Abstract
[Purpose] Optimization of post-training muscle recovery is important in clinical rehabilitation and sports science. In this study, we investigated the effects of local vibration stimulation on post-training muscle recovery and hypertrophy in healthy adults, focusing on the upper extremities. [Participants and Methods] The study included 20 healthy students categorized into the control and vibration stimulation groups. Both groups underwent training, including elbow flexion. The vibration stimulation group received immediate post-training local vibration stimulation. Evaluation included measurement of upper arm circumference, muscle strength, muscle hardness, and ultrasonographic imaging. [Results] Our results showed that local vibration stimulation increased muscle luminosity but had no significant effect on muscle strength, hardness, or thickness. [Conclusion] Post-training vibration stimulation may promote muscle growth and recovery by stimulating blood flow and improving nutrient and oxygen supply to muscles.
Keywords: Vibration stimulation, Muscle recovery, Ultrasound imaging
INTRODUCTION
In modern rehabilitation and sports science, the recovery from muscle fatigue and an increase in muscle strength after training are important focal points. In muscle training, it has been shown that muscle protein synthesis increases after training along with increased damage and inflammation in structures within muscle cells, contributing to muscle hypertrophy through the recovery process after training-induced muscle injury1). In other words, optimizing post-training recovery may be critically important for maintaining and improving performance in various clinical and athletic settings. Post-training muscle damage is thought to be associated with decreased performance and an increased risk of injury, and effective management of this condition is essential for rehabilitation, health promotion, and improved performance in athletic competition. In particular, for the upper extremities, muscle fatigue affects the ability to perform movements, suggesting the need for recovery from muscle fatigue in performing upper extremity tasks in patients with rotator cuff injury2). Therefore, it is important to provide appropriate post-training muscle recovery and strengthening to the upper extremities.
Several studies on muscle recovery after training have been reported. A study on muscle recovery by post-training massage in amateur boxers reported that a 20-minute upper and lower extremity massage increased psychological recovery, but had no clear effect on physiological recovery or performance, such as punching power, and no clear effect on improvement3). In a study examining the effects of compression on muscle recovery in cricketers, wearing compression garments reduced post-exercise muscle soreness and creatine kinase levels the following day and promoted muscle recovery after exercise4). A study examining the effects of cooling on muscle recovery suggested that cold-water bathing of the lower extremities after endurance training promoted mitochondrial generation within the skeletal muscle5). Various exercise and physical therapy interventions have been investigated during the training recovery process. Many recent studies have also examined the effects of both whole-body and local vibration stimulation on muscles. In a study of whole-body vibration stimulation in healthy elderly participants, maximum knee extension muscle strength improved under normal oxygen concentration6). In addition, a study on local vibration stimulation after training in healthy adults compared local vibration stimulation of one arm and not the other in the same participant and found that vibration stimulation reduced delayed-onset muscle soreness7). Previous studies using local vibration stimulation post-training have evaluated muscle strength, delayed onset muscle soreness, and range of motion, but have not examined how it affects skeletal muscle morphology itself. Therefore, it is not clear whether post-training vibration stimulation promotes hypertrophy of the skeletal muscles themselves and aids in muscle recovery. To clarify this uncertainty, future research should directly investigate how local vibration stimulation affects skeletal muscle structure and intramuscular fat distribution. Detailed image analysis techniques could be used for this type of research to gain a better understanding of how vibration stimulation acts on the muscle regeneration process. It is also important to determine the optimal frequency, intensity, and duration of vibration stimulation during the muscle recovery process. This would allow for the development of more effective vibration stimulation protocols in the fields of sports science and rehabilitation medicine.
These findings indicate that vibration stimulation promotes muscle hypertrophy and muscle recovery after training; however, research on the effects of vibration stimulation is limited, and clarifying the effects of vibration stimulation is considered important for rehabilitation programs. Therefore, the effects of vibration stimulation require clarification when considering rehabilitation programs.
Recently, the validity of evaluations using ultrasound imaging devices has been investigated. Regarding ultrasound imaging findings after exercise, a relationship between decreased supraspinatus muscle thickness and muscle fatigue has been suggested after wheelchair running in women8). Evaluation using ultrasound imaging devices is considered clinically valid because it allows the examination of not only quantitative changes in skeletal muscle, but also qualitative changes using muscle luminance.
