Abstract
Background
Many hospitals introduced procalcitonin (PCT) testing to help diagnose bacterial coinfection in individuals with COVID-19, and guide antibiotic decision-making during the COVID-19 pandemic in the UK.
Objectives
Evaluating cost-effectiveness of using PCT to guide antibiotic decisions in individuals hospitalized with COVID-19, as part of a wider research programme.
Methods
Retrospective individual-level data on patients hospitalized with COVID-19 were collected from 11 NHS acute hospital Trusts and Health Boards from England and Wales, which varied in their use of baseline PCT testing during the first COVID-19 pandemic wave. A matched analysis (part of a wider analysis reported elsewhere) created groups of patients whose PCT was/was not tested at baseline. A model was created with combined decision tree/Markov phases, parameterized with quality-of-life/unit cost estimates from the literature, and used to estimate costs and quality-adjusted life years (QALYs). Cost-effectiveness was judged at a £20 000/QALY threshold. Uncertainty was characterized using bootstrapping.
Results
People who had baseline PCT testing had shorter general ward/ICU stays and spent less time on antibiotics, though with overlap between the groups’ 95% CIs. Those with baseline PCT testing accrued more QALYs (8.76 versus 8.62) and lower costs (£9830 versus £10 700). The point estimate was baseline PCT testing being dominant over no baseline testing, though with uncertainty: the probability of cost-effectiveness was 0.579 with a 1 year horizon and 0.872 with a lifetime horizon.
Conclusions
Using PCT to guide antibiotic therapy in individuals hospitalized with COVID-19 is more likely to be cost-effective than not, albeit with uncertainty.
Introduction
The COVID-19 pandemic has been a global health crisis, with millions of cases and fatalities worldwide. One of the critical issues that has emerged during the pandemic is the inappropriate use of antibiotics in the management of individuals with COVID-19, particularly in those hospitalized.1 Determining whether COVID-19 patients have a bacterial coinfection and who therefore may benefit from antibiotics is challenging, particularly because many of the frequently used biomarkers of infection, such as C-reactive protein (CRP), are often elevated in individuals with COVID-19.2 Inappropriate and excessive use of antibiotics can contribute to antimicrobial resistance (AMR), which can cause infections that are difficult or impossible to treat, and therefore interventions to support appropriate antibiotic prescribing decisions are needed.
Procalcitonin (PCT) is an inflammatory biomarker, measured in the blood, that rises when bacterial infection is present and falls in response to effective antimicrobial treatment. A Cochrane meta-analysis has demonstrated that PCT can guide antibiotic therapy in non-COVID-19 acute respiratory infections with reduced antibiotic exposure and improved survival.3 During the first wave of the COVID-19 pandemic in the UK, many hospitals introduced PCT testing to help diagnose bacterial coinfection in individuals with COVID-19 and guide antibiotic decision-making.4 This was at odds with US and UK national guidelines on the management of community-acquired pneumonia, which recommended against the use of PCT to guide antibiotic prescribing.5,6
The Procalcitonin Evaluation of Antibiotic use in COVID-19 Hospitalised patients (PEACH) study evaluated whether the use of PCT testing to guide antibiotic prescribing safely reduced antibiotic use among patients admitted to acute UK NHS hospitals with COVID-19.7 The study consisted of organization-level and individual patient-level analyses, both investigating the utility of PCT for guiding antibiotic prescribing. An initial survey of 148 (of 151; 98%) acute hospitals in England and Wales demonstrated increased use of PCT testing in emergency and acute admissions, which preceded development of the NICE guidance. The survey ascertained whether PCT testing was adopted during the pandemic and, if so, in which areas of the hospital, and factors relating to the test use and interpretation (e.g. cut-offs, testing algorithm).4 A retrospective analysis of organization-level data over time found that the introduction of PCT testing in emergency departments or acute medical admission units was associated with an initial, but non-sustained, reduction in total antibiotic use.8
The aim of this paper was to explore the cost-effectiveness of using PCT testing to guide antibiotic decisions in individuals hospitalized with COVID-19 based on a matched analysis of individual-level data collected from 11 UK NHS Trusts and Health Boards.
