Abstract
Initiation of productive infection by human herpes simplex virus type 1 (HSV-1) requires cell cycle-dependent protein kinase (cdk) activity. Treatment of cells with inhibitors of cdks blocks HSV-1 replication and prevents accumulation of viral transcripts, including immediate-early (IE) transcripts (26). Inhibition of IE transcript accumulation suggests that virion proteins, such as VP16, require functional cdks to activate viral transcription. In this report, we show that a cdk inhibitor, Roscovitine, blocks VP16-dependent IE gene expression. In the presence of Roscovitine, the level of virion-induced activation of a transfected reporter gene (the gene encoding chloramphenicol acetyltransferase) linked to the promoter-regulatory region of the ICP0 gene was reduced 40-fold relative to that of untreated samples. Roscovitine had little effect on the interaction of VP16 with VP16-responsive DNA sequences as measured by electrophoretic mobility shift assays. These data indicate that VP16-dependent activation of IE gene expression requires functional cdks and that this requirement is independent of the ability of VP16 to bind to DNA.
The human herpes simplex virus type 1 (HSV-1) regulatory protein, VP16, stimulates productive infection by activating transcription of viral immediate-early (IE) genes. VP16 activates transcription from IE promoters by indirectly binding to specific sequence elements (TAATGARAT) found in the promoter-regulatory regions of all IE genes (19, 33). VP16 is associated with the viral tegument and is released from the virion upon entry into susceptible cells. Inside the cell, VP16 interacts with two host proteins, host cell factor (HCF) and Oct-1, which together facilitate binding of the protein complex to VP16 response elements (14, 15, 30, 37). Formation of the protein-DNA complex is essential for transactivation of IE genes (19, 22, 33). Binding of VP16 to DNA through HCF and Oct-1 exposes the acidic activation domain of VP16, which interacts with host transcriptional proteins to increase the rate of transcription initiation (31). While in vitro reconstitution of VP16-dependent transcriptional activation using purified proteins has assisted in elucidating the molecular mechanism of VP16 action, the mechanism by which this process is regulated during viral infection is poorly understood (16, 17, 24).
Several lines of evidence suggest that VP16 and VP16-associated proteins rely on cell cycle-regulated activities to stimulate transcription. A temperature-sensitive form of HCF inhibits cell cycle progression at the nonpermissive temperature (5). Extracts prepared from these cells inhibit VP16-dependent DNA binding and transactivation in vitro (5). Domains of HCF that are required for cell cycle progression are also required for VP16-dependent transcriptional activation (36). In addition, the Oct-1 protein is phosphorylated in a cell cycle-dependent manner (23, 27). Finally, two inhibitors of cyclin-dependent kinases (cdks), Roscovitine and Olomucine, block accumulation of HSV-1 IE transcripts and inhibit viral replication when added 1 to 6 h postinfection (p.i.) (25, 26). Roscovitine is a specific inhibitor of cdk-1, cdk-2, cdk-5 (18), and cdk-7 (26a).
Inhibition of IE gene expression by cdk inhibitors suggests that these kinases are important for VP16-dependent transcriptional activation. Moreover, Roscovitine is the only drug that inhibits transcription of IE genes. Taken together, these observations indicate that regulation of VP16-dependent transactivation during viral infection requires cell cycle-dependent activities. In this study, we demonstrate that VP16-dependent transactivation of an IE promoter requires the activities of cellular cdks and that this requirement is independent of the ability of VP16 to bind to DNA.
Inhibition of virion-induced IE gene expression by Roscovitine.
