0.52 to 0.81) | 759 (4 studies) | ⊕⊕⊕⊕ high | |
| Progression/relapse at 3 years | 544 per 1000 | 397 per 1000 (343 to 463) | RR 0.73 (0.63 to 0.85) | 724 (4 studies) | ⊕⊕⊕⊕ high | |
| Neutropenia grade 3‐4 | 190 per 1000 | 368 per 1000 (277 to 485) | RR 1.94 (1.46 to 2.56) | 533 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Cardiotoxicity** | 2 per 1000 | 8 per 1000 (2 to 40) | RR 4.55 (0.92 to 22.49) | 1412 (3 studies) | ⊕⊝⊝⊝ very low4,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Includes all trials, irrespectively of the comparison ("same chemotherapy"; "different chemotherapy"). CI: confidence interval; RR: risk ratio; HR: hazard ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Small number of events 2 Moderate heterogeneity 3 Different reporting methods 4 Not consistently reported 5 Wide confidence interval
Background
Description of the condition
Follicular lymphoma (FL) is the most common indolent and the second most common non‐Hodgkin lymphoma (NHL) subtype in the Western world. It constitutes up to 30% of all NHL (NHLCP 1997), and its incidence has risen in the last decades and is currently 3.3 to 3.8 cases per 100,000 patient‐years, in the white US population (Morton 2006). It is defined as a group of malignancies composed of follicle center cells, usually a mixture of centrocytes (cleaved cells) and centroblasts (large non‐cleaved cells) (Vitolo 2008).
The Revised European‐American Classification of Lymphoid Neoplasms (REAL classification) (Harris 1994), and more recently the updated World Health Organization (WHO) classification (Harris 1999; Swerdlow 2008) propose the term follicle center lymphoma, and divide it into grades 1, 2, 3a, and 3b. The grades are distinguished by the presence of predominantly small, mixed small and large, and large cells, respectively. Pathologically, according to the 'Berard Criteria', the grades are defined by the number of centroblasts per high power field (Mann 1983). In grade 3a centrocytes are still present, while grade 3b involves centroblasts only. Grade 3 FL is biologically distinct from grades 1 and 2 in its clinical behavior and response to chemotherapy and is treated as aggressive lymphoma. Owing to imprecision in differentiating between grade 3a and 3b, its relative infrequency, and the nature of the trials involved, it is difficult to assess its natural history. Yet, some consider FL grades 1, 2, 3a as a single histologic entity, and keep it apart from grade 3b, which is treated closely to diffuse large B‐cell lymphoma (Chau 2003; Ganti 2006; Vitolo 2008). FL encompasses most malignancies previously classified as nodular lymphoma in the Working Formulation, most tumors classified as follicular center cell lymphoma in the Lukes‐Collins classification, and all cases in Kiel classification category of centroblastic/centrocytic (CB/CC) follicular or follicular CB lymphoma (Vitolo 2008).
The molecular hallmark of FL is the acquisition of translocation t(14;18) by pre‐B cells during an abnormal immunoglobulin rearrangement in the bone marrow, and the overexpression of bcl2 protein, which protects cells from apoptosis (Bendandi 2008). However, only 70% to 95% of FL patients are t(14;18) positive (Vitolo 2008), and t(14;18)‐positive cells may also be found in healthy individuals and patients with other malignancies. Staging of FL is done according to the Ann Arbor system, according to number of involved lymph‐node regions, presence of extra‐lymphatic involvement, and presence of B symptoms. Stages I/II are considered early disease, while stages III/IV are considered advanced. Advanced disease is present in more than 80% of FL patients, and bone marrow involvement in more than 60% (Vitolo 2008).
Contemporary scoring systems specific for FL are the Italian Lymphoma Intergroup Index (ILI) (Federico 2000) and the more widely accepted Follicular Lymphoma International Prognostic Index (FLIPI) (Solal‐Celigny 2004). The FLIPI designates prognostic groups as having low, intermediate, or high risk based on the presence or absence of five adverse prognostic factors: age > 60 years, Ann Arbor stage III/IV, hemoglobin level < 12 g/dL, involvement of more than four nodal sites, and elevated serum lactate dehydrogenase (LDH) level. The risk of transformation is higher in patients with advanced stage and higher FLIPI. Another scoring system was offered by the same group, FLIPI2, which is intended to stratify risk in the era of immunotherapy, and takes progression‐free survival (PFS) as the principal outcome measure. It takes into account β2‐microglobulin higher than the upper limit of normal, longest diameter of the largest involved node longer than 6 cm, bone marrow involvement, hemoglobin level lower than 12 g/dL, and age older than 60 years (Federico 2009). FL has had over the years a median survival of eight to 10 years. Its course is largely unpredictable, and it may undergo more aggressive histologic and clinical transformation to aggressive lymphoma at a rate of 3% per year (Bendandi 2008), which is usually poorly responsive to chemotherapy. The median survival from transformation is about one year (Vitolo 2008).
Treatment for FL is considered separately for early‐stage versus advanced‐stage disease and for newly diagnosed versus relapsed or resistant disease. Historically, advanced FL was considered incurable, with no difference in overall survival (OS) between early treatment and 'watch and wait' approach, and with relapse as a rule (Bendandi 2008). However, in recent years, new treatment approaches, and specifically the introduction of rituximab (a monoclonal anti‐CD20 antibody), have decreased transformation rate (Montoto 2007) and improved survival (Tilly 2008). Thus, the first challenge, especially in newly diagnosed patients with FL, is distinguishing those most likely to benefit from an aggressive, curative‐intent approach.
Early‐stage FL is curable in 30% to 40% of patients, and is usually treated with localized radiotherapy, with even better results with combined‐modality therapy (Bendandi 2008), especially in patients with high tumor burden (Vitolo 2008). In newly diagnosed advanced FL, observation is still an option, especially in high‐risk patients and provided there are no high tumor burden features (Horning 1984). In this case, usual indications for treatment in advanced FL are symptomatic disease, hematopoietic impairment, bulky disease, or rapid lymphoma progression. Specific criteria have been established to guide initiation of therapy, such as the Groupe d'Etute des Lymphomes Folliculire (GELF) criteria (Solal‐Celigny 1993). However, at least in principal, eradication should be the initial goal in the management of most patients. In relapsing or resistant FL, salvage therapy includes chemoimmunotherapy regimens not used in first‐line therapy, radioimmunotherapy and stem‐cell transplantation (SCT) (Bendandi 2008; Greb 2008; Vitolo 2008).
There are no standard guidelines for the initial treatment of advanced FL. The choice of chemotherapy largely depends on many factors such as: patient's age and performance status, comorbidity, the pace of disease, and the aim of the treatment (i.e. palliation or attempt to cure). Chemotherapy regimens may include: alkylating agents, anthracycline‐based chemotherapies, purine analogs, and regimens resembling the cyclophosphamide, vincristine, prednisone (CVP)‐like regimens (Vitolo 2008). The combination of rituximab with nearly any chemotherapy regimen is superior to the same chemotherapy regimen alone (Tilly 2008) as was shown in many randomized trials, and summarized in a meta‐analysis published in The Cochrane Library (Schulz 2007). Thus, the concomitant administration of rituximab and a chemotherapy regimen has rapidly become the first‐line standard of treatment in FL. It also has a role in maintenance therapy as well as in relapsed, recurrent, or resistant patients (Vidal 2009). According to the National LymphoCare Study (NLCS), most FL patients in the US (regardless of staging) were treated by chemotherapy and rituximab combination, 55% of whom with rituximab, cyclophosphamide, vincristine, adriamycin, prednisone (R‐CHOP) regimen, 23% with R‐CVP, and 15% with fludarabine‐based regimens (Friedberg 2009). High‐dose chemotherapy followed by autologous SCT is still a controversial modality in first‐line treatment of FL.
Response to therapy is monitored through history, physical examination, computed tomography (CT) or fluorodeoxyglucose positron emission tomography (FDG‐PET), and bone marrow biopsy in some patients. Complete response (CR) has been achieved when: there is no clinical evidence of disease or disease‐related symptoms; all lymph nodes are normal sized on CT scan; spleen and liver are non‐palpable and without nodules; previously involved bone marrow is negative on repeat biopsy. Partial response (PR) is defined as decrease in nodal size by at least 50% and no progression otherwise (Cheson 1999; Cheson 2007). These patients are treated as refractory disease. Molecular response, detecting minimal residual disease, by using polymerase chain reaction (PCR) for bcl‐2/IgH translocation and clonally rearranged IgH genes, correlates well with outcome as shown by several prospective studies. However, it is still unclear whether eradicating the t(14;18)‐bearing clone is an important goal of therapy, and is not routinely performed (Vitolo 2008).
Description of the intervention
We are going to assess the role of anthracyclines in the treatment of FL.
Anthracyclines are antibiotic drugs that are among the most important antitumor agents. They include doxorubicin (adriamycin) and daunorubicin, and the analogous idarubicin and epirubicin. Mitoxantrone is an anthracenedione, but is practically considered in this group. These drugs intercalate with deoxyribonucleic acid (DNA), directly affecting transcription and replication. Moreover, they form a tripartite complex with topoisomerase II and DNA, and inhibit the religation of broken DNA strands leading to apoptosis. They also generate free radicals that damage DNA (Goodman 2006).
The toxic manifestations of these agents include: myelotoxicity; stomatitis; alopecia; gastrointestinal (GI) disturbances; and dermatological manifestations such as 'adriamycin flare' at the site of injection, facial flushing, conjunctivitis, and lacrimation. Cardiac toxicity is a unique and the most important adverse event. Two types of cardiomyopathy may occur. An acute form, develops within 24 hours of treatment, and is characterized by abnormal electrocardiogram (ECG) findings and even transient reduction in ejection fraction, elevation of troponin, and pericardial effusion. A subacute/chronic, cumulative dose‐related toxicity (usually above 550 mg/m2) is manifested as congestive heart failure. Its incidence ranges widely, but is approximately 7.5% at a cumulative dose above 550 mg/m2. Elderly people, females, children and patients with a history of cardiac disease are at increased risk, as are patients treated with chest irradiation, and with the administration of high‐dose cyclophosphamide or another anthracycline, and concomitant trastuzumab or paclitaxel (Outomuro 2007).
In FL, anthracycline‐based regimens are the most frequently employed first‐line treatment in the US, and are considerably utilized even in early‐stage disease (Friedberg 2009), yet there is no proof of their superiority over regimens without anthracyclines. Even trials comparing single‐agent versus combination chemotherapy including anthracyclines have not consistently shown a benefit in response, relapse rate, or survival to combination therapy (Lister 1978). In some, a benefit was significant only in higher‐grade FL (Peterson 2003) or in elderly patients (Coiffier 1999). Furthermore, it is undetermined whether the benefit may be attributed to anthracyclines, other drugs, or the combination in itself.
In a retrospective study, 633 FL patients treated with anthracycline‐containing regimens (ACR) were compared to 128 comparable patients treated with combination chemotherapy not containing anthracyclines. The former group had better complete remission, five‐year OS, and failure‐free survival (FFS) (Rigacci 2003). This stands in contrast to previous survival data of patients with low‐grade lymphoma entered into Southwest Oncology Group (SWOG) lymphoma trials, where doxorubicin‐containing treatment did not prolong the median OS, in comparison to less aggressive programs (Dana 1993). Most other data come from indirect comparisons, comparing treatment arms from different trials (Brandt 2001; Bendandi 2008; Tilly 2008; Vitolo 2008; Siddhartha 2009). An analysis of five consecutive studies at MD Anderson Cancer Center, involving advanced FL patients has shown improvement in OS and FFS over a 25‐year period (Liu 2006). The fact that all protocols involved anthracyclines underscores the methodologic difficulty in comparing outcome rates in one trial with previous trials or historical cohorts, with versus without anthracyclines as is often done. Previous studies suggested that ACRs are advantageous specifically among patients with grade 3 FL (Wendum 1997; Chau 2003; Ganti 2006), and show a plateau in FFS following ACR (Bartlett 1994). As a result, the recommended treatment for patients with grade 3 FL (both 3a and 3b) is now R‐CHOP, even in early‐stage disease (Buske 2008).
Why it is important to do this review
FL is the most common indolent NHL, yet there is no standard guideline for its management. It has been considered an incurable progressive disease, but in recent years, implementation of combination therapy and treatment with rituximab have prolonged survival and decreased transformation rate. ACRs are of the most prevalent first‐line therapies, especially in advanced disease, but also in limited or relapsed cases. However, there is no proof that they are superior to other, non‐ACRs, or even single‐agent therapy. Observational studies show conflicting results and other data are based on indirect comparisons that are not reliable. However, the optimal design for assessment of interventions is randomized controlled trials (RCTs). Anthracycline use is often limited to younger patients with more advanced or high‐grade disease, owing to concern of their adverse effects, especially cardiotoxicity, although it may be diminished in face of preventive strategies (van Dalen 2009).
In this systematic review we assessed the evidence on the role of anthracyclines in the treatment of FL. This question is still important in the rituximab era, since the preferred combination chemotherapy used with it has not been elucidated.
Objectives
To compare the efficacy of ACRs to other chemotherapy regimens, in the treatment of FL.
Methods
Criteria for considering studies for this review
Types of studies
We considered any published or unpublished RCT eligible for inclusion in this review, without language restriction.
Types of participants
We included adult patients over 18 years of age, with a histologically confirmed diagnosis of FL, without gender or ethnicity restriction. If the trial had recruited patients with FL in addition to patients with other types of lymphoma, we included the study and extracted data for patients with FL separately. Where data were not provided separately for FL, we included the study and reported the number of patients with FL; we analyzed data only for studies with over 75% FL patients. We excluded studies that did not specify the number of FL patients enrolled.
We considered all stages and grades of FL. Classification systems for FL varied between trials, as they were conducted at different times. Considering the different classification systems, we unified various nomenclatures in the following manner:
grade 1 equivalent to follicular small cell lymphoma or CB/CC follicular or nodular lymphocytic poorly differentiated;
grade 2 equivalent to mixed small and large cell FL or CB/CC follicular and diffuse or nodular mixed;
grade 3 equivalent to large cell FL or CB/CC diffuse or nodular histiocytic. No equivalents to grade 3a and 3b in previous classifications.
We included both trials assessing treatment‐naive patients and previously treated patients (with or without relapse).
Types of interventions
The main intervention was an ACR compared to a non‐ACR, including any chemotherapy or immunochemotherapy.
We defined the interventions as follows:
intervention: ACR, regardless of additional agents, with or without radiotherapy;
control: non‐ACR, as a single agent or multiple agents, regardless of dose.
Anthracyclines considered in this review included doxorubicin (adriamycin), daunorubicin, idarubicin, epirubicin, mitoxantrone, and pixantrone. We included trials involving rituximab, interferon, or other novel chemoimmunotherapy only if the same chemotherapy regimen was administered in both arms. We excluded trials in which the control arm included one of the following: watchful waiting, radiotherapy alone, high‐dose chemotherapy with SCT.
We separated trials according to the similarity of chemotherapeutic regimens, other than anthracyclines, between study arms. Trials with the same chemotherapy, where the main difference was the addition of an anthracycline, were summed together. Studies with different chemotherapies were described narratively.
Types of outcome measures
We based the outcomes of this review on the revised response criteria for malignant lymphoma published in 2007 (Cheson 2007). Given that some studies were conducted before these guidelines and that data were reported heterogeneously, we allowed for deviations from these definitions and documented the study definitions.
Primary outcomes
OS, defined as the time from entry to study until death of any cause (Cheson 2007) and assessed among all patients. In addition we assessed all‐cause mortality at three, five and 10 years.
Secondary outcomes
PFS: defined as the time from entry into the study until lymphoma progression (including relapse) or death of any cause (Cheson 2007). This outcome was analyzed for all patients. We accepted other outcome definitions (e.g. excluding non‐lymphoma‐related deaths) as long as all patients were accounted for. We also tried to extract the number of patients with progression (progression, relapse, or death from any cause) at three and five years out of all patients.
CR: we accepted CR definitions as defined in study. In this category we included both documented and uncertain CR (CRu).
Overall response rate (ORR), defined as CR + CRu + PR, as defined in study.
