Nuclear magnetic resonance (NMR) technology is accelerating a revolution in medical and scientific neuroimaging that began in the 1970s with the introduction of powerful new x ray methods—computed tomography, positron emission tomography, and single photon emission computed tomography. Refinement of these technologies continues as applications for which they are uniquely well suited are defined.
Most clinicians are familiar with magnetic resonance imaging, which has recently become the most generally useful imaging modality for medical diagnosis.1 But conventional magnetic resonance imaging is only the first of an array of methods that will eventually comprise the NMR diagnostic armamentarium. We use the generic term “nuclear magnetic resonance” to emphasise the versatility of the technology. New NMR methods are in various stages of implementation and, combined with conventional magnetic resonance imaging, they are on the verge of changing medical practice so extensively that to describe the process as a revolution may ultimately seem conservative.
Summary points
Magnetic resonance imaging has become the premier imaging technique in medicine
It is the first well known member of a large array of NMR techniques
Several newer NMR techniques have medical and scientific potential
Such techniques are minimally invasive
Their power and versatility are unprecedented
They can make medical care more cost effective
NMR technology differs fundamentally from x ray methods. It uses the magnetic properties of atomic nuclei that occur naturally in the body; mostly hydrogen (1H) in water and other molecules, although several other nuclei, including phosphorus (31P), carbon (13C), lithium (7Li), sodium (23Na), nitrogen (15N), and oxygen (17O), are in regular use for medical research, and diagnostic applications are on the horizon.2
As measurements are made by synchronising atomic nuclear signals already present in tissue, NMR methods are among the least invasive diagnostic techniques available.3 At the cost of adding a minor degree of invasiveness, drugs that enhance contrast by altering the magnetic properties of tissues improve diagnostic utility. Because it can distinguish among signals from several biochemical molecules NMR technology has inherent chemical specificity. Such features make NMR methods the richest source of information about living tissue available from any imaging technology.
Diffusion and perfusion NMR imaging
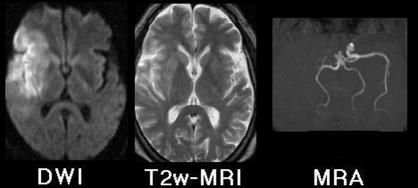
New NMR techniques with important consequences for daily medical practice include diffusion weighted imaging and magnetic resonance angiography in ischaemic stroke4,5; figure 1 shows the advantage of these over conventional magnetic resonance imaging for detection of early ischaemic stroke. When such a lesion is present, diffusion weighted imaging shows it clearly, and magnetic resonance angiography can often show the occluded artery without use of catheters, injected contrast agents, or ionising radiation.
Figure 1.
Magnetic resonance images 9.5 hours after onset of clinical stroke syndrome. Diffusion weighted imaging (DWI) shows hyperintense signal from physiologically damaged tissue surrounding right sylvian fissure—conventional T2 weighted imaging shows only subtle changes in same region. Magnetic resonance angiography (MRA) shows flow void in right internal carotid and middle cerebral arteries
The lesion in figure 1 is barely detectable by conventional magnetic resonance imaging. x Ray computed tomography would not have shown it at all, being characteristically negative in ischaemic stroke for many hours after the lesion is evident on conventional magnetic resonance imaging. When only x ray computed tomography is available time critical treatment decisions must be made either in the absence of precise information about the lesion or at the expense of invasive and costly x ray angiography. The advantages of diffusion weighted imaging and magnetic resonance angiography for diagnosis of early stroke are so great that much recent literature suggests that NMR methods will soon largely replace x ray computed tomography in the management of this condition.6–9
In addition to imaging of large cerebral arteries and veins by magnetic resonance angiogaphy, NMR perfusion imaging can measure blood flow through capillary beds.4,10 The state of brain perfusion associated with ischaemic and other lesions can be investigated. Both magnetic resonance angiography and perfusion imaging can be done without contrast enhancing agents, but contrast agents used for NMR imaging (for example, gadolinium chelates) cause allergic reactions less frequently than those used for x ray studies, and use of them increases anatomical resolution and sensitivity to several disease processes.
Functional magnetic resonance imaging
Functional magnetic resonance imaging can detect changes in blood oxygenation caused by increased metabolism of activated parts of the brain (fig 2).11 This has become a powerful tool for cognitive neuroscience research and brain activity mapping to preserve critical functions in patients needing neurosurgery.12,13
Figure 2.