Previous findings have suggested the possibility that cdks are important for expression of viral IE genes (25, 26). In order to measure the effects of the cdk inhibitor, Roscovitine, on VP16-dependent transcriptional activation, a transient-transfection/superinfection assay was utilized. Vero cells (2 × 105/60-mm-diameter dish) were transfected with 1 μg of a plasmid (pWRICP0-CAT) that contains the gene encoding chloramphenicol acetyltransferase (CAT) under the control of the promoter-regulatory region of the HSV IE gene, ICP0. At 48 h posttransfection, cultures were infected with the equivalent of 10 PFU of UV-inactivated HSV-1 KOS per cell in the presence and absence of 100 μM Roscovitine. At 3, 6, and 9 h p.i., the cultures were harvested and CAT activity was measured. UV inactivation of viral stocks inhibits viral gene expression but leaves the activities of virion proteins, including VP16, intact. Thus, in this assay, activation of the ICP0 promoter in the transfected plasmid by UV-inactivated virions is mediated by VP16 and possibly by other virion-associated proteins.
Addition of Roscovitine at the time of infection blocked the ability of UV-inactivated KOS to induce CAT expression from pWRICP0-CAT (Table 1, rows 1 to 4). In the presence of Roscovitine, the level of CAT activity in virus-infected cultures (row 4) was similar to that in mock-infected cultures (row 1). In the absence of Roscovitine, the level of CAT activity in virus-infected cultures at 9 h p.i. was 39-fold higher than that in mock-infected cultures (row 2). Addition of Roscovitine had no effect on the basal level of CAT expression in mock-infected cultures (row 3). These results demonstrate that Roscovitine inhibits virion-induced IE gene expression.
TABLE 1.
Roscovitine, but not K252a or Lovastatin, inhibits virion-induced IE gene expressiona
| Treatment | CAT activityb at indicated time p.i.
|
||
|---|---|---|---|
| 3 h | 6 h | 9 h | |
| Mock | 1.3 (0.6) | 0.9 (0.3) | 1.0 (0.3) |
| UV-KOS | 2.3 (1.3) | 17.7 (3.4) | 39.3 (5.1) |
| Rosco | 1.3 (0.6) | 0.8 (0.3) | 0.8 (0.3) |
| Rosco + UV-KOS | 0.8 (0.2) | 0.9 (0.4) | 0.9 (0.2) |
| Lova | 1.1 (0.6) | 1.0 (0.5) | 1.2 (0.6) |
| Lova + UV-KOS | 2.1 (1.2) | 21.4 (4.2) | 47.5 (6.0) |
| K252a | 0.8 (0.4) | 1.1 (0.3) | 1.0 (0.3) |
| K252a + UV-KOS | 2.3 (1.4) | 16.2 (3.7) | 26.6 (3.6) |
Vero cells (2 × 105/60-mm-diameter dish) were transfected with 1 μg of a plasmid (pWRICP0-CAT) that contains the gene encoding the CAT under the control of the promoter-regulatory region of the IE gene, ICP0. At 48 h posttransfection, the cultures were infected with the equivalent of 10 PFU of UV-inactivated HSV-1 KOS per cell in the presence and absence of 100 μM Roscovitine. At 3, 6, and 9 h p.i., the cultures were harvested and CAT activity was measured.
CAT activity was measured from three independent transfections, and data are expressed as fold activation relative to that of mock-infected cultures at the 3-h time point. The numbers in parentheses represent the standard errors of the means.
Kinetics of Roscovitine-dependent inhibition of IE gene expression.
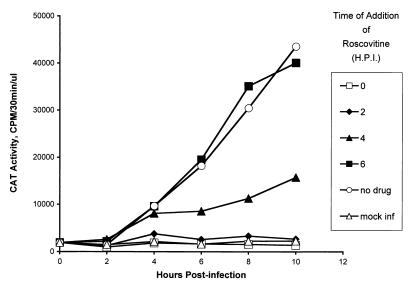
Since Roscovitine inhibits HSV replication even when added to infected cells at 6 h p.i. (7, 8), it was of interest to determine if cdk activity was required for activation of HSV IE promoters at different times after infection. For this purpose, Vero cells (2 × 105/60-mm-diameter dish) were transfected with 1 μg of pWRICP0-CAT and mock infected or infected with 10 PFU of UV-inactivated KOS per cell at 48 h posttransfection. The cultures were divided into six groups containing six dishes each. At 0, 2, 4, and 6 h p.i., the culture medium in a single group was removed and replaced with medium containing 100 μM Roscovitine. In addition, at 0, 2, 4, 6, 8, and 10 h p.i., one dish from each group was harvested and CAT activity was measured. The mock-infected group was not treated with Roscovitine.