RD: defined from the time when criteria for response (i.e. CR or PR) were met until the first documentation of relapse or progression (Cheson 2007). This outcome was analyzed for the subgroup of patients achieving remission. We accepted other outcome definitions counting the subgroup of patients achieving remission and defining events as progression, relapse, need for treatment, or death (e.g. relapse‐free survival (RFS), event‐free interval).
Relapse: number of patients with relapse out of those achieving CR. We extracted preferentially relapse at five years, but accepted other time points and documented these.
Disease control: a general measure including various outcome measures reported in trials (i.e. PFS, RFS, event‐free survival, time to treatment failure, time to progression, or RD). These measures were often ill‐defined, and were not necessarily compatible with common guidelines (Cheson 1999; Cheson 2007). The outcomes and their definitions are specified in the Characteristics of included studies table.
Quality of life (QoL) assessed using validated questionnaires.
-
Adverse events which were defined as follows:
cardiotoxicity ‐ clinical: defined on the basis of symptoms failure, confirmed by an abnormal diagnostic test; subclinical: defined as either histological abnormalities according to the Billingham score (Billingham 1978) on myocardial biopsy; or abnormalities in cardiac function measured by echocardiography or radionuclide ventriculography;
myelosuppression defined as number of patients developing grade III/IV neutropenia (Miller 1981);
infections, as defined in study;
alopecia (Miller 1981);
stomatitis (Miller 1981).
Search methods for identification of studies
We conducted a comprehensive search with the purpose of identifying all eligible trials regardless of language, year of publication, or status of publication (published in peer review journal, conference proceeding, thesis, or unpublished).
Electronic searches
We searched the electronic databases of Cochrane Central Register of Controlled Trials (CENTRAL, published in The Cochrane Library 2013, Issue 3) and MEDLINE (January 1966 to April 2013). We have provided the different search strategies in Appendix 1 and Appendix 2. We also searched the metaRegister of Controlled Trials (mRCT) for ongoing or unpublished trials.
Searching other resources
We searched conference proceedings available from 2004 to 2012 of the following: the American Society of Hematology (ASH), European Hematology Association (EHA), and American Society of Clinical Oncology (ASCO). We scanned references of all included trials.
Data collection and analysis
Selection of studies
One review author (Gilad Itchaki (GI)) scanned the results of the search. Two review authors (GI, Mical Paul (MP)) independently applied inclusion and exclusion criteria for possibly relevant studies.
Data extraction and management
Two review authors independently extracted the data from included trials into an electronic table (GI, MP). We extracted the following data.
Trial characteristics
Trial design
Setting and dates
Total duration of study
Duration of study follow‐up
Exclusion criteria
Statistical methods
Publication status
Funding
Ethical committee approval and informed consent
Risk of bias
We extracted data to assess the risk of bias, as specific below
Patient characteristics
Mean age and sex
Age over 60 years
Histologic confirmation of FL
Grade and stage (including B‐symptoms and bulky disease)
Bone marrow involvement
IPI or FLIPI score
Indication for initiation of therapy
Performance status (Eastern Cooperative Oncology Group (ECOG), Karnofsky)
Time from diagnosis to first treatment
Previous treatment
Other medical conditions, specifically: cardiac, hepatic, or renal dysfunction
Fulfillment of inclusion and exclusion criteria
Interventions
Setting
Type of chemotherapy, dose, number of cycles, possibility of dose reduction, addition of immunotherapy (type and dose) or radiation
Cumulative anthracycline dose, peak dose, infusion duration
Administration and timing of granulocyte‐colony stimulating factor (G‐CSF)
Compliance
Cross‐over and rate of completion
Outcomes
As defined above. We documented and reported the definitions of time‐to‐event outcomes (including the population assessed, event definition, and whether all‐cause or disease‐related deaths were included in the outcome).
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in included studies and extracted the data into the electronic table. We used a domain‐based evaluation as recommended by theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Review authors were not blinded to trial authors, its publication status, or other study characteristics. We assigned each domain a low or high risk of bias, using the definitions provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When there was insufficient information about the process, we assigned the domain an unclear risk of bias. We assessed the following domains for this review:
sequence generation;
allocation concealment;
blinding of participants, personnel, and outcome assessors;
incomplete outcome data: we assessed the number of exclusions and attrition for the primary outcomes and defined a priori high risk of bias when the number of randomized patients that were not included in outcome assessed was 30%;
selective outcome reporting: we assessed this domain if the trial's protocol was available and predefined outcomes could be compared to those reported. In all other cases we have classified the domain as unclear, unless there was a discrepancy between the outcome results reported in the publication and those specified in its methods section (where an assignment of inadequate was be given);
early stopping of the trial.
Measures of treatment effect
For dichotomous data (deaths, CR, adverse events) we extracted the number of patients with events and number of patients assessed, and compared study groups using risk ratios (RRs). For survival data we extracted the summary effect measures as reported in the primary study, preferably hazard ratios (HRs) with 95% confidence intervals (CIs). If HRs were not reported, we tried to calculate them from the values reported on survival curves. We used the number of patients and events per group and P value to calculate the HR with 95% CIs, and the observed‐expected with variance (the latter was used in the meta‐analysis). When a P value was not reported, we manually estimated the percentage of events for several time points on the Kaplan Meier curve (at least three) and calculated the same values from these data. This was done using the excel spreadsheet developed by Sydes and Tierney (Tierney 2007), which is based on the methods described in Parmar 1998 and Williamson 2002.
Dealing with missing data
We tried to complement missing data regarding review‐defined outcomes and risk of bias assessment by correspondence with trial authors. None of the authors replied or could complete missing data.
We have analyzed outcomes with missing data for some patients using available data only, since we were not able to impute missing values for time to event outcomes.
Assessment of heterogeneity
We assessed heterogeneity in each meta‐analysis using a Chi2 test of heterogeneity and the I2 statistic of inconsistency. Given the small number of studies in each of the analyses, we based the heterogeneity assessment mainly on the I2 statistic where a value greater than 50% represents substantial heterogeneity. We interpreted the importance of the observed I2 value by looking at the magnitude and the direction of effects.
Assessment of reporting biases
In analyses that include at least 10 trials we planned to draw funnel plots of effect estimates against study precision, and inspect asymmetry visually and indicate publication bias or other small study effects (Higgins 2011). However, all analyses were of five trials or less.
Data synthesis
We pooled RRs with 95% CIs using the fixed‐effect model. We recalculated all analyses using the random‐effects model, and reported significant discrepancies between the models. HRs were pooled according to Peto's method. Analyses were done in RevMan 5 (RevMan 2012).
Subgroup analysis and investigation of heterogeneity
As detailed above, we conducted a separate analysis for studies assessing directly the efficacy of anthracyclines by comparing the same chemotherapy regimens with or without the addition of an anthracycline. We set to investigate heterogeneity in each analysis based on the following preplanned subgroup analyses:
FL grade 3;
age over 60 years;
first‐line versus relapse or refractory;
regimens with or without monoclonal antibodies;
different anthracyclines.
However, the paucity of trials and lack of data in included studies allowed for subgroup analyses of the last count alone.
Sensitivity analysis
Allocation concealment (see above), which has been shown to affect subjective outcomes most strongly (Wood 2008). We planned to assess the effect of blinding, but all trials were open‐labelled.
Trials that encompassed a second randomization.
Publication status: published in a peer‐reviewed journal.
Given the paucity of included trials, we were able to fulfil only the first two counts, and did not undertake any further sensitivity analyses.
Results
Description of studies
Results of the search
See Characteristics of included studies; Characteristics of excluded studies; ; and Characteristics of ongoing studies tables.
A total of 1324 potentially relevant titles and abstracts were identified and screened for retrieval. Forty‐six were evaluated in more detail. Of these, 38 were excluded. Additional five ongoing trials were identified, of which two were terminated, two are ongoing and one was published in abstract form and excluded, since no randomization was done between ACR and non‐ACR (Flinn 2012). See Figure 1 for a flow diagram of the search.
1.

Study flow diagram.
Included studies
Eight RCTs were included in the systematic review, involving a total of 2636 randomized adult patients. Trials were conducted between 1974 and 2011, yet most were published in the 1980s and 1990s. Six studies recruited patients in Europe and two (Jones 1983; Peterson 2003) in the US. Six trials were conducted as part of a study group (Jones 1983; Kimby 1994; Unterhalt 1996; Peterson 2003; Taylor 2006; Federico 2013), and two were not (Lepage 1990; Zinzani 2000). All were multicenter trials. Time of follow‐up varied between three years and 18 years. Most trials reported outcomes at three to five years, while two trials (Peterson 2003; Taylor 2006) also reported outcomes at 10 years or more.
Type of participants
All trials involved patients with low‐grade NHL. Jones et al (Jones 1983) also included patients with high‐grade lymphoma, yet stratification and analysis was separate for patients with FL. All trials included patients with initial, untreated indolent NHL. In these trials, no prior chemotherapy was allowed. In three trials (Jones 1983; Taylor 2006; Federico 2013) prior radiotherapy to bulky regions was allowed, but was given in very few patients (not reported in Federico 2013).
Classification systems were not identical: three trials used REAL/WHO classification; three used Kiel classification initially, but two of those eventually reported results according to REAL classification; one used Working Formulation; and one used Rappaport classification.
In all studies recruitment was based on the pathologic diagnosis of the primary institution, yet central pathology reclassification was performed. Analysis was done according to initial classification, with subgroup analysis according to central pathology, except for one trial (Jones 1983) where changes in classification were frequent and analysis was based on central pathology alone.
Four trials ( Jones 1983; Kimby 1994; Unterhalt 1996; Peterson 2003) included only patients with advanced lymphoma (stage III/IV according to the Ann Arbor classification); three trials (Lepage 1990; Zinzani 2000; Federico 2013) included patients with stage II to IV NHL; and one trial (Taylor 2006) considered all patients eligible for treatment, yet only one patient with stage I disease was included. Of reported data, 96% of patients were stage III or IV.
All trials reported histology consistent with FL. They included 1520 evaluated patients with histologically confirmed FL. However, only two trials (Peterson 2003; Federico 2013) were designed specifically for FL patients. Other common indolent lymphomas included in studies were chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), and immunocytoma and mantle cell lymphoma (MCL). Grades of FL were reported in most trials, as specified in Additional Table 2. Most randomized FL patients were grade 1 or 2.
1. Studies included in meta‐analysis.
| Author | Randomized | Analyzed | FL analyzed | FL grade | Data specific for FL |
| Federico 2013 | 534 | 504 | 504 (333)* | 1, 2, 3a | For all outcomes |
| Jones 1983 | 652 | 497 | 226 (146)* | 1, 2, 3 | For all outcomes |
| Lepage 1990 | 113 | 113 | 101 | 1, 2 | For some outcomes |
| Taylor 2006 | 200 | 183 | 155 | Low grade | No |
| Zinzani 2000 | 208 | 199 | 102 | Indolent | For some outcomes |
| Kimby 1994 | 259 | 259 | 76 | 1, 2 | For some outcomes |
| Peterson 2003 | 228 | 228 | 189 | 1, 2 | For all outcomes |
| Unterhalt 1996 | 442 | 206 | 167 | 1, 2 | For some outcomes |
| Total | 2636 | 2189 | 1520 |
* number of FL patients analyzed in 2 (of 3) arms compared FL: follicular lymphoma
Common exclusion criteria were: severe cytopenias; significant kidney and liver disease; high ECOG score; cardiac failure contraindicating use of anthracyclines; and, where appropriate, any pulmonary dysfunction contraindicating use of bleomycin.
All patients were given first‐line treatment. Usual indications for treatment, when reported, were symptomatic, progressive or bulky disease, or cytopenia. In one trial (Peterson 2003) treatment was started within 100 days of diagnosis, regardless of symptoms. FLIPI/IPI score was reported in three trials (Zinzani 2000; Taylor 2006; Federico 2013). In the Taylor 2006 and Federico 2013 trials up to 37% of patients had a high FLIPI score. In Federico 2013, 27% of patients also had a high FLIPI2 score.
Study design
In four studies (Jones 1983; Lepage 1990; Unterhalt 1996; Taylor 2006) two consecutive randomization processes were performed (patients were randomized to type of induction therapy and then randomized again to maintenance therapy or observation). Our analyses refer to the first randomization.
Two studies had a triple‐arm design. Jones 1983 compared CHOP‐Bleo (cyclophosphamide, doxorubicin, vincristine, prednisone, bleomycin) versus CVP‐Bleo (cyclophosphamide, vincristine, prednisone, bleomycin) versus CHOP‐BCG (cyclophosphamide, doxorubicin, vincristine, prednisone, Bacillus Calmette Guerin). Since BCG was given also as part of induction therapy, and might be considered an immunotherapy, we did not extract data from this arm. Federico 2013 compared R‐CVP versus R‐CHOP versus R‐FM. We extracted data only for the first comparison, according to protocol.
Intervention
Five studies (Jones 1983; Lepage 1990; Zinzani 2000; Taylor 2006; Federico 2013) assessed the same chemotherapeutic regimen between study arms, yet only three trials reported OS data for FL patients specifically.
Importantly, three trials with 'same chemotherapies' were not exactly identical. Zinzani 2000 compared fludarabine with fludarabine‐idarubicin combination, yet fludarabine was given for five days in the control arm and only three days in the experimental arm. In Jones 1983 (CVP‐B versus CHOP‐B), the administration of cyclophosphamide varied by route, rate, and total dose between study arms, and there were also minor differences in bleomycin dose and frequency of cycles. In Federico 2013 (R‐CVP versus R‐CHOP), eight versus six cycles were given between non‐ACR and ACR arms, but the number of rituximab cycles was identical. Since regimens were basically similar, and higher doses were administered in the control arms, we have decided to pool results of these trials as 'same chemotherapy'.
Three studies (Kimby 1994; Unterhalt 1996; Peterson 2003) compared different regimens in the ACR and non‐ACR arms. For baseline characteristics of interventions see Additional Table 3. For a detailed comparison of therapeutic regimens see Additional Table 4.