Materials and methods
All analyses were performed in R version 4.3.1.
Data
Retrospective individual-level data on patients hospitalized with COVID-19 were collected from 11 NHS acute hospital Trusts and Health Boards from England and Wales, some of which used PCT testing routinely in COVID-19 patients during the first wave of the pandemic and some of which did not. In line with the study protocol,7 patient characteristics such as age, gender, ethnicity and comorbidities were recorded, along with hospital admission and discharge dates, ICU admission and discharge dates, and survival time. Information was gathered on whether PCT testing and other diagnostics were performed, as well as antibiotic and antiviral administration. Data for all patients 16 years old or over who were admitted to hospital between 1 February 2020 and 30 June 2020 and who had a confirmed positive PCR COVID-19 test during this period were eligible for the study.5
Total length of stay was calculated as the days between either the date of positive COVID-19 test or the date of hospital admission, whichever was later, and hospital discharge date. Length of ICU stay was calculated as the days between either a positive COVID-19 test result or ICU admission date, whichever was later, and ICU discharge date. General ward length of stay was found by subtracting ICU length of stay from total length of stay. After propensity score matching (PSM), participants with missing total or ICU length of stay were excluded. In addition, observations where the ICU length of stay was greater than the total length of stay or where either was greater than survival time were assumed to be erroneous and discarded.
Treatment was defined as having PCT tested at baseline, defined as the day of the first positive sample for COVID-19 (±1 day). Balance of important confounders between treatment and control groups was achieved using PSM. Full details of this procedure are available in our companion paper.9 Patients were matched on age, sex, ethnicity, number of comorbidities, smoking status, index of multiple deprivation decile, quick SOFA (qSOFA), national early warning score 2 (NEWS2), confusion, uraemia, respiratory rate, blood pressure, age >65 score (CURB-65), 4C mortality score for COVID-19, early secondary bacterial infection, admission to ICU at baseline, baseline lung imaging category, the logarithms of baseline CRP level, neutrophil count, white cell count, D-dimer and troponin and indicator variables denoting missing blood test data.
Model structure
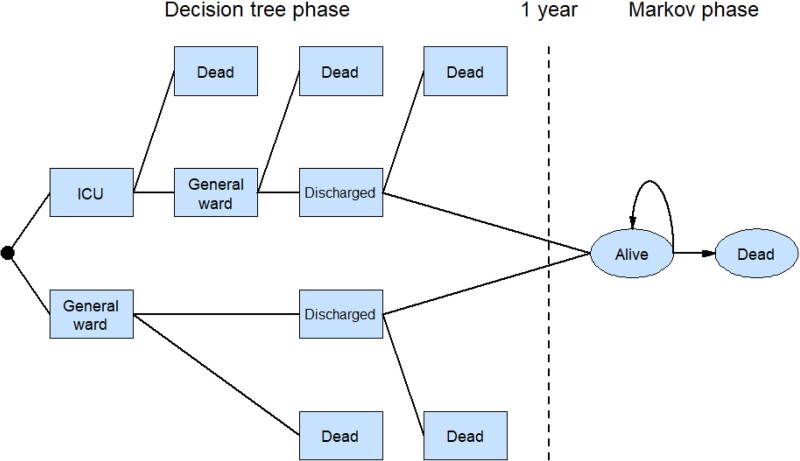
The model structure is shown in Figure 1. As with several previous studies of COVID-19-related interventions,10–12 the model had two phases. The first was a decision tree representing the acute phase following hospitalization with COVID-19. Patients’ PCT levels were either initially tested or not. Hospitalization could either be in a general ward or ICU, or a mixture of both over the course of a patient’s stay. Patients either died in hospital or were discharged. The decision tree phase had a time horizon of 1 year. Patients still alive at 1 year then entered a Markov phase with a lifetime horizon. This had two states: alive and dead, with the latter being an absorbing state. Results are reported separately for the decision tree phase alone and both decision tree and Markov phase combined.
Figure 1.