Inhibition of virion-induced CAT expression by Roscovitine was most efficient when drug was added at 0 and 2 h p.i. (Fig. 1). The level of CAT activity when Roscovitine was added at these times was similar to the basal levels in mock-infected samples. Roscovitine was less effective in inhibiting virion-induced CAT activity when added at 4 h p.i. Notably, however, the level of CAT activity did not change significantly after Roscovitine addition at this time, suggesting that the drug inhibited new CAT expression. By 6 h p.i., CAT activity in infected cultures was refractory to Roscovitine inhibition in that the levels of CAT activity in the presence of Roscovitine were comparable to those in the absence of drug. These results indicate either that (i) Roscovitine inhibits virion-induced CAT activity at a step that occurs prior to 6 h p.i. or (ii) by 6 h p.i., translation of CAT mRNA becomes rate limiting in the infected cell and blocking new synthesis of CAT mRNA does not affect translation of the remaining CAT message.
FIG. 1.
Kinetics of Roscovitine-dependent inhibition of IE gene expression. Vero cells (2 × 105/60-mm-diameter dish) were transfected with 1 μg of pWRICP0-CAT, and at 48 h posttransfection, the cultures were mock infected or infected with 10 PFU of UV-inactivated KOS per cell. The cultures were divided into six groups containing six dishes each. At 0, 2, 4, and 6 h p.i. (H.P.I.), the culture medium in a single group was removed and replaced with medium containing 100 μM Roscovitine. In addition, at 0, 2, 4, 6, 8, and 10 h p.i., one dish from each group was harvested and CAT activity was measured. The mock-infected group was not treated with Roscovitine. CAT activity was measured in the linear range of the assay, and a value of 40,000 cpm represents approximately 20% acetylation of the radiolabeled chloramphenicol substrate in the reaction mixtures.
Lovastatin and K252a do not inhibit virion-induced IE gene expression.
Roscovitine inhibits IE gene expression either by blocking cdk activity or by blocking the activities of downstream proteins which are both activated by cdks and required for cell cycle progression. We thus tested whether cell cycle inhibition or inhibition of other serine-threonine kinases blocked virion-induced activation of IE gene expression. A well-characterized cell cycle inhibitor, Lovastatin, and a broad-spectrum serine-threonine kinase inhibitor, K252a, were tested for their ability to inhibit virion-induced CAT expression (10). Lovastatin is an HMG-coenzyme A reductase inhibitor that blocks association of ras with the plasma membrane (9, 11). This interaction is required to transduce growth factor-dependent signaling to the nucleus (9). Blocking this signaling pathway arrests cells in the G1 phase of the cell cycle (9). Indeed, in control experiments, 10 μM Lovastatin blocked cell cycle progression, while higher doses were toxic (data not shown). Thus, although Roscovitine and Lovastatin inhibit cell cycle progression, their mechanisms of action are quite different.
To test the effects of Lovastatin and K252a on HSV-1 IE gene expression, Vero cells (2 × 105 cells/60-mm-diameter dish) were transfected with 1 μg of pWRICP0-CAT. At 48 h posttransfection, the cultures were infected with 10 PFU of UV-inactivated KOS per cell in the presence and absence of 100 μM Roscovitine, 10 μM Lovastatin, and 250 μM K252a (the highest nontoxic dose of this drug). At 3, 6, and 9 h p.i., infected cultures were harvested and CAT activity was measured.
As shown in Table 1, Lovastatin (row 8) and K252a (row 6) had little effect on virion-induced IE gene expression when added at the time of infection. Likewise, cultures treated with Lovastatin or K252a 24 h prior to infection had no effect on virion-induced IE gene expression (data not shown). Collectively, the results shown in Table 1 demonstrate that virion-induced IE gene expression requires activities (most likely cdks) that are sensitive to inhibition by Roscovitine but not Lovastatin or K252a.