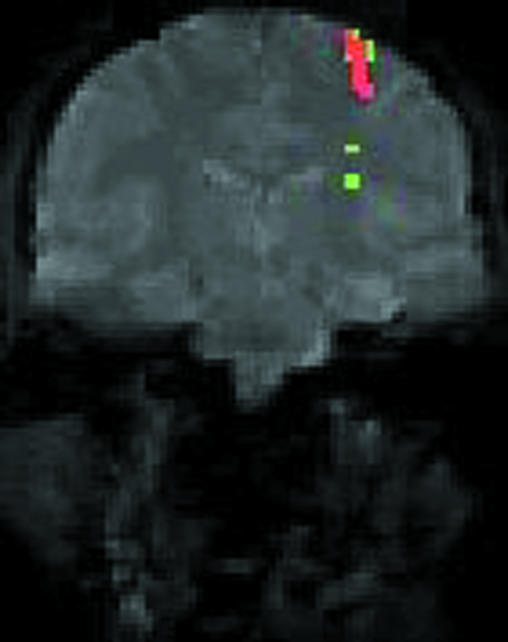
Functional magnetic resonance image of normal subject performing finger movements (conventional coronal magnetic resonance image in grey). Coloured regions identify tissue in which blood flow increases to meet extra metabolic requirements of task
Functional magnetic resonance imaging can be used to study any motor, sensory, or cognitive task that a patient can perform while in a scanner. Since functional magnetic resonance imaging requires no catheterisation or injection of contrast agent, observations can be repeated easily. It can be combined with contrasted studies for specific purposes, such as determination of the anatomical relation between a tumour mass and a brain region serving some key function. Functional magnetic resonance imaging is a powerful tool for studying the relation between brain and behaviour and how this is affected by disease.
Magnetisation transfer imaging
Magnetisation transfer imaging detects changes in the properties of water protons and some other magnetic nuclei as they move from one physical state or chemical configuration to another. The technique can be used to probe protein-water interaction and the rates of some key chemical reactions that are catalysed by enzymes. Its most productive medical application has been in the study of brain white matter disease, notably multiple sclerosis, where it shows lesions and aspects of pathophysiology opaque to other NMR methods and to x ray computed tomography.14–17
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy detects magnetic nuclei other than water protons.18 Lactate, some amino acids, and several other small metabolites in the brain can be measured in living tissue by detection of signals from their hydrogen nuclei. Magnetic resonance spectroscopy of 31P detects several small metabolites that contain phosphorus—ATP, phosphocreatine, and inorganic phosphate among them. Magnetic resonance spectroscopy for the detection of metabolites containing 13C, 15N, and 17O is becoming the premier technology for measurement of metabolic rates in living tissue. Brain pH can be measured by its effects on pH sensitive 31P and 1H signals.19
Magnetic resonance spectroscopy is unique among measurement technologies in the range of chemically specific studies that it can make on living undisturbed tissue—for example, analysis of antiepileptic drug effects on brain metabolism,20,21 demonstration of low γ-aminobutyric acid concentrations in the brains of patients with alcoholism and hepatic encephalopathy,22 and detection of chemical abnormalities in the white matter of patients with multiple sclerosis before changes can be seen on magnetic resonance images.23
Spectroscopic imaging
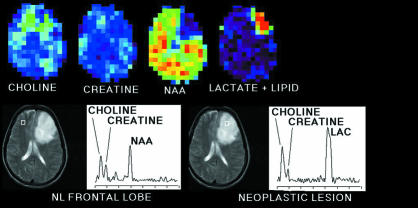
Spectroscopic imaging is an extension of magnetic resonance spectroscopy, which allows mapping of the distribution of specific compounds in the living brain. The technique is complex, but it is so powerful a way of studying normal and diseased brain chemistry that numerous laboratories are working to make it generally available. It has already been used to study several disease states including epilepsy,24 panic attacks,25 and brain tumours (fig 3).26
Figure 3.
(Top) Spectroscopic image of patient with glioblastoma multiforme. Colour images display topographical distribution of intensities of NMR signals from several cerebral metabolites including total choline, total creatine, N-acetylaspartate, lactate, and lipid. (Bottom) Tissue section examined displayed by conventional magnetic resonance imaging. Metabolite signals can be displayed as spectra from volumes (white squares) referenced to magnetic resonance image (graphs). Compared with right frontal lobe, neoplastic tissue in left frontal lobe shows increased choline, lactate, and lipid signals but decreased N-acetylaspartate signals
All the NMR techniques discussed above are feasible in humans with appropriately equipped NMR instruments only a little different from thousands of medical imaging scanners already in regular use worldwide. The combination of power, versatility, and non-invasiveness presented by these methods has no historical parallel. Only comparisons with all of microscopic technology from Leeuwenhoek's optical instrument in the 17th century to modern scanning tunnelling electron microscopes, or molecular biology, give an idea of the scale on which NMR technology is unfolding.