Therefore, this study was conducted to clarify the effects of vibration stimulation on the skeletal muscles of the upper limb on muscle strength and hypertrophy by comparing changes in muscle hardness and ultrasound imaging findings of skeletal muscles with and without local vibration stimulation after training performed on the upper limb, and to investigate the means to promote recovery after exercise in clinical rehabilitation.
PARTICIPANTS AND METHODS
The participants were 20 healthy students (10 males, 10 females), 21.1 ± 0.3 years , 165.4 ± 7.5 m, 58.2 ± 9.9 kg, and BMI 21.3 ± 0.3 kg/m2 (mean ± standard deviation). This study was approved by the Research Ethics Review Committee of the authors’ institution (Approval No. 2019007). This study was conducted in an ethical and privacy-conscious manner in accordance with the Declaration of Helsinki. Participants were fully informed of the purpose and methods of the study, and their consent to participate was obtained in advance.
The participants were divided into two groups, one with vibration stimulation immediately after exercise (“vibration stimulation group”) and the other without vibration stimulation (“control group”), each with 10 participants, with an equal ratio of male and female participants in each group. The evaluation consisted of upper arm circumference, muscle strength, and muscle hardness measurements, and observation of the subcutaneous morphology using ultrasound imaging before and after the training. The target of intervention and evaluation was the upper limb on the dominant hand side.
The training consisted of elbow joint flexion exercises under weight-bearing in the chair seating position. The posture was performed starting with the upper limb drooping and the forearm externally rotated, with the elbow joint fully flexed. The frequency of the training was based on the model adopted as the optimal strength training in previous studies9, 10), and was one set of 10 repetitions per day, with an interval of 48 hours between sets. The implementation period was one week, and subjects performed a total of three sets of training. The load was set at a 10-repetition maximum (hereafter referred to as “RM”), which was measured by performing elbow joint flexion exercises in 1 kg increments using weights.
Immediately after each training, the vibration stimulation group underwent vibration stimulation. Vibration stimulation was applied to the skeletal muscles at the center of the front upper arm at approximately 22 Hz for 5 min in the sitting position using a local vibration stimulator (Kinmaku mini drill gun, Global Japan, Inc., Tokyo, Japan). The control group was maintained in the resting position for 5 min.
A tape measure was used to measure upper arm circumference. The participant was instructed to relax in the supine position and measurements were taken at the maximal bulge of the upper arm.
A manual muscle testing device (Mutus, OG Wellness, Okayama, Japan) was used to measure isometric flexion muscle strength at the elbow joint. The measurement was taken with the upper limbs drooped, elbow joint flexed at 90°, and forearm in an external rotation position. A manual muscle strength measuring device fixed to a desk with a belt was placed on the distal forearm, and the flexion effort was continued for approximately 2 s until the maximum muscle strength was reached.
Muscle hardness was measured using a muscle (soft tissue) hardness tester (Neutone TDM-Z1, Try-All, Chiba, Japan). Measurements were taken at the maximal bulge of the upper arm with the participant in the supine and instructed to relax.
An ultrasound imaging device (FAMUBO-W, Seiko, Tokyo, Japan) was used for subcutaneous morphometric measurements. The participant was instructed to relax in the supine position, and images in the short-axis direction were captured at the maximal bulge of the upper arm. A sufficient amount of ultrasonic coupling agent was applied to the skin surface layer, and the ultrasonic probe was brought into contact with the skin without applying pressure, as described in a previous study11). The images were analyzed for muscle thickness and luminance using image analysis software (ImageJ, National Institutes of Health). For muscle thickness evaluation, the distance from the bone index to the fascia was measured perpendicular to the bone index of the elbow flexor muscle group on the anterior surface of the upper arm (Fig. 1). For muscle luminance, the largest possible rectangular area was specified for skeletal muscles, avoiding the fascia, using an 8-bit gray scale within the specified area, which was numerically represented by 256 shades (0–255) (Fig. 2). Higher values indicated higher muscle luminance, suggesting that connective tissues other than myofibers were present within the myoplasm11).
Fig. 1.