Roscovitine does not inhibit VP16-dependent DNA binding.
Binding of VP16 to the consensus sequence, TAATGARAT, is necessary for transcriptional activation of IE genes. To test whether Roscovitine inhibits binding of VP16 to DNA, gel mobility shift assays were performed. Nuclear extracts were prepared from cycloheximide-treated (50 μg/ml) Vero cells (107/T150 flask) that were either mock infected or infected with 20 PFU of KOS per cell in the presence or absence of 100 μM Roscovitine. Cycloheximide was used to inhibit viral gene expression so that only virion-associated activities would be measured in the nuclear extracts (22). In addition, nuclear, rather than whole-cell, extracts were used in the event that Roscovitine inhibits nuclear transport of VP16, HCF, and Oct-1. At 3 h p.i., the cultures were harvested and nuclear extracts were prepared by the method of Dignam et al. (3).
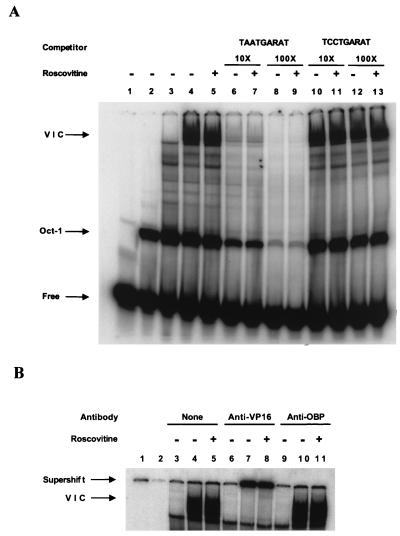
Three microliters of nuclear extract (12 μg of protein) was incubated in 12 μl of binding buffer [10 mM HEPES (pH 8.0), 1 mM EDTA, 5 mM dithiothreitol, 0.1% NP-40, 0.5% Ficoll, 50 ng of salmon sperm DNA/ml, 1.5 μg of poly(dIdC)/ml] for 5 min at 20°C. In addition, the binding reaction mixture was supplemented with histidine-tagged Oct-1 POU domain protein expressed in Escherichia coli and purified by nickel affinity chromatography. The binding reaction mixtures were supplemented with Oct-1 POU domain protein to enhance the VP16-dependent DNA binding activity because Oct-1 is limiting in Vero cell nuclear extracts. After 5 min of incubation, 0.5 ng of a 32P-end-labeled (∼5 × 105 cpm) 29-bp oligonucleotide probe (CCGTGCATGCTAATGATATTCCTTTGGGGG) containing the VP16 response element from the ICP0 promoter (underlined) was added to the reaction mixture, and the mixture was incubated for an additional 30 min at 20°C. The protein-DNA complexes were separated by native gel electrophoresis on a 5% polyacrylamide gel in TBE buffer (89 mM Tris-borate, 89 mM boric acid, 2 mM EDTA) and visualized by PhosphorImager analysis (Fig. 2A).
FIG. 2.