Owing to the brain's comparative immobility, NMR measurements are more advanced there than elsewhere in the body. As motion artefacts in thoracic and abdominal viscera are brought under control by methods that acquire images quickly the full range of NMR methods in use by neurologists and neurosurgeons will become available to a wider range of physicians—a movement already under way in cardiology.27,28
NMR methods have their own limitations, which must be taken into account. As with x ray computed tomography they are not, and will never be, bedside procedures. Patients undergoing them must be free of magnetic implants and instruments. Even non-magnetic metallic implants may degrade image quality near the implant and diminish the test's diagnostic value. Some patients are unable to tolerate the confines of a scanner or to keep still; sedative and antianxiety drugs can be helpful in such cases. Traditional routines of medical practice will need modification to take advantage of the opportunities offered by NMR technology; in particular closer consultation between referring and imaging physicians will be necessary to choose which of many possible measurements are most important for each patient.
But some limitations are more apparent than real. Many physicians may have heard that magnetic resonance imaging is too expensive for routine diagnostic use. We believe the opposite will prove to be true. In terms of technical maturity and familiarity to the medical profession NMR technology is today about where x ray computed tomography was 20 years ago—when it too was considered overly expensive—but the former is far more powerful. As NMR methods achieve technical and operational maturity over the next decade we predict that their power, versatility, and non-invasiveness will pay back their implementation costs many times over, by earlier and more accurate diagnosis leading to more effective treatment of diseases, fewer errors, and fewer admissions to hospital.
Important as those practical gains will be if our expectations are accurate, they are not the end of the story. Beyond them lie the benefits of reduced patient distress from onerous procedures and especially the time that more efficient diagnosis can recover for unhurried conversation between physician and patient.
Supplementary Material
Footnotes
Funding: Grants from the American Cancer Society (EDT-119), the American Heart Association (9650621N), and the United States Public Health Service (CA 76524, NS 34834, NS 07416).
Competing interests: None declared.
References
- 1.Stark DD, Bradley WG. Magnetic resonance imaging. St Louis: Mosby; 1992. [Google Scholar]
- 2.Grant DM, Harris RK. Encyclopedia of NMR. Chichester: Wiley; 1996. [Google Scholar]
- 3.Budinger TF. MR safety: past, present, and future from a historical perspective. Magn Reson Imaging Clin North Am. 1998;6:701–714. [PubMed] [Google Scholar]
- 4.Le Bihan D. Diffusion and perfusion magnetic resonance imaging: applications to functional MRI. New York: Raven; 1995. [Google Scholar]
- 5.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. 1995;37:231–241. doi: 10.1002/ana.410370214. [DOI] [PubMed] [Google Scholar]
- 6.Ay H, Buonanno FS, Rordorf G, Schaefer PW, Schwamm LH, Wu O, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784–1792. doi: 10.1212/wnl.52.9.1784. [DOI] [PubMed] [Google Scholar]
- 7.Fisher M, Albers GW. Applications of diffusion-perfusion magnetic resonance imaging in acute ischemic stroke. Neurology. 1999;52:1750–1756. doi: 10.1212/wnl.52.9.1750. [DOI] [PubMed] [Google Scholar]
- 8.Marks MP, Tong DC, Beaulieu C, Albers GW, de Crespigny A, Moseley ME. Evaluation of early reperfusion and IV tPA therapy using diffusion- and perfusion-weighted MRI. Neurology. 1999;52:1792–1798. doi: 10.1212/wnl.52.9.1792. [DOI] [PubMed] [Google Scholar]
- 9.Prichard JW, Grossman RI. New reasons for early use of MRI in stroke. Neurology. 1999;52:1733–1736. doi: 10.1212/wnl.52.9.1733. [DOI] [PubMed] [Google Scholar]