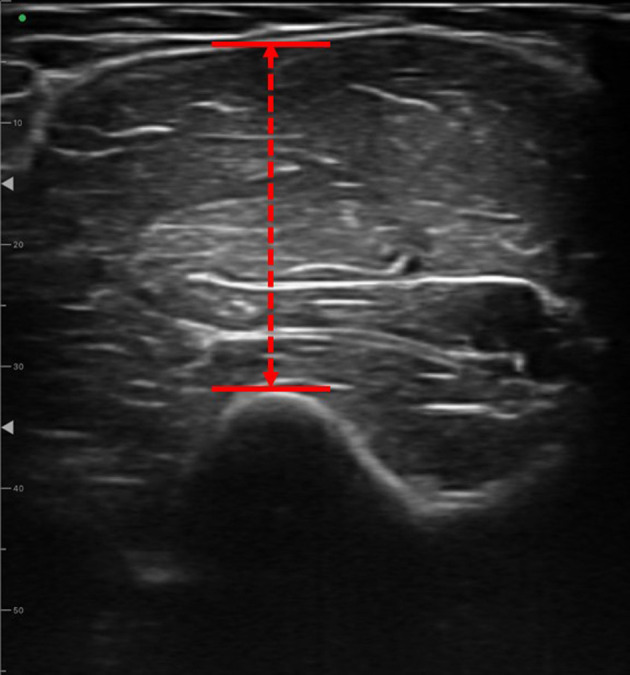
Measurement of muscle thickness.
Fig. 2.
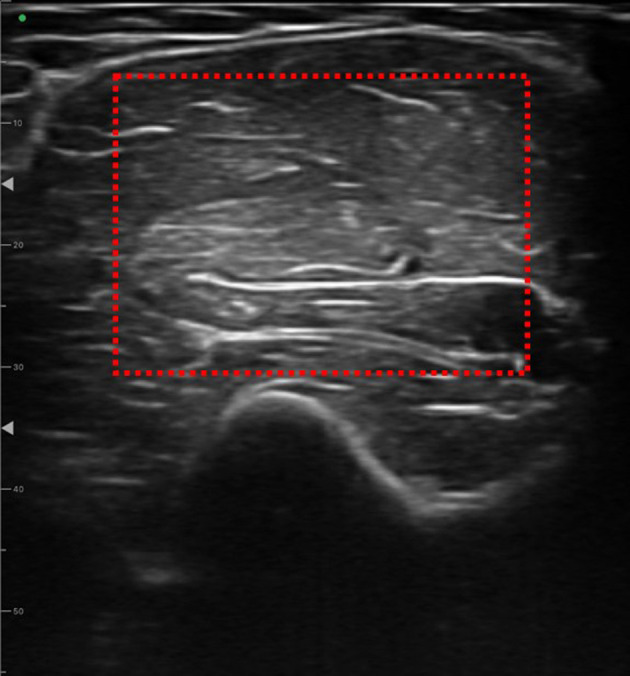
Measurement of muscle brightness.
Statistical analyses were performed using statistical analysis software (SPSS Statistics, IBM, Armonk, NY, USA). Data were checked for normality using the Shapiro–Wilk test to confirm that they did not follow a normal distribution. Wilcoxon’s signed-rank test was used to compare the upper arm circumference, muscle strength, muscle hardness, muscle thickness in the elbow flexor, tissue thickness including subcutaneous tissue, percentage of muscle thickness in the total tissue including subcutaneous tissue, and muscle luminosity before and after the intervention for the three sets of interventions. The significance level was set at 5%.
RESULTS
The basic attributes of the participants are presented in Table 1. Participants were 21.1 ± 0.3 years of age in both the vibration stimulation and control groups. The participants’ height was 165.8 ± 8.7 cm in the vibration stimulation group and 164.9 ± 6.6 cm in the control group, and body weight was 59.3 ± 10.8 kg in the vibration stimulation group and 57.1 ± 7.7 kg in the control group.
Table 1. Basic attributes of the participant.
| Age (years) | Height (cm) | Weight (kg) | |
| Vibration | 21.1 ± 0.3 | 164.9 ± 6.6 | 57.1 ± 7.7 |
| Control | 21.1 ± 0.3 | 165.8 ± 8.7 | 59.3 ± 10.8 |
Values are expressed as mean ± standard deviation.
Table 2 shows the results of the upper arm circumference measurements. The circumference of the maximal bulge of the upper arm was 25.9 ± 2.2 cm before and 26.0 ± 2.6 cm after the training in the vibration stimulation group, and 25.5 ± 2.9 cm before and 25.9 ± 3.1 cm after in the control group. In each group, there were no significant differences in the upper arm circumference before and after the intervention.
Table 2. Upper arm circumference (cm).
| Pre | Post | |
| Vibration | 25.9 ± 2.2 | 26.0 ± 2.6 |
| Control | 25.5 ± 2.9 | 25.9 ± 3.1 |
Values are expressed as mean ± standard deviation.