Economic evaluation model. Patients who did and did not receive a PCT test followed the same pathway. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Utilities
Quality-of-life data were not collected from patients, so previously published values were used to represent their utility. A review of the literature13 revealed a paucity of relevant data on quality of life for people hospitalized with COVID-19. Baseline utilities were calculated using the age/sex-specific UK population norms used by McNamara et al.,14 and in line with previous studies15 each day in a general ward/ICU was assigned a utility decrement. The disutility for general ward was taken from Wilcox et al.16 and that for ICU was taken from Hollman et al.17 Each day of antibiotic treatment was also assigned a decrement, taken from Oppong et al.,18 representing the potential for complications. Utility decrements are summarized in Table 1.
Table 1.
Utility decrements and costs by component of COVID pneumonia admission pathway
| Utility decrement | Cost (£) | |||
|---|---|---|---|---|
| Value | Source | Value | Source | |
| General ward | −0.36 | Wilcox et al.16 | £487.50 per day | Metry et al.19 |
| ICU | −0.58 | Hollmann et al.17 | £2386 per day | NHS Reference Costs20 |
| PCT | £15.20 per test | NICE21 | ||
| Antibiotics | −0.05 | Oppong et al.18 | Varies, see methods | eMIT;22 BNF23 |
| AMR | £2.12 per prescription | Oppong et al.24 | ||
Costs
The daily cost of a general ward stay was taken from NICE guidance on economic evaluation for COVID-19 therapeutics.19 The latest figures were for 2019/20, so an inflation uplift of 2.5% was applied.25 The daily cost of an ICU stay was obtained from NHS reference costs for clinical care for 2020/21.20
The average unit price for PCT testing has previously been estimated by NICE, based on list prices of the tests and no discounts assumed. This estimate incorporates overhead costs, including capital, service and maintenance, and calibration costs.21 As the cost estimate was for 2015/16, an inflation uplift of 10.4% was applied.20
To calculate the cost of antibiotics in our study, data on the name, dose and frequency of dose for antibiotics were collected. These data were interpreted with the assistance of a clinician. Data on names of prescribed antibiotics were provided in our dataset in two ways: as coded types of antibiotics for common types of antibiotic; and as free text. Inspection of the data showed that in many cases, the free text contained various different spellings of, shorthand versions of, and typographical errors in, names of both coded antibiotics and non-coded but common antibiotic types. It was furthermore established that some instances of free text referred to prescribed medication that was not an antibiotic.
As a result of this, regular expressions were used, where possible, to correct data contained in free text to standardized names of antibiotics. This reduced the number of unique values in the free text from 195 to 57. After removal of 19 non-antibiotics from this list, and merging with 49 coded antibiotic names, 63 unique valid antibiotics remained.
Information on dose was also provided as free text. Regular expressions were again used to ensure that, as far as possible, numeric and dose measures were consistently coded—for instance, reformatting ‘500 millilitres’ as ‘500 mL’.
Data on antibiotic name and dose were used to match observations in our dataset to publicly available data sources. As per NICE guidelines, medication was preferentially matched to a cost provided in the drugs and pharmaceutical electronic market information tool (eMIT)22 and, where this was not possible, to NHS indicative prices provided by NICE BNF records.23 An iterative process was followed to merge antibiotic records to these two datasets in order to correct idiosyncratic errors, and allow for instances where the prescribed dose was apparently unavailable but was present as a multiple of a recorded available dose in this costing data. This allowed the costing of 97.3% of antibiotic records in our data.
Excessive and inappropriate antibiotic administration raises the risk of AMR, with implication for future health expenditures. A per-dose cost representing the cost of AMR was estimated based on the method reported by Oppong et al.24
Transition probabilities
Transition probabilities for the decision-tree phase were estimated from patient data. For the Markov phase, age/sex-specific transition probabilities were taken from Office for National Statistics national life tables.26
Discounting
Utilities in the Markov phase were discounted at an annual rate of 3%, in line with NICE guidelines.27
Bootstrapping
Point estimates of quality-adjusted life years (QALYs), costs and incremental cost-effectiveness ratios (ICERs), including and excluding the Markov phase, were calculated using the whole dataset. Bootstrapping with 100 000 iterations was then used to generate 95% CIs. The bootstrapping results were also used to construct cost-effectiveness acceptability curves (CEACs) by estimating the probability of baseline PCT being cost-effective at cost-per-QALY thresholds between £0 and £50 000.