(A) Roscovitine does not affect induction of virion-induced TAATGARAT DNA binding activity. Three microliters of nuclear extract (12 μg of protein) was incubated in 12 μl of binding buffer [10 mM HEPES (pH 8.0), 1 mM EDTA, 5 mM dithiothreitol, 0.1% NP-40, 0.5% Ficoll, 50 ng of salmon sperm DNA/ml, 1.5 μg of poly(dIdC)/ml] for 5 min at 20°C. In addition, the binding reaction mixture was supplemented with histidine-tagged Oct-1 POU domain protein expressed in E. coli and purified by nickel affinity chromatography. After 5 min of incubation, 0.5 ng of a 32P-end-labeled (∼5 × 105 cpm) 29-bp oligonucleotide probe (see text for complete sequence) containing the wild-type VP16 response element from the ICP0 promoter (TAATGARAT) or a probe containing a mutant VP16 response element (TCCTGARAT) was added to the reaction mixture, and the mixture was incubated for an additional 30 min at 20°C. The protein-DNA complexes were separated by native gel electrophoresis on a 5% polyacrylamide gel in TBE buffer and visualized by PhosphorImager analysis. Lane 1, free probe; lane 2, Oct-POU domain (Oct-1); lane 3, mock-infected nuclear extract; lanes 4 to 13, KOS-infected nuclear extract in the presence (+) or absence (−) of 100 μM Roscovitine. The VIC is indicated. (B) VP16 antibody supershifts the VIC. Binding reactions were performed as described for panel A. Following the 30-min incubation period, 1 μl of binding buffer (lanes 3 to 5), polyclonal rabbit antibody directed against a Gal4-VP16 fusion protein (32) (anti-VP16) (lanes 6 to 8), or antibody directed against the HSV-1 origin binding protein (anti-OBP) (lanes 9 to 11) was added to the reaction mixtures. The binding reaction mixtures were incubated for 5 min prior to gel electrophoresis. Lane 1, free probe (not shown in figure); lane 2, Oct-1 (not shown in figure); lanes 3, 6, and 9, mock-infected extracts; lanes 4, 5, 7, 8, 10, and 11, KOS-infected nuclear extract in the presence (+) or absence (−) of 100 μM Roscovitine.
Mock-infected nuclear extracts supplemented with purified Oct-1 POU domain protein produced a complex that migrated more slowly than free probe (Fig. 2A, lane 2, Oct-1). In KOS-infected nuclear extracts, a second major complex, the virion-induced complex (VIC) containing VP16, HCF, and Oct-1 POU domain protein bound to the VP16 response element, was observed (Fig. 2A, lanes 4, 5, and 10 to 13, VIC). This complex was not present in mock-infected nuclear extracts (Fig. 2A, lane 3). To measure the specificity of the two protein-DNA complexes, binding was completed with 10- and 100-fold molar excesses of either unlabeled specific probe or a mutant probe in which the VP16 consensus site, TAATGARAT (R represents any purine), was changed to TCCTGARAT. The VIC band did not form with an oligonucleotide probe containing this mutation (data not shown). Formation of both VIC and (putative) Oct-POU domain complexes was efficiently inhibited by addition of unlabeled specific probe (Fig. 2A, lanes 6 to 9) but not by addition of unlabeled mutant probe (Fig. 2A, lanes 10 to 13). Moreover, LA2-3, a rabbit polyclonal antibody directed against a Gal4-VP16 fusion protein (32) and kindly provided by Steven Treizenberg (Michigan State University, East Lansing, Mich.), supershifted the VIC complex (Fig. 2B, lanes 7 and 8), while an antibody specific for the herpesvirus origin binding protein (OBP) did not (Fig. 2B, lanes 10 and 11). Based on these observations, it is likely that the virion-induced protein-DNA complex, VIC, represents VP16 bound to the consensus TAATGARAT site through interaction with the Oct-1 POU domain and possibly HCF. Notably, addition of Roscovitine had no measurable effect on the formation or mobility of either VIC or the putative Oct-1 protein DNA complex. Thus, Roscovitine does not affect the interaction of VP16 with its consensus binding site in this assay. These results suggest that inhibition of activation of IE gene expression by Roscovitine occurs at a step following VP16 binding to DNA. Whether individual components of the VIC complex are equally phosphorylated in the presence (Fig. 2A, lane 5) or absence (Fig. 2A, lane 4) of Roscovitine remains to be determined.
In this study, we have shown that Roscovitine, a specific inhibitor of cdk activity, blocks virion-induced activation of an IE promoter. Two other compounds, Lovastatin, an inhibitor of cell cycle progression, and K252a, a broad-spectrum serine-threonine kinase inhibitor, did not inhibit virion-dependent IE gene activation, demonstrating that the inhibitory effect is specific for Roscovitine. The ability of Roscovitine to inhibit transactivation of IE promoters indicates that the activity of one or more components of the VP16-containing transcription complex is affected by active cdks. Roscovitine does not inhibit formation of protein-DNA complexes with TAATGARAT elements or detectably change the mobility of these complexes measured by native polyacrylamide gel electrophoresis.