2. Baseline characteristics of interventions.
| Author | Publication status | Same/different intervention | Anthracycline | Control arm | Experimental arm |
| Federico 2013 | Full text | Same | Doxorubicin | R‐CVP | R‐CHOP |
| Jones 1983 | Full text | Same* | Doxorubicin | CVP‐B | CHOP‐B |
| Lepage 1990 | Full text | Same | Doxorubicin | PCOP | PACOP |
| Taylor 2006 | Full text | Same | Idarubicin | ChD | ChID |
| Zinzani 2000 | Full text | Same* | Idarubicin | F | FI |
| Kimby 1994 | Full text | Different | Doxorubicin | Ch‐D | CHOP |
| Peterson 2003 | Full text | Different | Doxorubicin | C | CHOP |
| Unterhalt 1996 | Full text | Different | Mitoxantrone | CVP | PmM |
* trials with higher non‐anthracycline dose in control‐arm
3. Detailed therapeutic regimens in included studies.
| Author | Control arm | Experimental arm |
| Federico 2013 | d 1 rituximab 375 mg/m2, d 1 cyclophosphamide 750 mg/m2 IV, d 1 vincristine 1.4 mg/m2 (max 2 mg) IV, d 1‐5 prednisone 40 mg/m2 PO, 8 cycles, every 21 d [Note: more treatment cycles] |
d 1 rituximab 375 mg/m2, d 1 cyclophosphamide 750 mg/m2 IV, d 1 doxorubicin 50 mg/m2 IV, d 1 vincristine 1.4 mg/m2 (max 2 mg) IV, d 1‐5 prednisone 100 mg/d PO 6 cycles, every 21 d. Added 2 cycles of rituximab every 21 d |
| Jones 1983 | d 1‐14 cyclophosphamide 125 mg/m2 PO, d 1, 8 vincristine 1.4 mg/m2 IV (max 2 mg/dose), d 1‐5 prednisone 100 mg/d PO, d 1, 8 bleomycin 4 mg/m2 IV. 8 cycles, every 21 d [Note: different route of administration for cyclophosphamide; total doses differ for all drugs; cycles are more frequent] |
d 1 cyclophosphamide 750 mg/m2 IV, d 1 doxorubicin 50 mg/m2 IV, d 1 vincristine 1.4 mg/m2 (max 2 mg) IV, d 1‐5 prednisone 100 mg/d PO, d 1 bleomycin 4 mg/m2 IV. 8 cycles, every 28 d |
| Lepage 1990 | d 1, 8 cyclophosphamide 400 mg/m2 IV, d 1, 8 vincristine 1.4 mg/m2 , d 1, 14 procarbazine 80 mg/m2 PO, d 1‐5 prednisone 60 mg/m2 PO. 6 cycles, every 28 d | same + d 1, 8 doxorubicin 20 mg/m2 IV |
| Taylor 2006 | d 1‐3 chlorambucil 20 mg/m2/d, d 1‐5 dexamethasone 4 mg bd. 6 cycles, every 21 d | same + d 1‐3 idarubicin 10 mg/m2/d |
| Zinzani 2000 | d 1‐5 fludarabine 25 mg/m2/d IV. 6 cycles, every 28 d [Note: total fludarabine dose is higher by 66%] | d 1‐3 fludarabine 25 mg/m2/d IV, d 1‐3, d 1 idarubicin 12 mg/m2 6 cycles, every 28 d |
| Kimby 1994 | d 1 chlorambucil 0.4 mg/kg PO, d 1‐3 prednisone 75 mg PO every 14 d, for 4‐8 months | d 1 cyclophosphamide 750 mg/m2 IV, d 1 doxorubicin 50 mg/m2 IV, d 1 vincristine 2 mg/m2 IV, d 1‐5 prednisone 50 mg/m2 PO 4‐8 cycles every 28 d |
| Peterson 2003 | d 1 cyclophosphamide 750 mg/m2 IV, d 1 doxorubicin 50 mg/m2 IV, d 1 vincristine 1.4 mg/m2 (max 2 mg) IV, d 1‐5 prednisone 60 mg/m2 PO, d 1 bleomycin 10 u/m2 IM every 21 d until complete response and then every 28 d up to 2 years, bleomycin up to 6 cycles | 100 mg/m2/d PO until 2 years from maximal response |
| Unterhalt 1996 | d 1‐5 cyclophosphamide 400 mg/m2/d IV, d 1 vincristine 1.4 mg/m2 (max 2 mg) IV, d 1‐5 prednisone 100 mg/m2/d PO 4‐6 induction cycles every 21 d | d 1‐5 prednimustine 100 mg/m2/d PO, d 1‐2 mitoxantrone 8 mg/m2/d IV. 4‐6 induction cycles every 28 d |
d: day; IM: intramuscular; IV: intravenous; PO: oral
Different anthracyclines were used in the studies: five used doxorubicin (Jones 1983; Lepage 1990; Kimby 1994; Peterson 2003; Federico 2013); two used idarubicin (Zinzani 2000; Taylor 2006); and one used mitoxantrone (Unterhalt 1996). Rituximab was used only in Federico 2013.
Outcome assessment
All but one trial (Unterhalt 1996) assessed OS, although not necessarily as the primary outcome. However, Federico 2013 did not eventually report OS data and Zinzani 2000 did not report OS separately in FL patients. Finally, only three trials with similar chemotherapeutic regimens were analyzed for survival (Jones 1983; Lepage 1990; Taylor 2006).
All trials assessed response rate, although definitions were not uniform across trials. Outcomes regarding disease control varied considerably across trials, and commonly were not compatible with the subsequently published criteria for reporting outcomes from clinical trials (Cheson 1999; Cheson 2007). The outcomes and their definitions are specified in the Characteristics of included studies table.
Excluded studies
Thirty‐nine trials were excluded (including one ongoing trial, Flinn 2012). Reasons for exclusion were as follows: eight were non‐randomized; five did not include patients with FL; four did not include anthracyclines in study arms and two included anthracyclines in both study arms (one study with multiple combinations); three trials compared irradiation to ACR; one trial had immunotherapy (interferon) only in one study arm; one involved different chemotherapeutic regimens in conjunction with immunotherapy (rituximab); two did not randomize between ACR and non‐ACR arms; one was a cross‐over trial; one article emphasized comparison of different diseases rather than therapeutic arms; two were reviews; in two relevant outcome data could not be retrieved; and five were double publications, that did not add information to the main articles. Three trials reported results for indolent NHL patients, but did not specify the number of FL patients included. Al‐Ismail 1987 randomized 47 low grade lymphoma patients between CVP and epirubicin, vincristine, prednisolone (EPV) regimen. There was no difference in response rates, response duration or OS. Prentice 1996, published only in abstract form, randomized 182 patients with low grade NHL, that were stage II or more, between chlorambucil‐prednisone combination and same therapy with mitoxantrone. The results indicated benefit for ACR, with a higher rate of CR (58% versus 44%, P = 0.02); longer disease‐free survival (2.98 years versus not reached); and longer OS (median of 3.31 years versus not reached). Lastly, Santoro 2006, also published in abstract form, compared rituximab as single agent with pixantrone and rituximab combination, in 38 relapsed and refractory indolent NHL patients. Response rates were higher in the immunochemotherapy combination, as was progression‐free survival, although data are missing from abstract, and we could not retrieve any further information from authors.
Risk of bias in included studies
All included studies were described as RCTs. Generation of randomization sequence was assessed as adequate for four trials, where it was done centrally (Jones 1983; Kimby 1994; Unterhalt 1996; Federico 2013). The other studies did not describe the methods used for generation of randomization and hence were classified as unclear. Allocation concealment was assessed as adequate in five trials (Jones 1983; Lepage 1990; Kimby 1994; Unterhalt 1996; Federico 2013) and not reported in the other trials. None of the trials were blinded (Figure 2).
2.
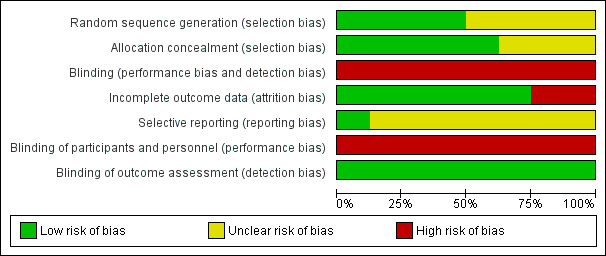
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Two trials were stopped early. In Jones 1983, the control arm (CVP‐B) was stopped early when inferiority was suspected. Moreover, 652 patients were randomized in this trial, but only 497 were centrally reclassified and analyzed. Unterhalt 1996 enrolled 442 patients, 42 of whom were excluded owing to protocol violation and NHL subtypes not in concert with trial. However, after only 246 patients were analyzed, the authors' basic assumption of a 20% improvement in overall response could be rejected. Hence, analysis of remaining patients was abandoned, and it was not specified in two double publications (Hiddemann 1994; Unterhalt 1994).
Trials stated that analysis was done on an intention‐to‐treat (ITT) basis, but in most cases, the provided data could not fully support this statement. Drop‐out rate was less than 10% in all but one trial (Unterhalt 1996). Incomplete outcome data could not be well assessed also for Jones 1983 where data were analyzed only for 497 patients with centrally reviewed pathologies, out of total of 636 eligible randomized patients. Thus, according to incomplete outcome data, six trials were considered as low risk for attrition bias and two as high risk.
In Peterson 2003, data were reported for 228 patients (six patients were excluded, three in each arm). Of those, a separate analysis was made for 189 patients with confirmed central pathology, in order to compare between grade 1 and grade 2 FL. In our analysis, we used data from the whole cohort, which is more elaborate and where outcomes were reported on an ITT basis.
Selective outcome reporting could not be assessed since most trials were old and trial registry or protocol were unavailable. The outcomes reported were in accordance with those specified in the methods section of the final publication.
Taylor 2006 reported a protocol violation that may have confounded results, as more patients in the experimental arm received radiotherapy, although they did not initially suffer from a bulky disease. They were included in the analysis on an ITT basis.
All trials were published in peer‐reviewed journals, and all reported ethics committee approval and that informed consent was obtained from the patients.
Three trials declared their funding resources, and were not supported by the pharmaceutical industry (Jones 1983; Taylor 2006; Federico 2013). Five studies did not report funding resources (Lepage 1990; Kimby 1994; Unterhalt 1996; Zinzani 2000; Peterson 2003).
Effects of interventions
See: Table 1
Studies comparing same chemotherapy
Five of the eight trials included in this review administered the same chemotherapy (other than anthracyclines). The following sections report outcome measures of these trials alone, and when possible, sum their total effect. We analyzed data specifically for FL patients, or from trials with over 75% FL patients. Three of the trials used higher doses of non‐anthracycline therapies in the control arm. When possible, we examined the effect of those trials separately. In Zinzani 2000, FL patients constituted only about a half of the cohort. Results were included in analysis, only for parameters reported separately for FL patients.
The three trials that used different chemotherapies in study arms, will be described separately. Since the regimens differ considerably between trials, we did not sum their effect, but rather summarized them in a table.
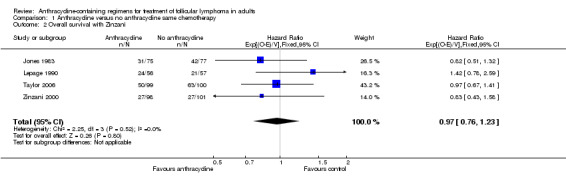
Overall survival
OS data were analyzed for 464 patients included in three studies (Jones 1983; Lepage 1990; Taylor 2006). The pooled HR for OS was 0.99 (95% CI 0.77 to 1.29; Figure 3), indicating that there was no advantage to ACR chemotherapy compared with other chemotherapy. There was no heterogeneity among trials (P = 0.37; I2 = 0%). Federico 2013 did not publish mortality data, as it was under‐powered to assess survival. We did not include Zinzani 2000 in the analysis, since survival data were reported for the whole cohort, and not specifically for FL. However, adding it did not change the pooled results (HR 0.97; 95% CI 0.76 to 1.23, Analysis 1.2).
3.

Forest plot of comparison: 1 Anthracycline versus no anthracycline same chemotherapy, outcome: 1.1 Overall survival.
1.2. Analysis.
Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 2 Overall survival with Zinzani.
All‐cause mortality
There was no difference between ACR and non‐ACR chemotherapy for mortality at three years (RR 0.92; 95% CI 0.67 to 1.26; three trials; Figure 4), and no heterogeneity among trials (P = 0.48; I2 = 0%). We found similar results for five‐year mortality (RR 0.95; 95% CI 0.77 to 1.18; three trials; 539 patients; Analysis 1.4). Data regarding 10‐year all‐cause mortality were reported only in Taylor 2006, where there was no benefit to anthracyclines.
4.

Forest plot of comparison: 1 Anthracycline versus no anthracycline same chemotherapy, outcome: 1.3 Mortality at 3 years.
1.4. Analysis.
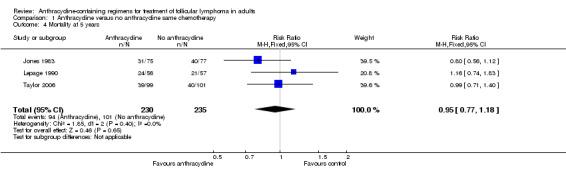
Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 4 Mortality at 5 years.
Response rate (CR and ORR)
All trials contributed to the analysis of CR, and all reported response data for FL patients separately. Use of ACR was not statistically significantly better than non‐ACR (RR 1.05; 95% CI 0.94 to 1.18; Figure 5), yet there was moderate heterogeneity between trials (P = 0.12; I2 = 46%). One trial, showing the opposite, contributed to the heterogeneity (Zinzani 2000). This discrepancy may arise from the higher dose of fludarabine in the control arm. Excluding Zinzani 2000 from the analysis did not change results (RR 1.11; 95% CI 0.99 to 1.25; I2 = 0%). Also, using the random‐effects model did not considerably change the analysis (RR 1.03; 95% CI 0.86 to 1.23).
5.
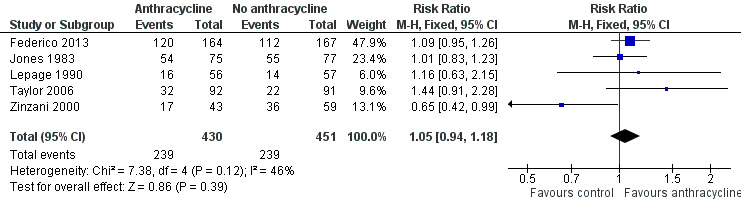
Forest plot of comparison: 1 Anthracycline versus no anthracycline same chemotherapy, outcome: 1.5 Complete response.
ORR data were available in three trials (Zinzani 2000; Taylor 2006; Federico 2013), analyzing 616 patients. The pooled RR was 1.06 (95% CI 1.00 to 1.12; I2 = 12%; Analysis 1.6), with a probable effect for ACR, although not statistically significant.
1.6. Analysis.

Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 6 Overall response.
Disease control
Disease control measures were reported in four trials using same chemotherapy (Jones 1983; Zinzani 2000; Taylor 2006; Federico 2013). End points, as specified in studies, were: PFS, RFS, TTTF, and PFS, respectively (for outcomes definitions see Characteristics of included studies table). However, these measures were often ill‐defined, or were not necessarily compatible with common guidelines. Zinzani 2000, for example, used PFS to describe remission duration (RD) in responding patients.
With respect to disease control, ACRs were superior to non‐ACRs, with a pooled HR of 0.65 (95% CI 0.52 to 0.81; Figure 6), with some heterogeneity (I2 = 46%; P = 0.14) resulting only from differential benefit for ACR.
6.
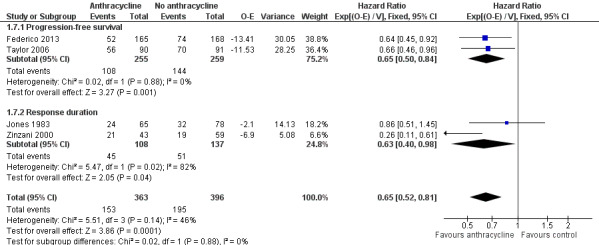
Forest plot of comparison: 1 Anthracycline versus no anthracycline same chemotherapy, outcome: 1.7 Disease control.
Disease control outcomes could be divided into two groups, according to time from which outcomes were counted: PFS, where outcomes were counted from randomization, and RD, which include only patients responding to treatment from end of therapy. ACRs retained their benefit for PFS (two studies; HR 0.65; 95% CI 0.50 to 0.84) and RD (two studies, HR 0.63; 95% CI 0.40 to 0.98), separately (Figure 6).
Progression or relapse
Progression or relapse was chosen as a complementary dichotomous measure of disease control, relevant in patients with FL. This outcome was assessed in four trials (Jones 1983; Zinzani 2000; Taylor 2006; Federico 2013), and could be extracted at three years in all trials. The pooled RR for previously untreated FL was 0.73 (95% CI 0.63 to 0.85; little heterogeneity I2 = 7%; Figure 7), indicating that therapy‐naive FL patients treated with ACR had a lower progression rate at three years.
7.
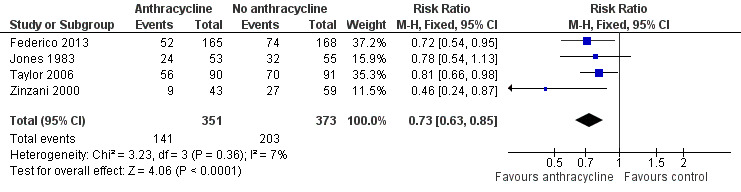
Forest plot of comparison: 1 Anthracycline versus no anthracycline same chemotherapy, outcome: 1.8 Progression or relapse at 3 years.
Toxicity
Neutropenia
Four trials reported neutropenia as a serious event, but only two (Taylor 2006; Federico 2013) were amenable for meta‐analysis, demonstrating a higher rate of grade 3‐4 neutropenia with ACR (RR 1.94; 95% CI 1.46 to 2.56; Analysis 1.9). Zinzani 2000 (fludarabine‐idarubicin‐ versus fludarabine) reported equal distribution of neutropenia (toxicity over treatment cycles rather than absolute episodes). However, a higher dose of fludarabine was administered in the control arm. Jones 1983 gave a qualitative description of more neutropenia in ACR‐treated patients.