Robustness tests
Initial inspection of the data revealed that a number of individuals in the ‘no PCT’ group spent an entire year in the general ward, whereas the longest general ward stay in the PCT group was 158 days. As a robustness test, the bootstrapping analysis was repeated after removing those outliers.
Results
Data from 6173 individuals with a positive COVID-19 test were collected. After quality control (e.g. removal of individuals with a COVID-19 test date outside of the study timeline, removal of those with inconsistent hospital admission/discharge dates), 6089 remained. Data from 5960 of 6089 (97.9%) people were used for the propensity-score-matched primary analysis, of whom 1548 (26.0%) had PCT tested at baseline and 4412 (74.0%) did not. This quality control process and matched analyses were conducted by the statistics team (D.G., P.P., R.W.) and are reported in more detail elsewhere.9
Due to missing data or inconsistencies in variables key to the health economic analysis, some further exclusions were necessary. Of those included in the primary matched analysis (n = 5960), 47 observations had missing ICU length of stay, 78 had missing total length of stay, and 38 were missing survival time. In addition, there were five cases in which ICU length of stay was longer than total length of stay, and 38 where total length of stay was greater than survival time. After these exclusions, there were 5771 people included in the analysis, of whom 1509 (26.1%) had PCT tested at baseline and 4262 (73.9%) did not. Table 2 summarizes the participants’ characteristics before and after these exclusions.
Table 2.
Participant characteristics, weighted using propensity score-matching weights
| All participants in original matched analysis (n = 5960) | Participants included in health economic analysis (n = 5771) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline PCT | No baseline PCT | Baseline PCT | No baseline PCT | ||||||
| Mean (SE) | Median (IQR) | Mean (SE) | Median (IQR) | Mean (SE) | Median (IQR) | Mean (SE) | Median (IQR) | ||
| Age (years) | 70.0 (0.425) | 73.0 (25.0) | 72.4 (0.249) | 76.0 (22.0) | 70.0 (0.429) | 72.5 (25.0) | 70.1 (0.597) | 71.5 (25.0) | |
| Sex, % | Male | 56.4 (1.26) | 55.3%(0.749) | 56.4 (1.28) | 58.6 (1.77) | ||||
| Ethnicity, % | White | 79.3 (1.03) | 76.6 (0.637) | 79.1 (1.05) | 80.6 (1.36) | ||||
| Black | 4.13 (0.506) | 1.95 (0.208) | 4.24 (0.519) | 3.77 (0.777) | |||||
| Asian | 6.78 (0.639) | 3.08 (0.260) | 6.83 (0.649) | 5.65% (0.83) | |||||
| Mixed | 0.711 (0.214) | 0.793 (0.134) | 0.663 (0.209) | 1.03 (0.421) | |||||
| Other | 3.36 (0.458) | 4.28 (0.305) | 3.38 (0.465) | 3.58 (0.552) | |||||
| IMD decile | 4.13 (0.0734) | 4.00 (5.00) | 4.50 (0.0452) | 4.00 (5.00) | 4.14 (0.0745) | 3.50 (5.00) | 4.14 (0.107) | 2.50 (6.00) | |
| Number of comorbidities | 1.99 (0.0491) | 2.00 (3.00) | 2.65 (0.0297) | 2.00 (3.00) | 1.99 (0.0493) | 1.50 (3.00) | 2.06 (0.0546) | 1.50 (2.00) | |
| 4C mortality score for COVID-19 | 9.73 (0.0967) | 10.0 (5.00) | 9.80 (0.0576) | 10.0 (4.00) | 9.73 (0.0981) | 9.50 (5.00) | 9.85 (0.137) | 9.50 (6.00) | |
| N | 5960 | 5771 | |||||||
Means for included participants weighted using propensity score-matching weights. SE, standard error.