There are several possible sites at which Roscovitine may act, and they are described in the following discussions: (i) In vitro studies have characterized the molecular mechanism of VP16-dependent transcriptional activation (13–17, 19, 22, 24, 30, 31, 37). These studies have identified an acidic activation domain in the C terminus of VP16 that is essential for transcriptional activation (16, 17, 24, 29, 31). The acidic activation domain, when fused to heterologous DNA binding domains, stimulates transcription from promoters that contain the binding site for the heterologous DNA binding protein (2). The acidic activation domain interacts with basal transcription factors, TFIIB and TFIID, as measured by vitro transcription-translation studies and by affinity chromatography (16, 17, 31). Mutations in the acidic activation domain that block the interaction of the VP16 with TFIIB and TFIID inhibit transcriptional activation (16, 24, 31). Taken together, these studies suggest that VP16 recruits basal transcription proteins to promoters through interactions with the acidic activation domain. Recruitment of basal transcription factors by the VP16 acidic activation domain may require cdk activity. Indeed, VP16 is phosphorylated in vitro at position 375 (20). Point mutations at this site block VP16-dependent protein-DNA complex assembly (20). Even if cdks do not phosphorylate VP16 directly, they may stimulate downstream kinases or other enzymes that activate the C-terminal acidic activation domain. Thus, inhibition of cdk activity by Roscovitine might block the ability of VP16 to recruit basal transcription factors to HSV-1 IE gene promoters.
(ii) While no obvious cdk phosphorylation sites map to the domains of VP16 that are phosphorylated in vivo, a strong cdk consensus site maps to the N-terminal portion of HCF at position 127 (25). Point mutations at amino acid 134 in HCF, near the cdk consensus site, block VP16-dependent transactivation and inhibit cell cycle progression (5). These observations suggest that phosphorylation of HCF may play a critical role in the activity of the protein with respect to VP16-induced transactivation and cell cycle progression. In addition, Oct-1 is phosphorylated in a cell cycle-dependent manner (23, 27). Moreover, Oct-1 DNA binding activity is regulated by phosphorylation (27). Phosphorylation of components of the VP16–HCF–Oct-1 complex, and not just the C-terminal acidic transactivation domain of VP16, may be required for transcriptional activation. Thus, Roscovitine may inhibit VP16-dependent IE gene activation by preventing the phosphorylation of one or more proteins in the VP16-induced complex. While phosphorylation may not affect the interaction of these proteins with VP16 and the TAATGARAT element, inhibition of phosphorylation may block the ability of these proteins to interact with basal transcription factors. The cell cycle-dependent phosphorylation of VP16 and VP16-associated proteins is currently under investigation.
(iii) Roscovitine may inhibit a component(s) of the basal transcription complex. Initiation of eukaryotic transcription involves the assembly of more than 30 proteins at a specific site on the promoter (12). Many of the proteins that form the transcription complex are regulated by cell cycle-dependent activities. Cell cycle-dependent phosphorylation of basal transcription factors has been observed for TFIID and TBP (4). Furthermore, cdk-7, a component of the basal transcription complex, phosphorylates the carboxy-terminal domain of RNA polymerase II. This phosphorylation appears to be required for basal and activated transcription (1, 6, 21). Recent studies suggest that Roscovitine directly inhibits cdk7 activity in vitro (26a). Thus, Roscovitine may inhibit VP16–HCF–Oct-1-dependent transcriptional activation by blocking assembly and/or function of the assembled transcription complex. If this is the primary mechanism of Roscovitine inhibition of HSV replication, however, one would predict that this drug would also affect basal transcription. While we observed little effect on the basal level of ICP0-CAT expression in the presence of Roscovitine, more-sensitive assays that directly measure basal transcription will be required to compare the effects of Roscovitine on both basal and activated transcription. It should be noted, however, that Vero and HEL cells survive for long periods (more than 48 h) in Roscovitine-containing medium, indicating that basal cellular transcription is not totally blocked by this drug.