1.9. Analysis.
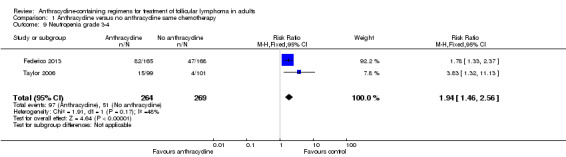
Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 9 Neutropenia grade 3‐4.
Infection
The types of infection reported varied considerably between any infection, serious infection, and neutropenic fever. Three trials were included in a meta‐analysis considering the infection reported in the study (Jones 1983; Taylor 2006; Federico 2013). The pooled RR of anthracyclines was 1.16 (95% CI 0.75 to 1.80; Figure 8) with considerable heterogeneity (I2 = 51%; P = 0.13).
8.

Forest plot of comparison: 1 Anthracycline versus no anthracycline same chemotherapy, outcome: 1.10 Infection.
Cardiotoxicity
Cardiotoxicity was reported in three trials that employed similar regimens. In Jones 1979, 11 cardiac events were recorded in 472 patients receiving ACR (including patients from a third arm of CHOP‐BCG). Zinzani 2000 had no cardiac events in both arms. Federico 2013 reported one grade 3‐4 event in each study arm.
Quality of life
QoL was not assessed in the included trials. However, nausea and vomiting, diarrhea, and mucositis were reported more often with ACR.
Sensitivity analysis
There was no evidence for OS benefit of anthracyclines in trials reporting adequate allocation concealment or in those with unclear allocation concealment (Analysis 2.1). ACR were superior to non‐ACR in terms of disease control, regardless of allocation concealment quality (HR 0.70; 95% CI 0.52 to 0.95 and HR 0.58; 95% CI 0.41 to 0.81, respectively; P = 0.38 for subgroup difference; Analysis 2.2).
2.1. Analysis.
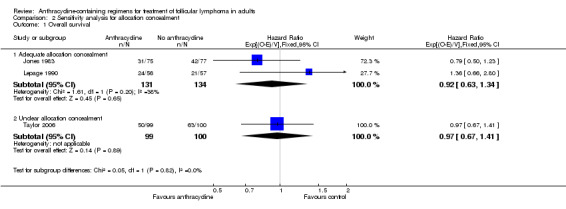
Comparison 2 Sensitivity analysis for allocation concealment, Outcome 1 Overall survival.
2.2. Analysis.
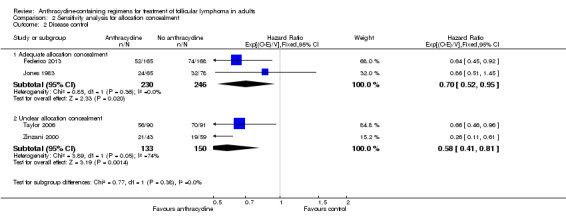
Comparison 2 Sensitivity analysis for allocation concealment, Outcome 2 Disease control.
All studies were published as peer‐reviewed journal articles.
As mentioned above, three studies proceeded to a second randomization (Jones 1983; Lepage 1990; Taylor 2006), and only these trials reported OS. Second randomization did not influence disease control (P value for subgroup difference was 0.26, Analysis 3.1).
3.1. Analysis.
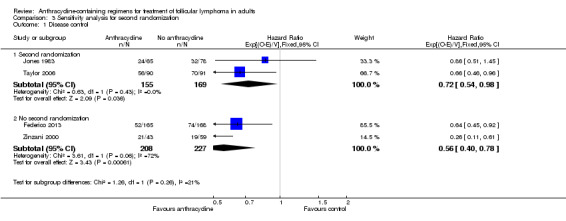
Comparison 3 Sensitivity analysis for second randomization, Outcome 1 Disease control.
Subgroup analysis
There were not sufficient outcome data for subgroup analysis according to grade of FL or age of patients. Also, all patients included had first‐line therapy. Only Federico 2013 had included monoclonal antibodies (rituximab) in study arms. Its results were consistent with those of the other studies.
We undertook subgroup analysis of the different anthracyclines used. Regarding OS, trials employing the same chemotherapies used either doxorubicin (Jones 1983; Lepage 1990) or idarubicin (Taylor 2006). There was no evidence of survival benefit to either type of anthracycline, as shown in Analysis 4.1. Disease control measures were reported in two trials employing doxorubicin (Jones 1983; Federico 2013) and two trials using idarubicin (Zinzani 2000; Taylor 2006). The advantage of anthracyclines in disease control was preserved regardless of type of anthracycline used, as shown in Figure 9.
4.1. Analysis.
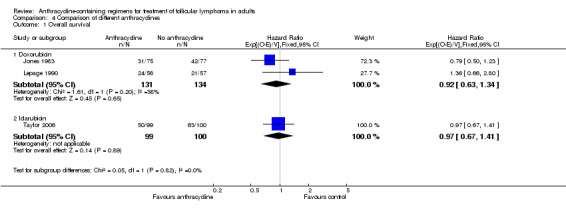
Comparison 4 Comparison of different anthracyclines, Outcome 1 Overall survival.
9.
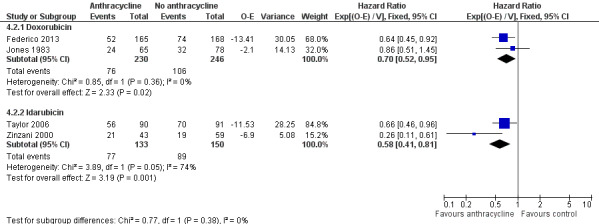
Forest plot of comparison: 4 Comparison of different anthracyclines, outcome: 4.2 Disease control.
Studies comparing different chemotherapies
Three trials used different regimens between study arms, and among trials themselves, and therefore could not be summed together.
Peterson 2003 compared CHOP‐B with continuous cyclophosphamide PO; Unterhalt 1996 compared prednimustine‐mitoxantrone with CVP regimen; and Kimby 1994 evaluated CHOP with chlorambucil‐dexamethasone, both given PO (Characteristics of included studies).
The effects of interventions are summarized in Additional Table 5, and graphically in Analysis 5.1; Analysis 5.2; Analysis 5.3; and Analysis 5.4. Consistent with pooled results presented for trials comparing the same chemotherapy, there was no evidence of effect on survival, three‐, and five‐year mortality for the ACR arms. All trials showed a trend in favour of anthracyclines for disease control. Results for CR and OR were heterogeneous.
4. Outcome measures in trials with different chemotherapeutic regimens.
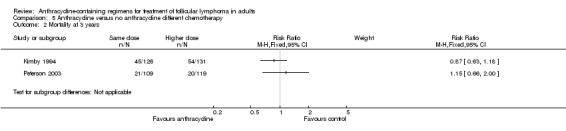
| OS | Mortality at 3 years | Mortality at 10 years | CR | ORR | Disease control | Progression or relapse | |
| Kimby 1994 | 0.89 (0.67 to 1.18) |
0.87 (0.63 to 1.18) |
NR |
3.42 (1.52 to 7.68) |
1.70 (1.30 to 2.23) |
0.66 (0.41 to 1.05) |
0.90 (0.67 to 1.20) |
| Peterson 2003 | 0.96 (0.70 to 1.31) |
1.15 (0.66 to 2.00) |
0.95 (0.74to 1.23) |
0.90 (0.74 to 1.10) |
1.04 (0.96 to 1.13) |
0.84 (0.63 to 1.11) |
1.03 (0.82 to 1.28) |
| Unterhalt 1996 | NR | NR | NR |
1.82 (1.10 to 3.01) |
1.02 (0.89 to 1.17) |
0.59 (0.36 to 0.98) |
NR |
CR: complete response; NR: not reported; ORR: overall response rate; OS: overall survival
5.1. Analysis.
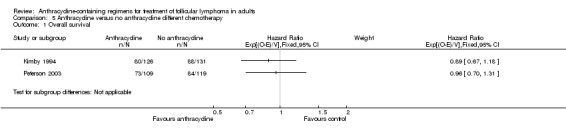
Comparison 5 Anthracycline versus no anthracycline different chemotherapy, Outcome 1 Overall survival.
5.2. Analysis.
Comparison 5 Anthracycline versus no anthracycline different chemotherapy, Outcome 2 Mortality at 3 years.
5.3. Analysis.
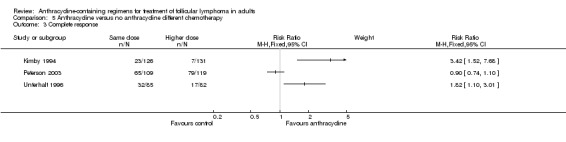
Comparison 5 Anthracycline versus no anthracycline different chemotherapy, Outcome 3 Complete response.
5.4. Analysis.
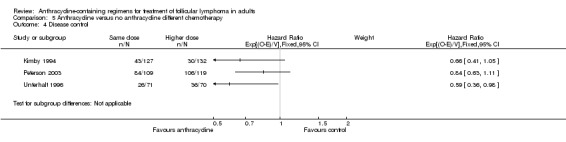
Comparison 5 Anthracycline versus no anthracycline different chemotherapy, Outcome 4 Disease control.
Toxicity
Owing to the different regimens used in the ACR and non‐ACR arm we did not proceed with a comparative analysis of adverse events from these trials, although a higher rate of myelosuppression was noted with ACR. Peterson 2003 (cyclophosphamide versus CHOP) have reported two cases of cardiotoxicity only in the ACR arm.
Additional analysis ‐ cardiotoxicity (all studies)
Since cardiotoxicity is mainly attributed to anthracyclines we pooled results from all trials reporting cardiotoxicity, regardless of regimen similarity (Jones 1983; Zinzani 2000; Peterson 2003; Federico 2013). The pooled RR of ACRs was 4.55 without heterogeneity, but with a very wide 95% CI (0.92 to 22.49) as shown in Analysis 6.1.
6.1. Analysis.
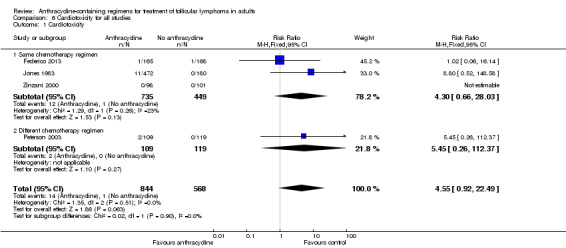
Comparison 6 Cardiotoxicity for all studies, Outcome 1 Cardiotoxicity.
Discussion
Summary of main results
This systematic review was undertaken to evaluate the role of anthracyclines in the treatment of patients with FL. Eight RCTs involving a total of 2636 patients were compatible with the inclusion criteria. Over a half of randomized patients had centrally confirmed FL and were evaluable for analysis. Outcomes were reported separately for FL patients in most trials. All patients were therapy‐naive, and most had low‐grade, advanced‐stage FL. We separated the analysis of trials comparing the same chemotherapy with or without an anthracycline and trials comparing different chemotherapies.
The following findings emerged.
There was no evidence that anthracyclines improved OS or mortality at three and five years.
FL patients treated with ACR had statistically significantly better disease control (e.g. PFS, RD).
Subgroup analysis showed that these effects do not necessarily depend on the type of anthracycline.
ACR‐treated patients had more side effects than non‐ACR‐treated patients, including myelotoxicity and cardiotoxicity.
Overall completeness and applicability of evidence
The first‐line treatment of patients with FL is still undetermined. In the US, most patients are treated with R‐CHOP, an ACR. However, there is no proof that ACRs are superior to non‐ACRs. To our knowledge, this is the first systematic review and meta‐analysis trying to answer this important question.
The contribution of anthracyclines is best elucidated in trials administering the same regimens in both study arms, as they were designed specifically for this question. We also included trials that involved different regimens in study arms, since anthracyclines are dominant antitumor agents. However, these trials were designed to compare different chemotherapy combinations, where anthracycline is only one of the components. It is impossible, then, to differentiate the effect of anthracyclines from other chemotherapeutic agents, or their combination. Hence, we have separated the analysis of trials according to similarity of chemotherapies in study arms, and outcome measures were summed only from studies that used the same chemotherapy.
Even among trials with 'same chemotherapy', regimens were not identical between study arms in three out of five studies. Nonetheless, similarity outweighed the difference (e.g. R‐CVP eight cycles versus R‐CHOP, six cycles in Federico 2013), and we have chosen to sum outcome data. Moreover, since control arms were more intense in all three trials discussed, any benefit of anthracyclines would only underscore their importance.
All trials, except for two (Peterson 2003; Federico 2013), randomized patients with NHL, and not specifically FL. However, most patients in these studies had FL, or alternatively, specific data regarding FL patients were reported. Moreover, although diagnosis of FL was usually based on initial pathology, it was confirmed centrally. Thus, even though different classification systems were applied in different trials, it is reasonable to assume that the diagnosis was correct. All FL patients in these trials were previously untreated, and most had advanced‐stage, low‐grade FL. There were not sufficient data to analyze the role of anthracyclines in untreated early‐stage disease, or in persistent or relapsing disease. Subgroup analysis regarding effect of anthracyclines in different grades of FL was not possible.
Follow‐up varied between three and 18 years, but most trials reported outcomes at five years, which is reasonable given that until recently the median survival of FL patients was considered to be eight to 10 years. With the introduction of immunotherapy, namely rituximab, longer follow‐up periods are desired.
A major limitation to our review is that the trials included were conducted mostly in the pre‐rituximab era. Encouraging data came from Federico 2013, which showed a statistically significant improvement in time to treatment failure with R‐CHOP compared to R‐CVP, at three years' follow‐up. An ongoing trial comparing R‐CHOP to R‐CVP with the addition of R‐maintenance (NCT00801281) is expected to further elucidate the role of anthracyclines in the rituximab era. Other trials are conducted to assess the utility of various R‐pixantrone‐based regimens in FL patients.
Other important questions that remained unanswered are: the benefit of anthracyclines in high‐risk FL patients, according to FLIPI score; their role in molecular remission and its relevance to prolonged PFS; their effect on FL transformation rate; QoL in patients treated with anthracyclines; and the association between anthracyclines and secondary malignancies.
Quality of the evidence
All trials were RCTs, which is the optimal design for assessment of interventions, yet they were not blinded. All of them were multicenter trials. Hence, we believe that the randomization process was adequate in most trials.
In randomized trials, an estimated intervention effect may be biased if some randomized participants are excluded from the analysis. ITT analysis is often recommended as the least biased way to assess an intervention. In our review, most trials claimed to have used ITT analysis, but in fact reported results for fewer patients than were initially randomized (e.g. only for patients with confirmed FL histology). However, the low drop‐out rate in seven trials supports the authors' claim. Protocol violation (Taylor 2006) and early stopping of trials (Jones 1983, and specifically Unterhalt 1996 who did not complete analysis of recruited patients), also weakens the strength of evidence contributed by them.
By using HR for analysis of time‐to‐event data, such as OS and disease control measures, we minimized the risk of bias related to measuring death or progression at specific time points. We complemented these results by extracting and calculating RRs for mortality at three and five years, and progression or relapse at three years.
We observed a lack of uniformity related to the reporting of treatment‐associated side effects. The most often reported grade 3 and 4 adverse events were myelosuppression, fever, infection, nausea and vomiting, and alopecia.
Potential biases in the review process
Only a few trials were suitable for meta‐analysis of the various outcome measures. Hence, publication of new large trials could substantially change the results of this meta‐analysis.
Many trials were conducted and published over a decade ago, which might have introduced bias for several reasons. Over the years different classification systems have been used for hematological malignancies, and there is no certainty that analyzed patients would be regarded as FL patients using present guidelines; methods for staging and the definition of response measures have changed as radiology and laboratory methods improved; standard of care including supportive care have changed over the years, allowing patients to withstand more aggressive therapies. Finally, we could not complete missing data in the older trials.
Various protocols, some of which are scarcely administered currently, and different types of anthracyclines have been used making it difficult to assess the role of a specific anthracycline within a given regimen (e.g. doxorubicin in CHOP versus CVP).
There was substantial variability in outcome definition for disease control measures. The definition was often partial and did not necessarily comply with the currently published criteria (Cheson 1999; Cheson 2007). Death was inconsistently included in trial definitions, and when reported, we had to assume it was death by any cause, and not related to lymphoma specifically.