The results of the muscle strength measurements are presented in Table 3. The elbow isometric flexor muscle strength was 17.6 ± 7.2 kgf before and 18.9 ± 8.5 kgf after the intervention in the vibration stimulation group and 14.9 ± 3.9 kgf before and 16.2 ± 5.6 kgf after the intervention in the control group. In each group, there were no significant differences in the isometric elbow flexion muscles before and after the intervention.
Table 3. Elbow isometric flexion muscle strength (kgf).
| Pre | Post | |
| Vibration | 17.6 ± 7.2 | 18.9 ± 8.5 |
| Control | 14.9 ± 3.9 | 16.2 ± 5.6 |
Values are expressed as mean ± standard deviation.
The results of the muscle hardness measurements are presented in Table 4. Muscle hardness at the maximal bulge of the upper arm was 28.8 ± 5.1 before and 30.2 ± 5.9 after the intervention in the vibration stimulation group and 25.0 ± 6.2 before and 26.3 ± 6.8 after the intervention in the control group. In each group, muscle hardness was not significantly different before and after intervention.
Table 4. Muscle stiffness.
| Pre | Post | |
| Vibration | 28.8 ± 5.1 | 30.2 ± 5.9 |
| Control | 25.0 ± 6.2 | 26.3 ± 6.8 |
Values are expressed as mean ± standard deviation.
Table 5 shows the results of the morphometric measurements using ultrasound imaging. Muscle thickness of the elbow flexor muscle group on the anterior surface of the upper arm was 27.7 ± 6.3 mm before and 29.5 ± 6.4 mm after the intervention in the vibration stimulation group and 28.3 ± 5.0 mm before and 26.8 ± 4.4 mm after the intervention in the control group. Tissue thickness, including subcutaneous tissue, was 29.3 ± 7.8 mm before and 32.6 ± 5.3 mm after the training in the vibration stimulation group and 31.8 ± 4.4 mm before and 29.2 ± 4.5 mm after the training in the control group. Muscle luminance was 48.2 ± 10.7 before and 74.2 ± 13.1 after the training in the vibration stimulation group and 38.7 ± 25.5 before and 61.3 ± 24.4 after the intervention in the control group. In each group, there was no significant difference in tissue thickness, including muscle thickness and subcutaneous tissue, before and after the intervention; however, there was a statistically significant difference in muscle luminance in the vibration stimulation group before and after the intervention (p<0.05).
Table 5. Morphometric measurements in ultrasound images.
| Muscle thickness of elbow flexor muscle group (mm) | ||
| Pre | Post | |
| Vibration | 27.7 ± 6.3 | 29.5 ± 6.4 |
| Control | 28.3 ± 5.0 | 26.8 ± 4.4 |
| Subcutaneous tissue including muscle (mm) | ||
| Pre | Post | |
| Vibration | 29.3 ± 7.8 | 32.6 ± 5.3 |
| Control | 31.8 ± 4.4 | 29.2 ± 4.5 |
| Muscle brightness | ||
| Pre | Post | |
| Vibration | 48.2 ± 10.7 | 74.2 ± 13.1 |
| Control | 38.7 ± 25.5 | 61.3 ± 24.3 |
Values are expressed as mean ± standard deviation. *; Significant difference between pre and post (p<0.05).
DISCUSSION
This study examined the effects of exercise on muscles by applying post-exercise vibration stimulation to the upper extremities. In this study, we investigated the quantitative and qualitative changes in skeletal muscle morphology, which had not been examined in previous studies, using new ultrasound images, and found new insights into the effects of local vibration stimulation on skeletal muscle hypertrophy and muscle recovery by more directly evaluating the changes that occur in skeletal muscle. The results of this study were as follows. The results suggest that vibration stimulation may contribute to increased muscle brightness in the elbow flexor muscle group. However, muscle strength, hardness, or thickness did not significantly differ between the two groups. This did not indicate a specific effect of vibration stimulation on these measurements. These findings suggest that vibration stimulation affects only certain aspects of the muscles.