Whatever the mechanism of Roscovitine-dependent inhibition of virion-induced IE gene expression, the demonstration that cdk activity is required for this process suggests that productive infection would be less efficient in cell types in which these enzymes are not active. Moreover, recent observations suggest that cdk activity is also required for viral E gene expression (26). Terminally differentiated neurons do not express most of the cdks whose expression has been studied (28). Indeed, induction of cdk-2 activity in terminally differentiated neurons correlates with induction of apoptosis in vitro (28). Thus, HSV infection of neurons not expressing cdk activity may promote the establishment of latent infection by limiting expression of IE and E genes. Indeed, low-multiplicity infection of neurons in culture leads to a latent-like infection (34, 35). These observations are consistent with the hypothesis that cdk activity is required for HSV IE and E gene expression during productive infection and reactivation from latency (25).
Acknowledgments
This work was supported in part by Public Health Service grant RO1 CA20260 from the National Cancer Institute and grant IRG-135R from the American Cancer Society.
We thank William Halford for helpful discussions and ideas and Timothy Block and Ying-Hsiu Su for critical reading of the manuscript. We also thank Steve Treizenberg and David Davido for providing VP16 antibody and Jennifer Isler for providing OBP antibody.
REFERENCES
- 1.Dahmus M E. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem. 1996;271:19009–19012. doi: 10.1074/jbc.271.32.19009. [DOI] [PubMed] [Google Scholar]
- 2.Das G, Hinkley C S, Herr W. Basal promoter elements as a selective determinant of transcriptional activator function. Nature. 1995;374:657–659. doi: 10.1038/374657a0. [DOI] [PubMed] [Google Scholar]
- 3.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1488. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dynlact B. Regulation of transcription by proteins that control the cell cycle. Nature. 1997;389:149–152. doi: 10.1038/38225. [DOI] [PubMed] [Google Scholar]
- 5.Goto H, Motomura S, Wilson A, Freiman R N, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T. A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 function. Gen Dev. 1997;11:726–737. doi: 10.1101/gad.11.6.726. [DOI] [PubMed] [Google Scholar]
- 6.Greenblatt J. RNA polymerase II holoenzyme and transcriptional regulation. Curr Opin Cell Biol. 1997;9:310–319. doi: 10.1016/s0955-0674(97)80002-6. [DOI] [PubMed] [Google Scholar]
- 7.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakobisiak M, Bruno S, Skierski J S, Darzynkiewicz Z. Cell cycle-specific effects of lovastatin. Proc Natl Acad Sci USA. 1991;88:3628–3631. doi: 10.1073/pnas.88.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 11.Keyomarsi K, Sandoval L, Band V, Pardee A B. Synchronization of tumor and normal cells from g1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- 12.Kingston R E, Bunker C A, Imbalzano A N. Repression and activation by multiprotein complexes that alter chromatin structure. Gen Dev. 1996;10:905–920. doi: 10.1101/gad.10.8.905. [DOI] [PubMed] [Google Scholar]
- 13.Kristie M T, Sharp P A. Interactions of the oct-1 POU subdomains with specific DNA sequences and with the HSV a-trans-activator protein. Gen Dev. 1990;4:2383–2396. doi: 10.1101/gad.4.12b.2383. [DOI] [PubMed] [Google Scholar]
- 14.Kristie T M, Sharp P A. Purification of the cellular c1 factor required for the stable recognition of the oct-1 homeodomain by the herpes simplex virus a-trans-induction factor (VP16) J Biol Chem. 1993;268:6525–6534. [PubMed] [Google Scholar]
- 15.LaMarco K L, McKnight S L. Purification of a set of cellular polypeptides that bind to the purine-rich cis-regulatory element of herpes simplex virus immediate early genes. Gen Dev. 1989;3:1372–1383. doi: 10.1101/gad.3.9.1372. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Green M R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991;64:971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Ha I, Maldonado E, Reinberg D, Green M R. Binding of general transcription factor TFIIB to an acidic activating region. Nature. 1991;353:569–571. doi: 10.1038/353569a0. [DOI] [PubMed] [Google Scholar]