Adverse events were reported in all trials, but inconsistently, and using different measures (i.e. episodes of fever per cycle or per patient; any infection versus severe infection). Therefore, reporting bias is likely. Qualitatively, adverse events were more often reported with ACR.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first systematic review and meta‐analysis regarding use of anthracyclines in FL patients. Conflicting results arose from retrospective studies regarding OS and FFS (Dana 1993; Rigacci 2003). Most other data came from indirect comparisons, comparing treatment arms to historical cohorts and published literature, indicating an improved response rate with anthracyclines, with inconsistency in relation to other end points (Brandt 2001; Bendandi 2008; Tilly 2008; Vitolo 2008; Siddhartha 2009).
A previous systematic review of chemotherapy effects in indolent NHL (Brandt 2001) neither focused on FL patients, nor on ACR. Three trials were identified (Kimby 1994 and Unterhalt 1996 were included in our review; Al‐Ismail 1987 was excluded), all of which compared different regimens in study arms. The authors concluded that there was no evidence that initial combination therapy prolonged survival, with no remark concerning PFS.
Siddhartha (Siddhartha 2009) tried to evaluate the difference between R‐CHOP and R‐CVP indirectly, based on trials comparing R‐CHOP versus CHOP and R‐CVP versus CVP. Results suggested better a CR rate with R‐CVP, but higher ORR with R‐CHOP. However, OS and disease control measures were not addressed.
Notably, our meta‐analysis is in agreement with almost all aforementioned studies, in that an improvement of disease control measures (i.e. PFS) did not translate into prolonged survival. This contradiction emphasizes the incurable nature of FL, on the one hand, while underscoring the plentitude of effective salvage therapies and improved supportive care, on the other hand. Another factor to consider that the somewhat more intense regimens in the control‐arms of three trials ( Jones 1983; Zinzani 2000; Federico 2013), may have underestimated the benefit of anthracyclines in the treatment of FL. Relatively short follow‐up periods in included trials and the older population of FL patients, may have a role as well. Yet, the place of PFS in directing therapy choices is still debatable.
Olin et al (Olin 2010) have used decision analysis methodology to show that PFS is the most important factor in maximizing quality‐adjusted life years, in advanced‐stage, low‐grade FL patients. Based on their estimates of PFS and QoL from available literature, R‐CHOP is the optimal regimen, followed by R‐Flu, while R‐CVP consistently generated fewer quality‐adjusted life years. QoL assessment could not be assessed in our systematic review.
Since there is no consensus on initial treatment of FL, the NLCS published a report of treatment trends of FL in the US (Friedberg 2009). Over 50% of patients were treated with R‐CHOP. Moreover, this has become the recommended treatment for patients with grade 3 FL (Buske 2008). Nonetheless, other authors have expressed doubt regarding the role of anthracyclines (Chau 2003; Nabhan 2007).
The debate concerning best chemotherapy regimen in the rituximab era may seem obsolete, as combination of rituximab with nearly any induction chemotherapy is superior in terms of OS to the same regimen alone (Schulz 2007). This is reinforced by the fact that maintenance rituximab prolongs OS in patients with relapsed, recurrent, or resistant FL (Vidal 2009). However, a clue regarding the importance of anthracyclines in induction therapy for FL came from the international Primary Rituximab and Maintenance (PRIMA) study. This study has shown significant reduction in lymphoma progression with rituximab maintenance as compared with observation. However, in a preplanned Cox regression multivariate analysis, longer PFS was associated with R‐CHOP induction therapy (75% of patients), but could not reach statistical significance for R‐CVP therapy (22% of patients) (Salles 2011).
As the role of anthracyclines with immunotherapy remains to be elucidated, an old drug that has recently re‐emerged puts the necessity of ACR in FL into question. In one large randomized trial comparing bendamustine‐rituximab with R‐CHOP, Rummel et al found the non‐ACR to be superior with respect to PFS and CR and less toxic, with a median follow‐up of 45 months (Rummel 2009; Rummel 2013).
Adverse events were reported more often with ACR, including cardiotoxicity. Various methods have been developed and variably studied in order to reduce them: use of anthracycline‐analogues; continuous infusion of adriamycin (as was also shown in one meta‐analysis, van Dalen 2009); use of liposomal preparations; or administration of various substances including calcium channel blockers and angiotensin receptor blockers (Outomuro 2007). In our meta‐analysis, cardiotoxicity was mostly reported in trials involving doxorubicin. No cardiotoxicity was reported with idarubicin in Zinzani 2000. Al‐Ismail et al had demonstrated a significantly lower rate of cardiotoxicity from epirubicin, in comparison with adriamycin, in high‐grade lymphoma patients (Al‐Ismail 1987). Zinzani et al (Zinzani 2004) compared CHOP with fludarabine‐mitoxantrone (FM), and showed higher CR and molecular remission rate with FM, with fewer adverse events, such as grade 3‐4 neutropenia. However, at 19 months of follow‐up, there was neither survival nor PFS benefit to either regimen. Moreover, no cardiotoxicity was registered.
Authors' conclusions
Implications for practice.
ACR, regardless of the chemotherapeutic regimen, increased PFS and RD, in first‐line treatment of advanced, low‐grade FL patients. There was no notable effect on OS, although disparities in regimens might weaken this result, in favour for ACR. Evidence is of moderate quality.
In an indolent disease with a remitting and relapsing long‐term course, importance of PFS prolongation needs to be considered, especially in light of increased toxicity with ACR.
Implications for research.
The role of anthracyclines in conjunction with immunotherapy needs to be elucidated in a large‐scale RCT. There are a few ongoing trials comparing R‐CHOP with R‐CVP or with other regimens, and they are most likely to answer this important question. RCTs assessing the role of anthracyclines for early‐disease, high‐grade FL or as second‐line treatment are needed. Prospective trials with long follow‐up periods (of 10 years or more) are needed in order to assess OS benefit in FL patients adequately. In addition, the effect of ACR on molecular remission, and its importance as a response measure requires investigation.
The efficacy and adverse events rate of various non‐doxorubicin ACR should be investigated, especially in more frail populations. However, the availability of novel and efficacious biologic agents makes it unlikely that new resources will be invested.
Acknowledgements
We thank all authors and investigators who provided us with additional information. We especially thank Professor Federico and his team for their co‐operation in providing the data from their important study, which was published in an abstract form. We thank the editors and the consumers of the Cochrane Hematological Malignancies Group for their support and meticulous comments.
Appendices
Appendix 1. PubMed search strategy
Part I AND part II AND part III
Part I:
((indolent OR follicular OR nodular OR follicle‐cell OR centroblastic OR centrocytic OR ((zentroblast* or zentrocytic*) and lymphom*) OR follicle‐center OR low‐grade OR Brill‐ symmer) AND lymphoma*) OR Lymphoma, Follicular [Mesh]
Part II:
Doxorubicin [Mesh] OR AD mycin OR adriacin OR adriamycin OR adriablastina OR adriablastine OR adriablastin OR adrimedac OR adrim OR adrosal OR axibin OR biorrub OR biorubina OR candria OR dobixin OR daxotel OR dicladox OR dox‐sl OR doxsl OR doxobin OR doxo‐cell OR doxolem OR doxomed OR doxotec OR doxotil OR farmiblastina OR fauldoxo OR ifadox OR myocet OR neoxane OR onkodox OR oxocina OR pallagicin OR rastocin OR ribodoxo OR rubex OR rubidox OR doxil OR caelyx OR Doxocris OR Doxokebir OR Doxonolver OR Doxopeg OR Doxorbin OR Doxtie OR Flavicina OR Lipo‐dox nagun OR Oncodox OR Oncodria OR Onkostatil OR Ranxas OR Rubinat OR Ribodoxo OR Roxorin OR Serodox OR Roxodox OR Tevadox OR Varidoxo OR daunorubicin [MeSH] OR Daunoblastine OR Daunobin OR Daunomycin OR Rubomycin OR Rubidomycin OR daunoxome OR cerubidine OR cerubidin OR daunoblastina OR daunoblastin OR daunocin OR oncodaunotec OR rubilem OR idarubicin [MeSH] OR Desmethoxydaunorubicin OR Demethoxydaunorubicin OR DMDR IDA OR imi‐30 OR imi30 OR nsc‐256439 OR nsc256439 OR idamycin OR idaralem OR zavedos OR epirubicin [MeSH] OR anthracin OR bioepicyna OR ciazil OR ellence OR epi‐cell OR epilem OR epi‐NC OR epirub OR E.P.Mycin OR farmorrubicina OR farmorubicina OR farmorubicine OR farmorubicin megarubicin OR nuovodox OR pharmorubicin OR riboepi OR rubina OR tecnomax OR Epi‐DXR OR Epi‐cell OR Epicell OR Epi‐ADR OR Epiadriamycin OR Axirubine OR Bendaepi OR imi‐28 OR imi28 OR nsc‐256942 OR nsc256942 OR mitoxantrone [MeSH] OR domitrone OR ebexantron OR elsep OR formyxan OR genefadrone OR haemato‐tron OR misostol OR mitaxis OR mitoxal OR mitoxan OR mitoxgen OR mitroxene OR mitroxone OR neotalem OR neoxantron OR norexan OR novantrone OR Mitozantrone OR Novatron OR Onkotrone OR Parlifan OR Ralenova OR anthracyclin* OR anthracenedione
Part III:
| #1 #2 #3 #4 #5 #6 #7 #8 #9 #10 #11 |
randomized controlled trial [pt] controlled clinical trial [pt] randomized [tiab] placebo [tiab] drug therapy [sh] randomly [tiab] trial [tiab] groups [tiab] #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 animals [mh] NOT humans [mh] #9 NOT #10 |
Appendix 2. CENTRAL search strategy
Part I AND part II
Part I:
((indolent OR follicular OR nodular OR follicle‐cell OR centroblastic OR centrocytic OR ((zentroblast* or zentrocytic*) and lymphom*) OR follicle‐center OR low‐grade OR Brill‐ symmer) AND lymphoma*) OR Lymphoma, Follicular [Mesh]
Part II:
Doxorubicin [Mesh] OR AD mycin OR adriacin OR adriamycin OR adriablastina OR adriablastine OR adriablastin OR adrimedac OR adrim OR adrosal OR axibin OR biorrub OR biorubina OR candria OR dobixin OR daxotel OR dicladox OR dox‐sl OR doxsl OR doxobin OR doxo‐cell OR doxolem OR doxomed OR doxotec OR doxotil OR farmiblastina OR fauldoxo OR ifadox OR myocet OR neoxane OR onkodox OR oxocina OR pallagicin OR rastocin OR ribodoxo OR rubex OR rubidox OR doxil OR caelyx OR Doxocris OR Doxokebir OR Doxonolver OR Doxopeg OR Doxorbin OR Doxtie OR Flavicina OR Lipo‐dox nagun OR Oncodox OR Oncodria OR Onkostatil OR Ranxas OR Rubinat OR Ribodoxo OR Roxorin OR Serodox OR Roxodox OR Tevadox OR Varidoxo OR daunorubicin [MeSH] OR Daunoblastine OR Daunobin OR Daunomycin OR Rubomycin OR Rubidomycin OR daunoxome OR cerubidine OR cerubidin OR daunoblastina OR daunoblastin OR daunocin OR oncodaunotec OR rubilem OR idarubicin [MeSH] OR Desmethoxydaunorubicin OR Demethoxydaunorubicin OR DMDR IDA OR imi‐30 OR imi30 OR nsc‐256439 OR nsc256439 OR idamycin OR idaralem OR zavedos OR epirubicin [MeSH] OR anthracin OR bioepicyna OR ciazil OR ellence OR epi‐cell OR epilem OR epi‐NC OR epirub OR E.P.Mycin OR farmorrubicina OR farmorubicina OR farmorubicine OR farmorubicin megarubicin OR nuovodox OR pharmorubicin OR riboepi OR rubina OR tecnomax OR Epi‐DXR OR Epi‐cell OR Epicell OR Epi‐ADR OR Epiadriamycin OR Axirubine OR Bendaepi OR imi‐28 OR imi28 OR nsc‐256942 OR nsc256942 OR mitoxantrone [MeSH] OR domitrone OR ebexantron OR elsep OR formyxan OR genefadrone OR haemato‐tron OR misostol OR mitaxis OR mitoxal OR mitoxan OR mitoxgen OR mitroxene OR mitroxone OR neotalem OR neoxantron OR norexan OR novantrone OR Mitozantrone OR Novatron OR Onkotrone OR Parlifan OR Ralenova OR anthracyclin* OR anthracenedione
Data and analyses
Comparison 1. Anthracycline versus no anthracycline same chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | 464 | Hazard Ratio (95% CI) | 0.99 [0.77, 1.29] |
| 2 Overall survival with Zinzani | 4 | 663 | Hazard Ratio (95% CI) | 0.97 [0.76, 1.23] |
| 3 Mortality at 3 years | 3 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.26] |
| 4 Mortality at 5 years | 3 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.18] |
| 5 Complete response | 5 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.18] |
| 6 Overall response | 3 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| 7 Disease control | 4 | 759 | Hazard Ratio (95% CI) | 0.65 [0.52, 0.81] |
| 7.1 Progression‐free survival | 2 | 514 | Hazard Ratio (95% CI) | 0.65 [0.50, 0.84] |
| 7.2 Response duration | 2 | 245 | Hazard Ratio (95% CI) | 0.63 [0.40, 0.98] |
| 8 Progression/relapse at 3 years | 4 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.63, 0.85] |
| 9 Neutropenia grade 3‐4 | 2 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.46, 2.56] |
| 10 Infection | 3 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.75, 1.80] |
1.1. Analysis.

Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 1 Overall survival.
1.3. Analysis.
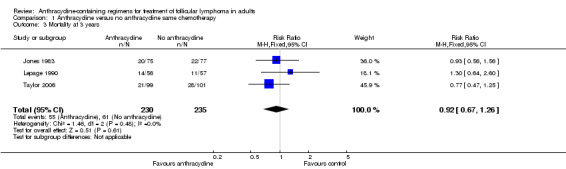
Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 3 Mortality at 3 years.
1.5. Analysis.
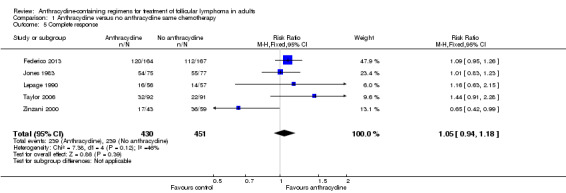
Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 5 Complete response.
1.7. Analysis.
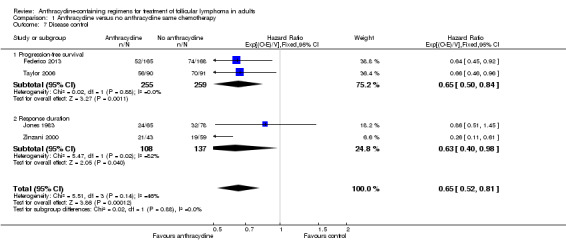
Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 7 Disease control.
1.8. Analysis.
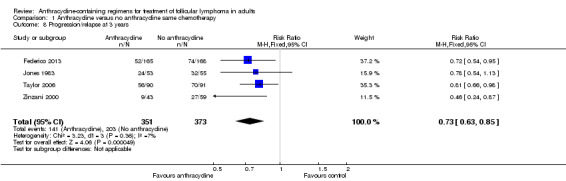
Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 8 Progression/relapse at 3 years.
1.10. Analysis.

Comparison 1 Anthracycline versus no anthracycline same chemotherapy, Outcome 10 Infection.