It is generally known that a combination of morphological and neural factors, such as muscle cross-sectional area and motor units, are important in increasing muscle strength. Many reports have shown that incorporating various training methods and performing multiple sets are effective for increasing muscle strength12). However, a literature review of the effects of local vibration stimulation on muscle strength reported that there are not enough studies to support the effects of vibration stimulation on muscle strength13). In addition, a previous study on muscle training and vibration stimulation showed that adding vibration stimulation during training did not change the outcome from that during normal training, indicating that vibration stimulation did not provide additional benefits14). In the present study, when an training was set with a baseline of 10 RM, and the effect of adding vibration stimulation after the training was examined, there was no difference in muscle strength between the vibration stimulation and control groups. In the present study, vibration stimulation was applied for recovery after training rather than during training, as reported in previous studies. However, vibration stimulation applied after training did not have the same effect on muscle strength as that applied during training. In the present study, no muscle strength gain was observed, even in the control group, suggesting that the amount of exercise load and duration of the exercise should be reexamined.
A literature review on the effects of vibration stimulation on flexibility reported that it is effective in improving muscle flexibility due to decreased motor nerve excitability, decreased pain sensation, increased blood flow, and muscle relaxation. In particular, combining stretching and vibration stimulation can have a larger effect15). Regarding the effect of vibration stimulation on muscle hardness after training, Pournot et al. investigated the hardness of the biceps brachii after exercise by performing four sets of barbell curls and reported that local vibration stimulation had no effect on muscle hardness16). In the present study, when muscle hardness was assessed after three days of training, rather than immediately after only one day of exercise, there was no difference in muscle hardness between the vibration stimulation and control groups. This indicates that, similar to previous studies, the vibration stimulation used in this study was not effective in the recovery effect on muscle hardness after exercise. This suggests that when vibration stimulation is used for post-training muscle hardness, it may be appropriate to include stretching, rather than vibration stimulation alone, to achieve muscle relaxation and decrease motor nerve excitability as well as for post-training recovery.
Regarding muscle morphology, muscle luminosity increased in the vibration-stimulated group compared with that in the control group, with a greater increase in muscle luminosity in the vibration-stimulated group after three days of training. An increase in muscle luminance reflects an increase in non-contractile tissues other than muscle fibers, such as connective tissue and fat within the muscle, and it is difficult to say whether the increase in muscle luminance itself has a positive effect on muscle condition17, 18). On the other hand, it has been suggested that temporary muscle damage occurs during the regeneration process after exercise loading, and that an increase in connective tissue during the repair process after injury is necessary for muscle recovery and regeneration19). In addition, a literature review that investigated the effects of vibration stimulation on muscle blood flow reported that vibration increases the blood volume in the muscle, arterial diameter, and other factors20). Muscle training has been reported to cause a temporary increase in muscle blood flow owing to sympathetic vasomotor regulation and an increase in muscle blood flow owing to intramuscular vasodilation caused by perfusion within the muscle owing to muscle contraction21, 22). Therefore, the reason why muscle brightness increased in the vibration stimulation group in the present study may be that the vibration stimulation of the muscle after training increased blood flow, which supplied nutrients and oxygen to the muscle, thereby promoting recovery from muscle damage, which may have served as the basis for muscle growth. Therefore, the application of vibration stimulation after training may efficiently promote muscle recovery and increase muscle size due to training.
One limitation of this study is that the vibration stimulation conditions and number of participants were limited. Although local vibration stimuli were used in this study, the duration and frequency of the stimuli were fixed, and the various conditions within the vibration stimuli were not compared. In addition, the participants were healthy adults, and the results were limited in scope. Future studies should compare these results under various vibration-stimulus conditions. In addition, by examining the effects in different age groups and health conditions, the scope of the applicable post-exercise vibration stimulation could be expanded. In particular, the effects of vibration stimulation should be examined in people with different clinical backgrounds such as elderly people with reduced mobility and those with specific diseases. In addition, longer follow-up periods should be considered to assess the long-term effects. These approaches provide a deeper understanding of the effects of vibration stimulation and have broad applications in a wide range of areas.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare no competing interests.