- 10.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prichard JW, Cummings JL. The insistent call from functional MRI. Neurology. 1997;48:797–800. doi: 10.1212/wnl.48.4.797. [DOI] [PubMed] [Google Scholar]
- 13.Redpath TW. MRI developments in perspective. Br J Radiol. 1997;70:70–80S. doi: 10.1259/bjr.1997.0010. [DOI] [PubMed] [Google Scholar]
- 14.Filippi M, Rocca MA, Minicucci L, Martinelli V, Ghezzi A, Bergamaschi R, et al. Magnetization transfer imaging of patients with definite MS and negative conventional MRI. Neurology. 1999;52:845–848. doi: 10.1212/wnl.52.4.845. [DOI] [PubMed] [Google Scholar]
- 15.Poser CM. Serial magnetization transfer imaging to characterize the early evolution of new MS lesions. [Letter.] Neurology. 1999;52:1717. doi: 10.1212/wnl.52.8.1717. [DOI] [PubMed] [Google Scholar]
- 16.Richert ND, Ostuni JL, Bash CN, Duyn JH, McFarland HF, Frank JA. Serial whole-brain magnetization transfer imaging in patients with relapsing-remitting multiple sclerosis at baseline and during treatment with interferon beta-1b. Am J Neuroradiol. 1998;19:1705–1713. [PMC free article] [PubMed] [Google Scholar]
- 17.Silver NC, Lai M, Symms MR, Barker GJ, McDonald WI, Miller DH. Serial magnetization transfer imaging to characterize the early evolution of new MS lesions. Neurology. 1998;51:758–764. doi: 10.1212/wnl.51.3.758. [DOI] [PubMed] [Google Scholar]
- 18.Prichard JW. Brain MRS of human subjects. In: Grant DM, Harris RK, Young IR, editors. Encyclopedia of NMR. Vol. 2. Chichester: Wiley; 1996. pp. 1023–1030. [Google Scholar]
- 19.Prichard JW, Rothman DL, Petroff OAC. Brain pH measurements by NMR spectroscopy. In: Kaila K, Ransom BR, editors. pH and brain function. New York: Wiley; 1998. pp. 149–165. [Google Scholar]
- 20.Petroff OA, Rothman DL. Measuring human brain GABA in vivo: effects of GABA-transaminase inhibition with vigabatrin. Mol Neurobiol. 1998;16:97–121. doi: 10.1007/BF02740605. [DOI] [PubMed] [Google Scholar]
- 21.Petroff OA, Hyder F, Mattson RH, Rothman DL. Topiramate increases brain GABA, homocarnosine, and pyrrolidinone in patients with epilepsy. Neurology. 1999;52:473–478. doi: 10.1212/wnl.52.3.473. [DOI] [PubMed] [Google Scholar]
- 22.Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, et al. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- 23.Fu L, Matthews PM, De Stefano N, Worsely KJ, Narayanan S, Francis GS, et al. Imaging axonal damage of normal-appearing white matter in multiple sclerosis. Brain. 1998;121:103–113. doi: 10.1093/brain/121.1.103. [DOI] [PubMed] [Google Scholar]
- 24.Kuzniecky R, Hugg JW, Hetherington H, Butterworth E, Bilir E, Faught E, et al. Relative utility of 1H spectroscopic imaging and hippocampal volumetry in the lateralization of mesial temporal lobe epilepsy. Neurology. 1998;51:66–71. doi: 10.1212/wnl.51.1.66. [DOI] [PubMed] [Google Scholar]
- 25.Dager SR, Friedman SD, Heide A, Layton ME, Richards T, Artru A, et al. Two-dimensional proton echo-planar spectroscopic imaging of brain metabolic changes during lactate-induced panic. Arch Gen Psychiatry. 1999;56:70–77. doi: 10.1001/archpsyc.56.1.70. [DOI] [PubMed] [Google Scholar]
- 26.Gupta RK, Sinha U, Cloughesy TF, Alger JR. Inverse correlation between choline magnetic resonance spectroscopy signal intensity and the apparent diffusion coefficient in human glioma. Magn Reson Med. 1999;41:2–7. doi: 10.1002/(sici)1522-2594(199901)41:1<2::aid-mrm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Boothroyd A. Magnetic resonance—its current and future role in paediatric cardiac radiology. Eur J Radiol. 1998;26:154–162. doi: 10.1016/s0720-048x(97)00091-0. [DOI] [PubMed] [Google Scholar]
- 28.Passariello R, De Santis M. Magnetic resonance imaging evaluation of myocardial perfusion. Am J Cardiol. 1998;81(12A):68–73. doi: 10.1016/s0002-9149(98)00057-5. G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.