Comparison 2. Sensitivity analysis for allocation concealment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Adequate allocation concealment | 2 | 265 | Hazard Ratio (95% CI) | 0.92 [0.63, 1.34] |
| 1.2 Unclear allocation concealment | 1 | 199 | Hazard Ratio (95% CI) | 0.97 [0.67, 1.41] |
| 2 Disease control | 4 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Adequate allocation concealment | 2 | 476 | Hazard Ratio (95% CI) | 0.70 [0.52, 0.95] |
| 2.2 Unclear allocation concealment | 2 | 283 | Hazard Ratio (95% CI) | 0.58 [0.41, 0.81] |
Comparison 3. Sensitivity analysis for second randomization.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease control | 4 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Second randomization | 2 | 324 | Hazard Ratio (95% CI) | 0.72 [0.54, 0.98] |
| 1.2 No second randomization | 2 | 435 | Hazard Ratio (95% CI) | 0.56 [0.40, 0.78] |
Comparison 4. Comparison of different anthracyclines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Doxorubicin | 2 | 265 | Hazard Ratio (95% CI) | 0.92 [0.63, 1.34] |
| 1.2 Idarubicin | 1 | 199 | Hazard Ratio (95% CI) | 0.97 [0.67, 1.41] |
| 2 Disease control | 4 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Doxorubicin | 2 | 476 | Hazard Ratio (95% CI) | 0.70 [0.52, 0.95] |
| 2.2 Idarubicin | 2 | 283 | Hazard Ratio (95% CI) | 0.58 [0.41, 0.81] |
4.2. Analysis.
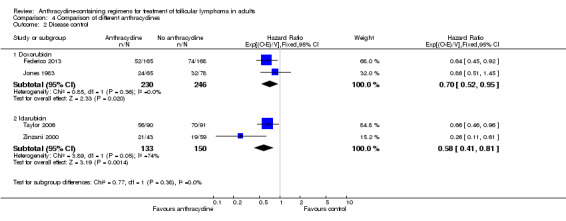
Comparison 4 Comparison of different anthracyclines, Outcome 2 Disease control.
Comparison 5. Anthracycline versus no anthracycline different chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 2 | Hazard Ratio (95% CI) | Subtotals only | |
| 2 Mortality at 3 years | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Complete response | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Disease control | 3 | Hazard Ratio (95% CI) | Subtotals only |
Comparison 6. Cardiotoxicity for all studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiotoxicity | 4 | 1412 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [0.92, 22.49] |
| 1.1 Same chemotherapy regimen | 3 | 1184 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.66, 28.03] |
| 1.2 Different chemotherapy regimen | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.45 [0.26, 112.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Federico 2013.
| Methods | RCT Location: Italy, multicenter Years: recruited 2006 to 2010 Follow‐up: median 34 months Pharmaceutical sponsorship: no Classification system: WHO Central pathology: only for grade 3 FL |
|
| Participants | Number of patients randomized: 534 Number of patients analyzed: 504 Numbers of patients with FL: 504 Number of patients included in our analysis: 333 Lymphoma: previously untreated stage II to IV FL, grade 1 to 3a, age 18 to 75 years, ECOG 0 to 2 Line of treatment: first Patients: median age (range) 56 years (30 to 75 years) |
|
| Interventions | Same: R‐CHOP x 6 + R x 2 versus R‐CVP x 8 Note number of courses in each arm |
|
| Outcomes | Time to treatment failure Response rate Progression‐free survival Overall survival Toxicity |
|
| Notes | Unpublished data from authors This was a triple arm study: R‐CVP versus R‐CHOP versus R‐FM. We extracted data for the first comparison, according to protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization arm was assigned automatically by the EDC system upon verification of eligibility criteria. Arm was communicated on screen and confirmed by email to the investigator. Randomization was based on prepopulated tables that were prepared and uploaded by the IT staff on the EDC system at study start |
| Allocation concealment (selection bias) | Low risk | Randomization tables could not be accessed or modified, or both, by any investigator or study co‐ordinator |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | No change from protocol |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open study; review authors did not believe this will introduce bias |
Jones 1983.
| Methods | RCT Location: US, multicenter, SWOG study Years: 1974 to 1977 Follow‐up: maximum 6 years Pharmaceutical sponsorship: no Classification system: Rappaport Central pathology: yes |
|
| Participants | Number of patients randomized: 652 Number of patients analyzed: 497 (after central pathology) Numbers of patients with FL: 226 (nodular lymphoma) Lymphoma: advanced‐stage NHL Line of treatment: first or recurrence after RTx Patients: of 226 patients with nodular lymphoma, grade classification was as follows: NLPD (nodular lymphocytic poorly differentiated or follicular small cleaved cell lymphoma), 160 patients; NM (nodular mixed or follicular small cleaved and large cell lymphoma), 42 patients; NH (nodular 'histiocytic' or follicular large cell lymphoma), 24 patients |
|
| Interventions | Same: CHOP‐Bleo x 8 versus CVP‐Bleo x 8 Control arm: d 1‐14 cyclophosphamide 125 mg/m2 PO, d 1, 8 vincristine 1.4 mg/m2 IV (max 2 mg/dose), d 1‐5 prednisone 100 mg/d PO, d 1,8 bleomycin 4 mg/m2 IV. 8 cycles, every 21 d Experimental arm: d 1 cyclophosphamide 750 mg/m2 IV, d 1 doxorubicin 50 mg/m2 IV, d 1 vincristine 1.4 mg/m2 (max 2 mg) IV, d 1‐5 prednisone 100 mg/d PO, d 1 bleomycin 4 mg/m2 IV. 8 cycles, every 28 d |
|
| Outcomes | Overall survival Response rate Relapse‐free survival Toxicity |
|
| Notes | Published: journal article Double publication of preliminary data: Jones 1979 Triple‐arm design: CHOP‐BCG versus CHOP‐Bleo versus CVP‐Bleo. Second randomization in patients achieving partial response was: no treatment versus BCG maintenance CVP‐Bleo arm terminated early |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned at random by the SWOG Statistical Office" Comment: assumed proper sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were assigned at random by the SWOG Statistical Office" Comment: assumed concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 652 patients were randomized; 636 were evaluable (Quote: "16 were not evaluable ‐ insufficient data submitted or major protocol violation"); only 497 had central pathology, according to which analysis was made. Data were reported only for 468 patients with chosen diagnoses Data were reported for 226 FL patients. The number of patients with FL at randomization could not be inferred |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open study; review authors do not believe this will introduce bias |
Kimby 1994.
| Methods | RCT Location: Sweden, multicenter, LGCS study Years: 1982 to 1988 Follow‐up: minimum 57 months Pharmaceutical sponsorship: NR Classification system: Kiel Central pathology: yes |
|
| Participants | Number of patients randomized: 259 Number of patients analyzed: 259 Numbers of patients with FL: 76 Lymphoma: advanced‐stage, symptomatic, low‐grade NHL (CLL, immunocytoma, centrocytic lymphoma, centroblastic/centrocytic lymphoma) Line of treatment: first Patients: mean age 64 years |
|
| Interventions | Different: CHOP x 4‐8 cycles versus CD x 4‐8 cycles | |
| Outcomes | Overall survival Response rate Freedom from progression defined as survival without progression, measured from randomization Response duration ‐ time from CR or PR to relapse or progression Toxicity |
|
| Notes | Published: journal article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was centralized" Comment: assumed proper sequence generation, since done by the LGCS |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was centralized" Comment: assumed proper allocation concealment, since done by the LGCS |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open study; review authors do not believe this will introduce bias |
Lepage 1990.
| Methods | RCT Location: France, single center Years: 1981 to 1984 Follow‐up: median 59 months Pharmaceutical sponsorship: NR Classification system: working‐formulation Central pathology: yes |
|
| Participants | Number of patients randomized: 113 Number of patients analyzed: 113 Numbers of patients with FL: 101 Lymphoma: stage II/IV and stage II bulky, low‐grade NHL Line of treatment: first Patients: median age (range) 51 years (26 to 70 years), over 90% were advanced stage, 40% with bulky disease, and 65% with bone‐marrow involvement |
|
| Interventions | Same: PCOP x 6 cycles versus PACOP x 6 cycles | |
| Outcomes | Overall survival Response rate Freedom from progression defined as survival without progression Freedom from relapse defined as time from CR to relapse Toxicity |
|
| Notes | Published: journal article Double publication: Gisselbrecht 1987 Second randomization in responding patients to maintenance with chlorambucil versus CVP for 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | None reported |
| Allocation concealment (selection bias) | Low risk | Comment: assumed proper since done as central randomization in a multicenter trial |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open study; review authors do not believe this will introduce bias |
Peterson 2003.
| Methods | RCT Location: USA, multicenter, CALGB study Years: 1980 to1985 Follow‐up: maximum 18 years Pharmaceutical sponsorship: NR Classification system: REAL Central pathology: yes |
|
| Participants | Number of patients randomized: 228 Number of patients analyzed: 228 Numbers of patients with FL: 189 after reclassification Lymphoma: advanced FL grade 1 (follicular small cell lymphoma) and grade 2 (follicular mixed‐cell lymphoma) Line of treatment: first Patients: median age 55 years, 36% of patients were over 60 years, 60% had bone marrow involvement, 50% were asymptomatic at initiation of treatment |
|
| Interventions | Different: CHOP‐Bleo for a median of 26 months (Bleo only 6 cycles) versus daily cyclophosphamide PO for a median of 28 months | |
| Outcomes | Overall survival Response rate TTTP ‐ time to first progression, relapse or recurrence, death from any cause, or discontinuation of treatment for non‐responders CR duration ‐ from first CR to relapse or death by any cause Toxicity |
|
| Notes | Published: journal article Subgroup analysis of FSCL and FML patients, after reclassification, indicated better outcomes in FML patients treated with CHOP regimen, including overall survival, TTTF and complete remission duration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. No statement regarding where randomization actually took place |
| Allocation concealment (selection bias) | Unclear risk | Not reported. No statement regarding where randomization actually took place |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 of 234 randomized patients were cancelled, 3 in each arm (reasons not stated). Data were reported for 228 patients. Another analysis was made to compare between grades of FL (FSCL versus FML), and included 189 patients with after central pathology review |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open study; review authors do not believe this will introduce bias |
Taylor 2006.
| Methods | RCT Location: UK, multicenter, SNLG study Years: 1993 to 2000 Follow‐up: median 58 months Pharmaceutical sponsorship: no Classification system: Kiel/REAL Central pathology: yes |
|
| Participants | Number of patients randomized: 200 Number of patients analyzed: 183 Numbers of patients with FL: 155 Lymphoma: low‐grade NHL Line of treatment: first, but previous radiotherapy for localized low‐grade NHL was allowed Patients: age was matched across study arms, 34% of patients were over age 60 years; 83% and 94% of patients were stage III/IV, in CID and CD arms, respectively; 15% and 32% had a high FLIPI score |
|
| Interventions | Same: CD (Chlorambucil + dexamethasone) x 6 cycles versus CID (same + idarubicin) x 6. Radiotherapy to bulky disease allowed | |
| Outcomes | Overall survival Response rate Time to treatment failure ‐ time from entry to the start of subsequent treatment or relapse Toxicity |
|
| Notes | Published: journal article Second randomization in responding patients: no treatment versus IFNα Protocol violation in CID arm with access radiotherapy might have introduced bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17 patients were not analyzed: 2 withdrew consent (1 in each arm); 15 were ineligible on pathology review (9 in CID arm, 6 in CD arm) |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open study; review authors do not believe this will introduce bias |
Unterhalt 1996.
| Methods | RCT Location: Europe, multicenter, GLGLSG study Years: 1989 to 1995 Follow‐up: NR Pharmaceutical sponsorship: NR Classification system: Kiel/REAL Central pathology: yes |
|
| Participants | Number of patients randomized: 442 Number of patients analyzed: 246 Numbers of patients with FL: 200 Lymphoma: advanced‐stage FL grade 1‐2 and MCL Line of treatment: first Patients: median (range) age 54 years (28 to 77 years), 67% had bone marrow involvement |
|
| Interventions | Different: PmM x 4‐6 cycles versus CVP x 4‐6 cycles | |
| Outcomes | Response rate Event‐free interval measured from response to the recurrence of lymphoma or death Toxicity |
|
| Notes | Published: journal article Triple publication: Hiddemann 1994; Hiddemann 1998 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible patients underwent a central randomization procedure" Comment: assumed proper sequence generation, since done by the GLGLSG |
| Allocation concealment (selection bias) | Low risk | Quote: "eligible patients underwent a central randomization procedure" Comment: assumed proper allocation concealment, since done by the GLGLSG |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Out of 344 randomized FL patients, only 200 were fully evaluable for central pathology, 33 of whom withdrew early from study (reasons stated in study), leaving only 167 FL patients for analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open study; review authors do not believe this will introduce bias |
Zinzani 2000.
| Methods | RCT Location: Italy, multicenter Years: 1995 to 1998 Follow‐up: median 19 months (range 6 to 38 months) after end of chemotherapy Pharmaceutical sponsorship: NR Classification system: REAL Central pathology: yes |
|
| Participants | Number of patients randomized: 208 Number of patients analyzed: 199 Numbers of patients with FL: 102 Lymphoma: stage II‐IV, indolent lymphoma (including FL, immunocytoma, and small lymphocytic lymphoma) or MCL Line of treatment: first Patients: median (range) age 54 years (25 to 65 years), 86% were stage III/IV, 12% had bulky disease, all had an IPI of 3 or less, 62% had IPI 0 or 1 |
|
| Interventions | Same: FI x 6 cycles versus fludarabine x 6 cycles Fludarabine was given for 5 days in the control arm versus 3 days in the experimental arm |
|
| Outcomes | Overall survival Response rate Progression‐free survival measured from date of any response until relapse or progression Relapse‐free survival measured from CR to relapse or death Toxicity |
|
| Notes | Published: journal article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the nine patients who were excluded were so because of incorrect diagnosis (three patients), loss to follow‐up (three patients), and protocol violations (three patients)" Comment: data were reported for all other 199 patients |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open study; review authors do not believe this will introduce bias |
BCG: Bacillus Calmette Guerin; CALGB: Cancer and Leukemia Group B; CD: chlorambucil, dexamethasone; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; CHOP‐Bleo: cyclophosphamide, doxorubicin, vincristine, prednisone, bleomycin; CHOP‐BCG: cyclophosphamide, doxorubicin, vincristine, prednisone, Bacillus Calmette Guerin; CID: chlorambucil, idarubicin, dexamethasone; CLL: chronic lymphocytic leukemia; CVP: cyclophosphamide, vincristine, prednisone; CVP‐Bleo: cyclophosphamide, vincristine, prednisone, bleomycin; CR: complete response; d: day; ECOG: Eastern Cooperative Oncology Group; EDC: electronic data capture; FI: fludarabine, idarubicin; FL: follicular lymphoma; FLIPI: Follicular Lymphoma International Prognostic Index; FML: follicular mixed lymphoma; FSCL: follicular small cleaved lymphoma; GLGLSG: German Low Grade Lymphoma Study Group; IPI: International Prognostic Index; IT: information technology; IV: intravenous; LGCS: Lymphoma Group of Central Sweden; MCL: mantle‐cell lymphoma; NHL: non‐Hodgkin lymphoma; NR: not reported; PACOP: cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin; PCOP: cyclophosphamide, vincristine, procarbazine, prednisone; PmM: prednimustine, mitoxantrone; PO: oral; R: rituximab; R‐CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R‐CVP: rituximab, cyclophosphamide, vincristine, prednisone; R‐FM: rituximab, fludarabine, mitoxantrone; RCT: randomized controlled trial; REAL: Revised European‐American Classification of Lymphoid Neoplasms; SNLG: Scotland and Newcastle Lymphoma Group; SWOG: South‐West Oncology Group; TTTF: time to treatment failure; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acker 1983 | No anthracyclines in study arms |
| Al‐Ismail 1987 | Did not state the number of FL patients out of low‐grade lymphomas |
| Andersen 1990 | Not FL |
| Ben‐Shahar 1998 | Did not report relevant outcomes (see Methods) |
| Bonadonna 1975 | Cross‐over trial |
| Cabanillas 1978 | Anthracyclines in both study arms |
| Coiffier 1999 | Immunotherapy only in 1 study arm |
| Engelhard 1986 | Not FL |
| Flinn 2012 | No randomization between ACR and non‐ACR arms |
| Gad 1976 | No anthracyclines in study arms |
| Gams 1985 | Not low‐grade lymphoma |
| Gisselbrecht 1987 | Double publication |
| Ha 2005 | No chemotherapy on control arm (irradiation only) |
| Hainsworth 2005 | Non‐randomized trial |
| Hiddemann 1994 | Double publication |
| Hiddemann 1998 | Comparison between FL and MCL, not between therapeutic study arms |
| Inoue 1993 | Non‐randomized trial, few patients with low‐grade lymphoma |
| Jones 1979 | Double publication |
| Junmin 2005 | Non‐randomized trial |
| Meerwaldt 1991 | No chemotherapy on control arm (irradiation only) |
| Meusers 1989 | Not FL |
| Mukherji 2009 | Review article |
| Niitsu 1997 | Non‐randomized trial |
| Parlier 1981 | Non‐randomized trial |
| Parlier 1982 | Non‐randomized trial |
| Peterson 1985 | Double publication |
| Pettengell 2009 | Not FL |
| Pott 1994 | Data could not be retrieved |
| Prentice 1996 | Did not state the number of FL patients out of low‐grade lymphomas |
| Robak 2000 | Non‐randomized trial |
| Rummel 2008 | Review article |
| Rummel 2009 | Different chemotherapeutic regimens in conjunction with immunotherapy (rituximab) |
| Rummel 2013 | Different chemotherapeutic regimens in conjunction with immunotherapy (rituximab) |
| Santini 2001 | Non‐randomized trial |
| Santoro 2006 | Did not state the number of FL patients out of low‐grade lymphomas |
| Somers 1987 | Report of a number of studies. No anthracyclines in study arms or anthracyclines in both study arms |
| Unterhalt 1994 | Double publication |
| Yahalom 1993 | No chemotherapy on control arm (irradiation only) |
| Young 1977 | No anthracyclines in study arms |
ACR: anthracycline‐containing regimens; FL: follicular lymphoma; MCL: mantle‐cell lymphoma;
Characteristics of ongoing studies [ordered by study ID]
NCT00551239.