Table 3 gives decision-tree transition probabilities. The probability of transitioning from hospitalized to discharged was a little over two-thirds, with a 0.008 lower probability for people administered PCT at baseline. Conditional on being discharged, there was a probability of around 0.6 of surviving to 1 year and entering the Markov phase. This probability was 0.1 higher for people administered PCT at baseline.
Table 3.
Decision-tree phase transition probabilities for patients who had a PCT performed at baseline and those who did not
| Transitions | Probabilities | ||
|---|---|---|---|
| From state | To state | PCT at baseline | No PCT at baseline |
| Hospitalized | Dead | 0.309 | 0.301 |
| Discharged | 0.691 | 0.699 | |
| Discharged | Dead | 0.385 | 0.395 |
| Markov phase | 0.615 | 0.605 | |
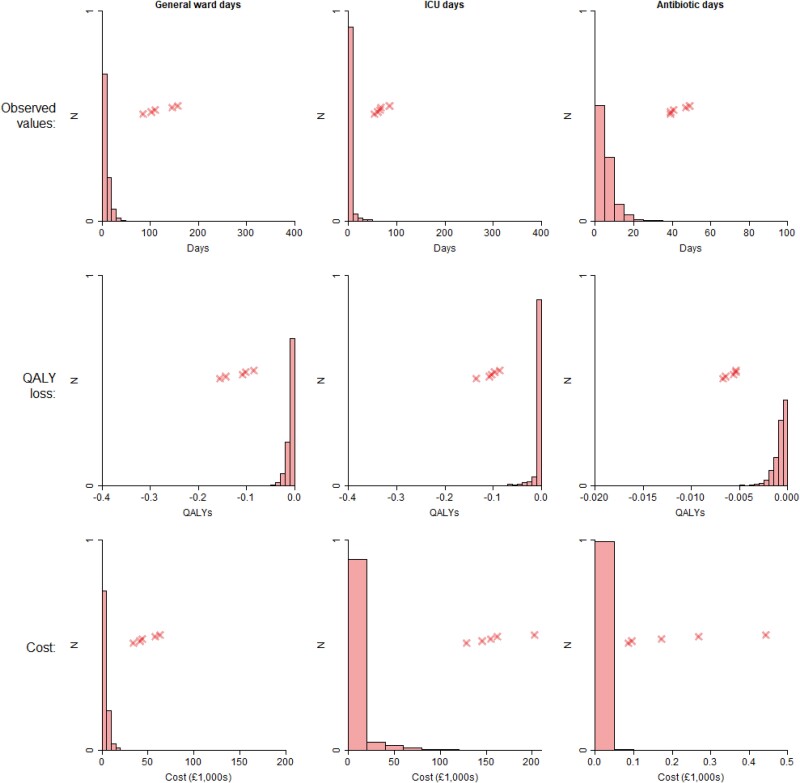
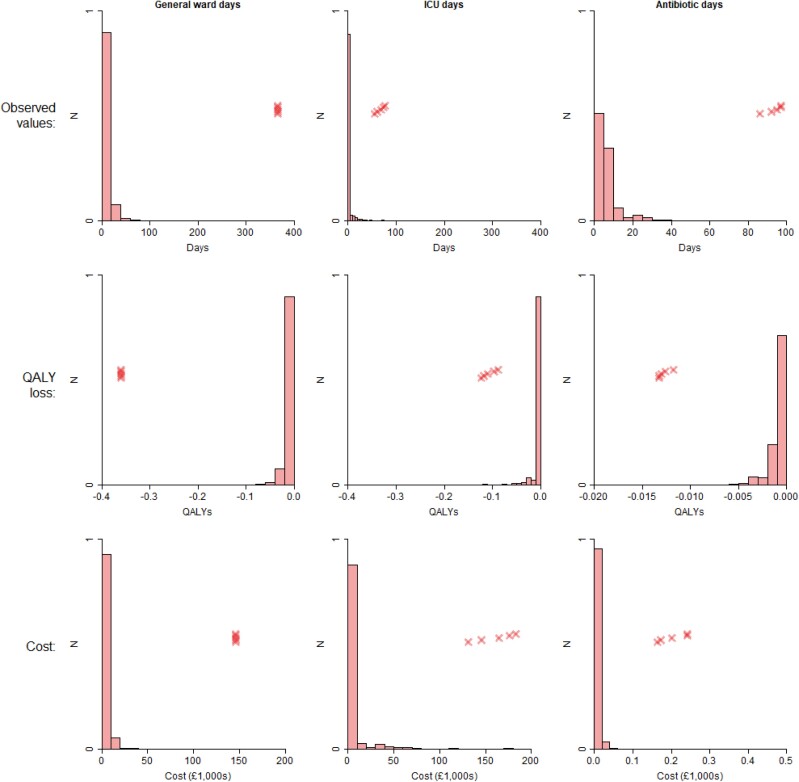
Table 4 shows the average days spent in a general ward and in ICU, days on antibiotics, and number of PCT tests, along with associated QALY losses and costs. People who had a PCT test at baseline had shorter general ward stays, as well as shorter ICU stays, and spent less time on antibiotics, though note the considerable overlap in 95% CIs in each case. The ‘baseline PCT’ group received 1.5 more PCT tests on average compared with the ‘no PCT’ group. The biggest QALY losses were associated with general ward days, and the greatest costs were associated with general ward and ICU days, with these being orders of magnitude greater than the impacts of PCT testing and antibiotic treatments. For example, in the ‘baseline PCT’ group, the QALY loss associated with being in a general ward was −9.15 × 10−3, which is 11 times greater than the loss of −8.45 × 10−4 associated with being on antibiotics. Similarly, in the ‘no baseline PCT’ group, the average cost of their ICU stay was £6400, which is 604 times greater than the £10.60 cost