- 18.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Biochemical and cellular effects of Roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Hare P, Goding C R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988;52:435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- 20.O’Reilly D, Hanscombe O, O’Hare P. A single serine residue at position 375 of VP16 is critical for complex assembly with Oct-1 and HCF and is a target of phosphorylation by casein kinase II. EMBO J. 1997;16:2420–2430. doi: 10.1093/emboj/16.9.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 22.Preston C M, Frame M C, Campbell M E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988;52:425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 23.Roberts S B, Segil N, Heintz N. Differential phosphorylation of the transcription factor Oct-1 during the cell cycle. Science. 1991;253:1022–1026. doi: 10.1126/science.1887216. [DOI] [PubMed] [Google Scholar]
- 24.Roberts S G E, Ha I, Maldonado E, Reinberg D, Green M R. Interaction between an acidic activation and transcription factor tfIIB is required for transcriptional activation. Nature. 1993;363:741–744. doi: 10.1038/363741a0. [DOI] [PubMed] [Google Scholar]
- 25.Schang L M, Phillips J, Schaffer P A. Requirement of cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schang L M, Rosenberg A, Schaffer P A. Transcription of herpes simplex virus immediate-early and early genes is inhibited by Roscovitine and inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999;73:2161–2172. doi: 10.1128/jvi.73.3.2161-2172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Schang, L. M., P. A. Schaffer, and R. Shiekhattar. Unpublished obserations.
- 27.Segil N, Roberts S B, Heintz N. Mitotic phosphorylation of the Oct-1 homeodomain and regulation of Oct-1 DNA binding activity. Science. 1991;254:1814–1816. doi: 10.1126/science.1684878. [DOI] [PubMed] [Google Scholar]
- 28.Shirvan A, Ziv I, Zilkha-Falb R, Machlyn T, Barzilai A, Melamed E. Expression of cell cycle-related genes during neuronal apoptosis: is there a distinct pattern. Neurochem Res. 1998;23:767–777. doi: 10.1023/a:1022415611545. [DOI] [PubMed] [Google Scholar]
- 29.Stargell L A, Struhl K. The TBP-TFIIA interaction in the response to acidic activators in vivo. Science. 1995;269:75–78. doi: 10.1126/science.7604282. [DOI] [PubMed] [Google Scholar]
- 30.Stern S, Tanaka M, Herr W. The Oct-1 homeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989;341:624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- 31.Stringer K F, Ingles C J, Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990;345:783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan S M, Horn P J, Olson V A, Koop A H, Miu W, Ebright R H, Triezenberg S. Mutational analysis of a transcriptional activation region of the VP16 protein of herpes simplex virus. Nucleic Acids Res. 1998;26:4487–4496. doi: 10.1093/nar/26.19.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Triezenberg S J, LaMarco K L, McKnight S L. Evidence of DNA: protein interactions that mediate HSV-1 immediate early gene activation by Vp16. Gen Dev. 1988;2:730–742. doi: 10.1101/gad.2.6.730. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox C L, Johnson E M. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol. 1987;61:2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilcox C L, Johnson E M. Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol. 1988;52:393–399. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson A C, Freiman R N, Goto H, Nishimoto T, Herr W. VP16 targets an amino-terminal domain of HCF involved in cell cycle progression. Mol Cell Biol. 1997;17:6139–6146. doi: 10.1128/mcb.17.10.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson A C, LaMarco K, Peterson M G, Herr W. The VP16 accessory protein HCF is a family of polypeptides processed from a large precursor protein. Cell. 1993;74:115–125. doi: 10.1016/0092-8674(93)90299-6. [DOI] [PubMed] [Google Scholar]