| Trial name or title | Fludarabine and rituximab with or without pixantrone in treating patients with relapsed or refractory indolent non‐Hodgkin lymphoma |
| Methods | RCT, single blind (outcomes assessor) |
| Participants | Patients with relapsed or refractory indolent non‐Hodgkin lymphoma |
| Interventions | Combination BBR 2778, fludarabine, and rituximab with the combination fludarabine and rituximab |
| Outcomes | Primary: progression‐free survival |
| Starting date | August 2007 |
| Contact information | Cell Therapeutics, Inc (Igor Gorbatchevsky, Medical Director) |
| Notes | Ongoing but not recruiting |
NCT00801281.
| Trial name or title | First‐line R‐CVP vs R‐CHOP induction immunochemotherapy for indolent lymphoma and R maintenance (PLRG4) |
| Methods | Multicenter, Phase III randomized study |
| Participants | First‐line indolent NHL |
| Interventions | R‐CVP versus R‐CHOP and R maintenance |
| Outcomes | Primary: event‐free survival |
| Starting date | February 2007 |
| Contact information | Polish Lymphoma Research Group |
| Notes | Recruiting |
FL: follicular lymphoma; NHL: non‐Hodgkin lymphoma; R‐CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R‐CVP: rituximab, cyclophosphamide, vincristine, prednisone; R‐FM: rituximab, fludarabine, mitoxantrone
Differences between protocol and review
Exclusion of studies that did not specify the number of FL patients enrolled.
Data analysis only from trials with over 75% FL patients or in case data were provided specifically for FL patients.
Explaining use of the outcome: disease control.
Calculation of analysis with both fixed‐effect and random‐effects models, to demonstrate differences in results in heterogeneous analysis.
Documenting previous histologic classifications for FL.
Contributions of authors
Gilad Itchaki: conception and design, protocol development, searching and selection of trials, data extraction, analysis and interpretation, eligibility and quality assessment, writing the review, and final approval.
Anat Gafter‐Gvili: content expert in hematology.
Meir Lahav: conception and design, clinical and scientific advice, and content input.
Liat Vidal: content expert in hematology.
Pia Raanani: content expert in hematology.
Ofer Shpilberg: conception and design, content expert in hematology, clinical advice, and final approval.
Mical Paul: design and protocol development, methodologic advice, data extraction, provision of statistical analysis, writing the review, and final approval.
Sources of support
Internal sources
Rabin Medical Center, Beilinson Hospital, Not specified.
External sources
No funding, Not specified.
Declarations of interest
No conflicts of interests known.
New
References
References to studies included in this review
Federico 2013 {published and unpublished data}
- Federico M, Luminari S, Dondi A, Tucci A, Vitolo U, Rigacci L, et al. R‐CVP versus R‐CHOP versus R‐FM for the initial treatment of patients with advanced‐stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. Journal of Clinical Oncology 2013;31(12):1506‐13. [DOI] [PubMed] [Google Scholar]
Jones 1983 {published data only}
- Jones SE, Grozea PN, Metz EN, Haut A, Stephens RL, Morrison FS, et al. Improved complete remission rates and survival for patients with large cell lymphoma treated with chemoimmunotherapy. A Southwest Oncology Group Study. Cancer 1983;51(6):1083‐90. [DOI] [PubMed] [Google Scholar]
Kimby 1994 {published data only}
- Kimby E, Bjorkholm M, Gahrton G, Glimelius B, Hagberg H, Johansson B, et al. Chlorambucil/prednisone vs. CHOP in symptomatic low‐grade non‐Hodgkin's lymphomas: a randomized trial from the Lymphoma Group of Central Sweden. Annals of Oncology 1994;5 Suppl 2:67‐71. [PubMed] [Google Scholar]
Lepage 1990 {published data only}
- Lepage E, Sebban C, Gisselbrecht C, Coiffier B, Harousseau JL, Bryon PA, et al. Treatment of low‐grade non‐Hodgkin's lymphomas: assessment of doxorubicin in a controlled trial. Hematological Oncology 1990;8(1):31‐9. [DOI] [PubMed] [Google Scholar]
Peterson 2003 {published data only}
- Peterson BA, Petroni GR, Frizzera G, Barcos M, Bloomfield CD, Nissen NI, et al. Prolonged single‐agent versus combination chemotherapy in indolent follicular lymphomas: a study of the cancer and leukemia group B. Journal of Clinical Oncology 2003;21(1):5‐15. [DOI] [PubMed] [Google Scholar]
Taylor 2006 {published data only}
- Taylor PR, White JM, Prescott RJ, Angus B, Galloway MJ, Jackson GH, et al. The addition of oral idarubicin to a chlorambucil/dexamethasone combination has a significant impact on time to treatment failure but none on overall survival in patients with low grade non‐Hodgkin's lymphoma: results of the Scotland and Newcastle Lymphoma Group randomized NHL VIII trial. Leukemia & Lymphoma 2006;47(11):2321‐30. [DOI] [PubMed] [Google Scholar]
Unterhalt 1996 {published data only}
- Unterhalt M, Herrmann R, Tiemann M, Parwaresch R, Stein H, Trumper L, et al. Prednimustine, mitoxantrone (PmM) vs cyclophosphamide, vincristine, prednisone (COP) for the treatment of advanced low‐grade non‐Hodgkin's lymphoma. German Low‐Grade Lymphoma Study Group. Leukemia 1996;10(5):836‐43. [PubMed] [Google Scholar]
Zinzani 2000 {published data only}
- Zinzani PL, Magagnoli M, Moretti L, Renzo A, Battista R, Zaccaria A, et al. Randomized trial of fludarabine versus fludarabine and idarubicin as frontline treatment in patients with indolent or mantle‐cell lymphoma. Journal of Clinical Oncology 2000;18(4):773‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acker 1983 {published data only}
- Acker B, Hoppe RT, Colby TV, Cox RS, Kaplan HS, Rosenberg SA. Histologic conversion in the non‐Hodgkin's lymphomas. Journal of Clinical Oncology 1983;1(1):11‐6. [DOI] [PubMed] [Google Scholar]
Al‐Ismail 1987 {published data only}
- Al‐Ismail SA, Whittaker JA, Gough J. Combination chemotherapy including epirubicin for the management of non‐Hodgkin's lymphoma. European Journal of Cancer & Clinical Oncology 1987;23(9):1379‐84. [DOI] [PubMed] [Google Scholar]
Andersen 1990 {published data only}
- Andersen J, Thorling K, Bentzen SM, Brincker H, Christensen BE, Pedersen M. Phase III trial of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) versus cisplatin, etoposide, bleomycin and prednisone (CisEBP) for the treatment of advanced non‐Hodgkin's lymphoma of high grade malignancy. The Danish Lymphoma Study Group. Acta Oncologica 1990;29(8):995‐9. [DOI] [PubMed] [Google Scholar]
Ben‐Shahar 1998 {published data only}
- Ben‐Shahar M, Gepstein R, Haim R, Polliack A. Phase III trial of a combination of chlorambucil (L) and prednisone (P) with or without mitoxantrone (M) in advanced low grade non‐Hodgkin's lymphoma (NHL) and CLL. Proceedings of the American Society of Clinical Oncology (Annual Meeting of the American Society of Clinical Oncology). Los Angeles, CA: Annual Meeting of the American Society of Clinical Oncology, 1998; Vol. 17:20a Abstract No 75.
Bonadonna 1975 {published data only}
- Bonadonna G, Lena M, Lattuada A, Milani F, Monfardini S, Beretta G. Combination chemotherapy and radiotherapy in non‐Hodgkin's lymphomata. The British Journal Cancer. Supplement 1975;2:481‐8. [PMC free article] [PubMed] [Google Scholar]
Cabanillas 1978 {published data only}
- Cabanillas F, Rodriguez V, Freireich EJ. Improvement in complete response rate, duration of response and survival with adriamycin combination chemotherapy for non‐Hodgkin lymphoma: a prognostic factor comparison of two regimens. Medical and Pediatric Oncology 1978;4(4):321‐31. [DOI] [PubMed] [Google Scholar]
Coiffier 1999 {published data only}
- Coiffier B, Neidhardt‐Berard EM, Tilly H, Belanger C, Bouabdallah R, Haioun C, et al. Fludarabine alone compared to CHVP plus interferon in elderly patients with follicular lymphoma and adverse prognostic parameters: a GELA study. Groupe d'Etudes des Lymphomes de l'Adulte. Annals of Oncology 1999;10(10):1191‐7. [DOI] [PubMed] [Google Scholar]
Engelhard 1986 {published data only}
- Engelhard M, Bartels H, Binder T, Fulle HH, Gunzer U, Havemann K, et al. Multicenter randomized trial of the polychemotherapy of advanced centrocytic lymphoma: no prognostic advantage by anthracycline. Blut 1986;53(3):220. [Google Scholar]
Flinn 2012 {published data only}
- Flinn EW, Jagt RH, Kahl BS, Wood P, Hawkins TE, MacDonald D, et al. An open‐label, randomized study of bendamustine and rituximab (BR) compared with rituximab, cyclophosphamide, vincristine, and prednisone (R‐CVP) or rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) in first‐line treatment of patients with advanced indolent non‐Hodgkin’s lymphoma (NHL) or mantle cell lymphoma (MCL): The BRIGHT Study. Blood (ASH Annual Meeting Abstracts) 2012;120(21):Abstract No 902. [Google Scholar]
Gad 1976 {published data only}
- Gad EMN. Chemotherapeutic management of non Hodgkin's lymphoma: comparative study of various combinations. Chemotherapy ‐ Proceedings of the 9th International Congress of Chemotherapy. New York: Plenum press, 1976; Vol. 2:581‐3.