REFERENCES
- 1.Damas F, Libardi CA, Ugrinowitsch C: The development of skeletal muscle hypertrophy through resistance training: the role of muscle damage and muscle protein synthesis. Eur J Appl Physiol, 2018, 118: 485–500. [DOI] [PubMed] [Google Scholar]
- 2.Kang JI, Moon YJ, Choi H, et al. : The effect of exercise types for rotator cuff repair patients on activities of shoulder muscles and upper limb disability. J Phys Ther Sci, 2016, 28: 2772–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemmings B, Smith M, Graydon J, et al. : Effects of massage on physiological restoration, perceived recovery, and repeated sports performance. Br J Sports Med, 2000, 34: 109–114, discussion 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffield R, Portus M: Comparison of three types of full-body compression garments on throwing and repeat-sprint performance in cricket players. Br J Sports Med, 2007, 41: 409–414, discussion 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ihsan M, Markworth JF, Watson G, et al. : Regular postexercise cooling enhances mitochondrial biogenesis through AMPK and p38 MAPK in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol, 2015, 309: R286–R294. [DOI] [PubMed] [Google Scholar]
- 6.Camacho-Cardenosa M, Camacho-Cardenosa A, Brazo-Sayavera J, et al. : Evaluation of 18-week whole-body vibration training in normobaric hypoxia on lower extremity muscle strength in an elderly population. High Alt Med Biol, 2019, 20: 157–164. [DOI] [PubMed] [Google Scholar]
- 7.Lau WY, Nosaka K: Effect of vibration treatment on symptoms associated with eccentric exercise-induced muscle damage. Am J Phys Med Rehabil, 2011, 90: 648–657. [DOI] [PubMed] [Google Scholar]
- 8.Bossuyt FM, Boninger ML, Cools A, et al. SwiSCI study group: Changes in supraspinatus and biceps tendon thickness: influence of fatiguing propulsion in wheelchair users with spinal cord injury. Spinal Cord, 2020, 58: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fish DE, Krabak BJ, Johnson-Greene D, et al. : Optimal resistance training: comparison of DeLorme with Oxford techniques. Am J Phys Med Rehabil, 2003, 82: 903–909. [DOI] [PubMed] [Google Scholar]
- 10.Toselli S, Badicu G, Bragonzoni L, et al. : Comparison of the effect of different resistance training frequencies on phase angle and handgrip strength in obese women: a randomized controlled trial. Int J Environ Res Public Health, 2020, 17: 1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagae M, Umegaki H, Yoshiko A, et al. : Muscle ultrasound and its application to point-of-care ultrasonography: a narrative review. Ann Med, 2023, 55: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suchomel TJ, Nimphius S, Bellon CR, et al. : The importance of muscular strength: training considerations. Sports Med, 2018, 48: 765–785. [DOI] [PubMed] [Google Scholar]
- 13.Masud AA, Shen CL, Luk HY, et al. : Impact of local vibration training on neuromuscular activity, muscle cell, and muscle strength: a review. Crit Rev Biomed Eng, 2022, 50: 1–17. [DOI] [PubMed] [Google Scholar]
- 14.Drummond MD, Couto BP, Oliveira MP, et al. : Effects of local vibration on dynamic strength training. J Strength Cond Res, 2021, 35: 3028–3034. [DOI] [PubMed] [Google Scholar]
- 15.Osawa Y, Oguma Y: Effects of vibration on flexibility: a meta-analysis. J Musculoskelet Neuronal Interact, 2013, 13: 442–453. [PubMed] [Google Scholar]
- 16.Pournot H, Tindel J, Testa R, et al. : The acute effect of local vibration as a recovery modality from exercise-induced increased muscle stiffness. J Sports Sci Med, 2016, 15: 142–147. [PMC free article] [PubMed] [Google Scholar]
- 17.Naimo MA, Varanoske AN, Hughes JM, et al. : Skeletal muscle quality: a biomarker for assessing physical performance capabilities in young populations. Front Physiol, 2021, 12: 706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goryachev I, Tresansky AP, Ely GT, et al. : Comparison of quantitative ultrasound methods to classify dystrophic and obese models of skeletal muscle. Ultrasound Med Biol, 2022, 48: 1918–1932. [DOI] [PubMed] [Google Scholar]
- 19.Mizumura K, Taguchi T: Neurochemical mechanism of muscular pain: insight from the study on delayed onset muscle soreness. J Physiol Sci, 2024, 74: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller JT, Thomson RL, Howe PR, et al. : Effect of vibration on muscle perfusion: a systematic review. Clin Physiol Funct Imaging, 2013, 33: 1–10. [DOI] [PubMed] [Google Scholar]
- 21.Hamann JJ, Valic Z, Buckwalter JB, et al. : Muscle pump does not enhance blood flow in exercising skeletal muscle. J Appl Physiol, 2003, 94: 6–10. [DOI] [PubMed] [Google Scholar]
- 22.Katayama K, Ishida K, Saito M, et al. : Enhanced muscle pump during mild dynamic leg exercise inhibits sympathetic vasomotor outflow. Physiol Rep, 2014, 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]