Gams 1985 {published data only}
- Gams RA, Rainey M, Dandy M, Bartolucci AA, Silberman H, Omura G. Phase III study of BCOP v CHOP in unfavorable categories of malignant lymphoma: a Southeastern Cancer Study Group trial. Journal of Clinical Oncology 1985;3(9):1188‐95. [DOI] [PubMed] [Google Scholar]
Gisselbrecht 1987 {published data only}
- Gisselbrecht C, Lepage E, Sebban C, Coiffier B. Low‐grade non‐Hodgkin's lymphoma in the adult: new therapeutic approaches [Lymphome non hodgkinien de l'adulte de faible evolutivite: nouvelles approches therapeutiques]. Nouvelle Revue Francaise d'Hematologie 1987;29(1):77‐81. [PubMed] [Google Scholar]
Ha 2005 {published data only}
- Ha CS, Cabanillas F, Lee MS, Tucker SL, McLaughlin P, Rodriguez MA, et al. A prospective randomized study to compare the molecular response rates between central lymphatic irradiation and intensive alternating triple chemotherapy in the treatment of stage I‐III follicular lymphoma. International Journal of Radiation Oncology, Biology, Physics 2005;63(1):188‐93. [DOI] [PubMed] [Google Scholar]
Hainsworth 2005 {published data only}
- Hainsworth JD, Litchy S, Morrissey LH, Andrews MB, Grimaldi M, McCarty M, et al. Rituximab plus short‐duration chemotherapy as first‐line treatment for follicular non‐Hodgkin's lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. Journal of Clinical Oncology 2005;23(7):1500‐6. [DOI] [PubMed] [Google Scholar]
Hiddemann 1994 {published data only}
- Hiddemann W, Unterhalt M, Koch P, Nahler M, Herrmann R. New aspects in the treatment of advanced low‐grade non‐Hodgkin's lymphomas: prednimustine/mitoxantrone versus cyclophosphamide/vincristine/prednisone followed by interferon alfa versus observation only ‐ a preliminary update of the German Low‐Grade Lymphoma Study Group. Seminars in Hematology 1994;31 Suppl 3(2):32‐5. [PubMed] [Google Scholar]
Hiddemann 1998 {published data only}
- Hiddemann W, Unterhalt M, Herrmann R, Woltjen HH, Kreuser ED, Trumper L, et al. Mantle‐cell lymphomas have more widespread disease and a slower response to chemotherapy compared with follicle‐center lymphomas: results of a prospective comparative analysis of the German Low‐Grade Lymphoma Study Group. Journal of Clinical Oncology 1998;16(5):1922‐30. [DOI] [PubMed] [Google Scholar]
Inoue 1993 {published data only}
- Inoue T, Fujiyama Y, Kitoh K, Niwakawa M, Andoh A, Hodohara K, et al. Effect of combination chemotherapy for elderly patients with malignant lymphoma. Nippon Ronen Igakkai Zasshi 1993;30(10):864‐8. [DOI] [PubMed] [Google Scholar]
Jones 1979 {published data only}
- Jones SE, Grozea PN, Metz EN, Haut A, Stephens RL, Morrison FS, et al. Superiority of adriamycin‐containing combination chemotherapy in the treatment of diffuse lymphoma: a Southwest Oncology Group study. Cancer 1979;43(2):417‐25. [DOI] [PubMed] [Google Scholar]
Junmin 2005 {published data only}
- Junmin L. Fludarabine‐based regimens in the treatment of indolent non‐Hodgkin’s lymphoma patients: a clinical study of the Fludarabine Cooperation Group in 16 hospitals in Eastern China. Blood (ASH Annual Meeting Abstracts) 2005;106:Abstract No 4753. [Google Scholar]
Meerwaldt 1991 {published data only}
- Meerwaldt JH, Carde P, Burgers JM, Monconduit M, Thomas J, Somers R, et al. Low‐dose total body irradiation versus combination chemotherapy for lymphomas with follicular growth pattern. International Journal of Radiation Oncology, Biology, Physics 1991;21(5):1167‐72. [DOI] [PubMed] [Google Scholar]
Meusers 1989 {published data only}
- Meusers P, Engelhard M, Bartels H, Binder T, Fulle HH, Gorg K, et al. Multicentre randomized therapeutic trial for advanced centrocytic lymphoma: anthracycline does not improve the prognosis. Hematological Oncology 1989;7(5):365‐80. [DOI] [PubMed] [Google Scholar]
Mukherji 2009 {published and unpublished data}
- Mukherji D, Pettengell R. Pixantrone maleate for non‐Hodgkin's lymphoma. Drugs Today (Barc) 2009;45(11):797‐805. [DOI] [PubMed] [Google Scholar]
Niitsu 1997 {published data only}
- Niitsu N, Umeda M. Prognostic factors of follicular lymphoma treated with combination chemotherapy. Rinsho Ketsueki [The Japanese Journal of Clinical Hematology] 1997;38(6):496‐504. [PubMed] [Google Scholar]
Parlier 1981 {published data only}
- Parlier Y, Gorin NC, Stachowiak J, Najman A, Duhamel G. Adriamycin for the treatment of non Hodgkin's lymphomas [Interet de l'adriamycine dans le traitement des lymphomes non Hodgkiniens]. La Seminae de Hospitaux 1981;57(41‐42):1685‐90. [PubMed] [Google Scholar]
Parlier 1982 {published data only}
- Parlier Y, Gorin NC, Najman A, Stachowiak J, Duhamel G. Combination chemotherapy with cyclophosphamide, vincristine, prednisone and the contribution of adriamycin in the treatment of adult non‐Hodgkin's lymphomas a report of 131 cases. Cancer 1982;50(3):401‐9. [DOI] [PubMed] [Google Scholar]
Peterson 1985 {published and unpublished data}
- Peterson BA, Anderson JR, Frizzera G, Bloomfield CD, Gottlieb AJ, Holland JF. Nodular mixed lymphoma (NML): a comparative trial of cyclophosphamide adriamycin vincristine prednisone and bleomycin (CAVPB). Blood 1985;5 Suppl 1:216a. [Google Scholar]
Pettengell 2009 {published data only}
- Pettengell R, Narayanan G, Mendoza FH, Digumarti R, Gomez H, Cernohous P, et al. Randomized phase III trial of pixantrone compared with other chemotherapeutic agents for third‐line single‐agent treatment of relapsed aggressive non‐Hodgkin's lymphoma. Journal of Clinical Oncology (ASCO Annual Meeting) 2009;27(15s):Abstract No 8523. [Google Scholar]
Pott 1994 {published data only}
- Pott C, Unterhalt M, Sandford D, Markert H, Freund M, Engert A, et al. Fludarabine as single agent and in combination with mitoxantrone and dexamethasone in relapsed and refractory low‐grade non‐Hodgkin‐lymphoma. Annals of Hematology 1994;68 Suppl 1:A2. [Google Scholar]
Prentice 1996 {published data only}
- Prentice AG, Johnson SAN, Harper M, Hewins P, Tyrrell CJ, Copplestone JA, et al. A randomised, prospective trial of chlorambucil and prednisolone (CP) v mitozantrone, chlorambucil and prednisolone (MCP) in de novo, stage II or more advanced, low‐grade non‐Hodgkins lymphoma (LG‐NHL). British Journal of Haematology (Annual Meeting of the British Society for Haematology) 1996;93 Suppl 1:74 Abstract No 286. [Google Scholar]
Robak 2000 {published data only}
- Robak T, Gora‐Tybor J, Chojnowski K. Cladribine as monotherapy or combined with dexamethasone and idarubicin or mitoxantrone in previously treated patients with low‐grade lymphoid malignancies. Haematologica 2000;85(2):215‐6. [PubMed] [Google Scholar]
Rummel 2008 {published and unpublished data}
- Rummel MJ. Bendamustine in chronic lymphocytic leukemia and refractory lymphoma. Seminars in Hematology 2008;45 Suppl 2(3):S7‐10. [DOI] [PubMed] [Google Scholar]
Rummel 2009 {published and unpublished data}
- Rummel MJ, Gruenhagen U, Niederle N, Ballo H, Weidmann E, Welslau M, et al. Bendamustine plus rituximab versus CHOP plus rituximab in the first‐Line‐treatment of patients with follicular, indolent and mantle cell lymphomas: results of a randomized phase III study of the Study Group Indolent Lymphomas (StiL). Blood (ASH Annual Meeting Abstracts) 2008;112:Abstract No 2596. [Google Scholar]
Rummel 2013 {published and unpublished data}
- Rummel MJ, Niederle N, Maschmeyer G, Banat GA, Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: an open‐label, multicentre,randomised, phase 3 non‐inferiority trial. Lancet 2013;381:1203‐10. [DOI] [PubMed] [Google Scholar]
Santini 2001 {published data only}
- Santini G, Nati S, Spriano M, Gallamini A, Pierluigi D, Congiu AM, et al. Fludarabine in combination with cyclophosphamide or with cyclophosphamide plus mitoxantrone for relapsed or refractory low‐grade non‐Hodgkin's lymphoma. Haematologica 2001;86(3):282‐6. [PubMed] [Google Scholar]
Santoro 2006 {published data only}
- Santoro A, Voglova J, Gabrail N, Ciuleanu T, Liberati M, Hancock BW, et al. Comparative trial of BBR 2778 (pixantrone) + rituximab vs single agent rituximab in the treatment of relapsed/refractory indolent non‐Hodgkin's lymphoma (NHL). Journal of Clinical Oncology (ASCO Annual Meeting Proceedings) 2006;24 Suppl(18):Abstract No 7578. [Google Scholar]
Somers 1987 {published data only}
- Somers R, Burgers JM, Qasim M, Glabbeke M, Duez N, Hayat M. EORTC trial non‐Hodgkin lymphomas. European Journal of Cancer & Clinical Oncology 1987;23(3):283‐93. [DOI] [PubMed] [Google Scholar]
Unterhalt 1994 {published data only}
- Unterhalt M, Koch P, Pfreundschuh M, Maschmeyer G, Freund M, Thiel F, et al. Prednimustine, mitoxantrone (PMM) versus cyclophosphamide, vincristine, prednisone (COP) for the treatment of advanced low grade non‐Hodgkin's lymphoma. Blood 1994;84 Suppl 1:Abstract No 656. [Google Scholar]
Yahalom 1993 {published data only}
- Yahalom J, Varsos G, Fuks Z, Myers J, Clarkson BD, Straus DJ. Adjuvant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy after radiation therapy in stage I low‐grade and intermediate‐grade non‐Hodgkin lymphoma. Results of a prospective randomized study. Cancer 1993;71(7):2342‐50. [DOI] [PubMed] [Google Scholar]
Young 1977 {published data only}
- Young RC, Johnson RE, Canellos GP, Chabner BA, Brereton HD, Berard CW, et al. Advanced lymphocytic lymphoma: randomized comparisons of chemotherapy and radiotherapy, alone or in combination. Cancer Treatment Reports 1977;61(6):1153‐9. [PubMed] [Google Scholar]
References to ongoing studies
NCT00551239 {published and unpublished data}
- NCT00551239. Fludarabine and rituximab with or without pixantrone in treating patients with relapsed or refractory indolent non‐Hodgkin lymphoma. ClinicalTrials.gov/show/NCT00551239 (accessed 17 April 2013).
NCT00801281 {published and unpublished data}
- NCT00801281. First‐line R‐CVP vs R‐CHOP induction immunochemotherapy for indolent lymphoma and R maintenance (PLRG4). ClinicalTrials.gov/show/NCT00801281 (accessed 17 April 2013).
Additional references
Bartlett 1994
- Bartlett NL, Rizeq M, Dorfman RF, Halpern J, Horning SJ. Follicular large‐cell lymphoma: intermediate or low grade?. Journal of Clinical Oncology 1994;12(7):1349‐57. [DOI] [PubMed] [Google Scholar]
Bendandi 2008
- Bendandi M. Aiming at a curative strategy for follicular lymphoma. CA: A Cancer Journal for Clinicians 2008;58(5):305‐17. [DOI] [PubMed] [Google Scholar]
Billingham 1978
- Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treatments Reports 1978;62(6):865‐72. [PubMed] [Google Scholar]
Brandt 2001
- Brandt L, Kimby E, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in indolent non‐Hodgkin's lymphoma. Acta Oncologica 2001;40(2‐3):213‐23. [DOI] [PubMed] [Google Scholar]
Buske 2008
- Buske C, Gisselbrecht C, Gribben J, Letai T, McLaughlin P, Wilson W. Refining the treatment of follicular lymphoma. Leukemia & Lymphoma 2008;49 Suppl 1:18‐26. [DOI] [PubMed] [Google Scholar]
Chau 2003
- Chau I, Jones R, Cunningham D, Wotherspoon A, Maisey N, Norman AR, et al. Outcome of follicular lymphoma grade 3: is anthracycline necessary as front‐line therapy?. British Journal of Cancer 2003;89(1):36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheson 1999
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
Cheson 2007
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. Journal of Clinical Oncology 2007;25(5):579‐86. [DOI] [PubMed] [Google Scholar]
Dana 1993
- Dana BW, Dahlberg S, Nathwani BN, Chase E, Coltman C, Miller TP, et al. Long‐term follow‐up of patients with low‐grade malignant lymphomas treated with doxorubicin‐based chemotherapy or chemoimmunotherapy. Journal of Clinical Oncology 1993;11(4):644‐51. [DOI] [PubMed] [Google Scholar]
Federico 2000
- Federico M, Vitolo U, Zinzani PL, Chisesi T, Clo V, Bellesi G, et al. Prognosis of follicular lymphoma: a predictive model based on a retrospective analysis of 987 cases. Intergruppo Italiano Linfomi. Blood 2000;95(3):783‐9. [PubMed] [Google Scholar]
Federico 2009
- Federico M, Bellei M, Marcheselli L, Luminari S, Lopez‐Guillermo A, Vitolo U, et al. Follicular Lymphoma International Prognostic Index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. Journal of Clinical Oncology 2009;27(27):4555‐62. [DOI] [PubMed] [Google Scholar]
Friedberg 2009
- Friedberg JW, Taylor MD, Cerhan JR, Flowers CR, Dillon H, Farber CM, et al. Follicular lymphoma in the United States: first report of the national LymphoCare study. Journal of Clinical Oncology 2009;27(8):1202‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganti 2006
- Ganti AK, Weisenburger DD, Smith LM, Hans CP, Bociek RG, Bierman PJ, et al. Patients with grade 3 follicular lymphoma have prolonged relapse‐free survival following anthracycline‐based chemotherapy: the Nebraska Lymphoma Study Group Experience. Annals of Oncology 2006;17(6):920‐7. [DOI] [PubMed] [Google Scholar]
Goodman 2006
- Goodman LS, Gilman A, Brunton LL, Lazo JS, Parker KL. Goodman & Gilman's the Pharmacological Basis of Therapeutics. New York: McGraw‐Hill, 2006. [Google Scholar]
Greb 2008
- Greb A, Bohlius J, Schiefer D, Schwarzer G, Schulz H, Engert A. High‐dose chemotherapy with autologous stem cell transplantation in the first line treatment of aggressive Non‐Hodgkin Lymphoma (NHL) in adults. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004024.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 1994
- Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A Revised European‐American classification of Lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994;84(5):1361‐92. [PubMed] [Google Scholar]
Harris 1999
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller‐Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting‐Airlie House, Virginia, November 1997. Journal of Clinical Oncology 1999;17(12):3835‐49. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horning 1984
- Horning SJ, Rosenberg SA. The natural history of initially untreated low‐grade non‐Hodgkin's lymphomas. New England Journal of Medicine 1984;311(23):1471‐5. [DOI] [PubMed] [Google Scholar]
Lister 1978
- Lister TA, Cullen MH, Beard ME, Brearley RL, Whitehouse JM, Wrigley PF, et al. Comparison of combined and single‐agent chemotherapy in non‐Hodgkin's lymphoma of favourable histological type. BMJ 1978;1(6112):533‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2006
- Liu Q, Fayad L, Cabanillas F, Hagemeister FB, Ayers GD, Hess M, et al. Improvement of overall and failure‐free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. Journal of Clinical Oncology 2006;24(10):1582‐9. [DOI] [PubMed] [Google Scholar]
Mann 1983
- Mann RB, Berard CW. Criteria for the cytologic subclassification of follicular lymphomas: a proposed alternative method. Hematology and Oncology 1983;1(2):187‐92. [DOI] [PubMed] [Google Scholar]
Miller 1981
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47(1):207‐14. [DOI] [PubMed] [Google Scholar]
Montoto 2007
- Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, Amess J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B‐cell lymphoma. Journal of Clinical Oncology 2007;25(17):2426‐33. [DOI] [PubMed] [Google Scholar]
Morton 2006
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992‐2001. Blood 2006;107(1):265‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabhan 2007
- Nabhan C. It is follicular... so, why CHOP?. Journal of Clinical Oncology 2007;25(7):915‐6. [DOI] [PubMed] [Google Scholar]
NHLCP 1997
- NHLCP. A clinical evaluation of the International Lymphoma Study Group classification of non‐Hodgkin's lymphoma. The Non‐Hodgkin's Lymphoma Classification Project. Blood 1997;89(11):3909‐18. [PubMed] [Google Scholar]
Olin 2010
- Olin RL, Kanetsky PA, Have TR, Nasta SD, Schuster SJ, Andreadis C. Determinants of the optimal first‐line therapy for follicular lymphoma: a decision analysis. American Journal of Hematology 2010;85(4):255‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Outomuro 2007
- Outomuro D, Grana DR, Azzato F, Milei J. Adriamycin‐induced myocardial toxicity: new solutions for an old problem?. International Journal of Cardiology 2007;117(1):6‐15. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5,2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rigacci 2003
- Rigacci L, Federico M, Martelli M, Zinzani PL, Cavanna L, Bellesi G, et al. The role of anthracyclines in combination chemotherapy for the treatment of follicular lymphoma: retrospective study of the Intergruppo Italiano Linfomi on 761 cases. Leukemia & Lymphoma 2003;44(11):1911‐7. [DOI] [PubMed] [Google Scholar]
Salles 2011
- Salles G, Seymour JF, Offner F, Lopez‐Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011;377(9759):42‐51. [DOI] [PubMed] [Google Scholar]
Schulz 2007
- Schulz H, Bohlius J, Skoetz N, Trelle S, Kober T, Reiser M, et al. Chemotherapy plus Rituximab versus chemotherapy alone for B‐cell non‐Hodgkin's lymphoma. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003805.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siddhartha 2009
- Siddhartha G, Vijay P. R‐CHOP versus R‐CVP in the treatment of follicular lymphoma: a meta‐analysis and critical appraisal of current literature. Journal of Hematology and Oncology 2009;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solal‐Celigny 1993
- Solal‐Celigny P, Lepage E, Brousse N, Reyes F, Haioun C, Leporrier M, et al. Recombinant interferon alfa‐2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d'Etude des Lymphomes de l'Adulte. New England Journal of Medicine 1993;329(22):1608‐14. [DOI] [PubMed] [Google Scholar]
Solal‐Celigny 2004
- Solal‐Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz‐Saez R, et al. Follicular lymphoma international prognostic index. Blood 2004;104(5):1258‐65. [DOI] [PubMed] [Google Scholar]
Swerdlow 2008
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th Edition. Vol. 2, Lyon, France: IARC Press, 2008. [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tilly 2008
- Tilly H, Zelenetz A. Treatment of follicular lymphoma: current status. Leukemia & Lymphoma 2008;49 Suppl 1:7‐17. [DOI] [PubMed] [Google Scholar]
van Dalen 2009
- Dalen EC, Pal HJ, Caron HN, Kremer LCM. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005008.pub3] [DOI] [PubMed] [Google Scholar]
Vidal 2009
- Vidal L, Gafter‐Gvili A, Leibovici L, Shpilberg O. Rituximab as maintenance therapy for patients with follicular lymphoma. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006552.pub2] [DOI] [PubMed] [Google Scholar]
Vitolo 2008
- Vitolo U, Ferreri AJ, Montoto S. Follicular lymphomas. Critical Reviews in Oncology/Hematology 2008;66(3):248‐61. [DOI] [PubMed] [Google Scholar]
Wendum 1997
- Wendum D, Sebban C, Gaulard P, Coiffier B, Tilly H, Cazals D, et al. Follicular large‐cell lymphoma treated with intensive chemotherapy: an analysis of 89 cases included in the LNH87 trial and comparison with the outcome of diffuse large B‐cell lymphoma. Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology 1997;15(4):1654‐63. [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson P, Tudur Smith C, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21:3337‐51. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zinzani 2004
- Zinzani PL, Pulsoni A, Perrotti A, Soverini S, Zaja F, Renzo A, et al. Fludarabine plus mitoxantrone with and without rituximab versus CHOP with and without rituximab as front‐line treatment for patients with follicular lymphoma. Journal of Clinical Oncology 2004;22(13):2654‐61. [DOI] [PubMed] [Google Scholar]