for PCT testing. Figures 2 and 3 show the distribution of general ward/ICU length of stay, and of antibiotic days, along with the associated QALY losses and costs. There are long tails in general ward and ICU distributions, with the extreme outliers being more prevalent in the ‘no baseline PCT’ group than in the ‘baseline PCT’ group.
Table 4.
Mean length of stay, PCT tests and antibiotics for patients who had a PCT performed at baseline and those who did not
| Baseline PCT | No baseline PCT | Baseline PCT | No baseline PCT | Baseline PCT | No baseline PCT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean QALY loss | 95% CI | Mean QALY loss | 95% CI | Mean cost | 95% CI | Mean cost | 95% CI | |
| General ward days | 9.28 | (8.85–9.69) | 10.7 | (9.81–11.5) | −0.00915 | (−0.00955 to −0.00872) | −0.0105 | (−0.0114 to −0.00967) | £3710 | (£3540–£3870) | £4270 | (£3920–£4610) |
| ICU days | 2.55 | (2.24–2.85) | 2.68 | (1.96–3.28) | −0.00404 | (−0.00452 to −0.00355) | −0.00426 | (−0.00521 to −0.00311) | £6070 | (£5330–£6790) | £6400 | (£4670–£7820) |
| PCT tests | 2.24 | (2.10–2.37) | 0.694 | (0.495–0.859) | £34.10 | (£32.00–£36.10) | £10.60 | (£7.53–£13.10) | ||||
| Antibiotic days | 5.94 | (5.71–6.17) | 6.78 | (6.37–7.16) | −0.000814 | (−0.000845 to −0.000781) | −0.000927 | (−0.000980 to −0.000873) | £5.56 | (£4.90–£6.14) | £4.85 | (£4.44–£5.26) |
| AMR | £5.61 | (£5.41–£5.81) | £6.14 | (£5.83–£6.44) | ||||||||
Figure 2.
Weighted histograms of ward/ICU stays and antibiotic days for patients receiving a PCT test at baseline, and associated QALY losses and costs. Crosses indicate the five highest values. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Weighted histograms of ward/ICU stays and antibiotic days for patients not receiving a PCT test at baseline, and associated QALY losses and costs. Crosses indicate the five highest values. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
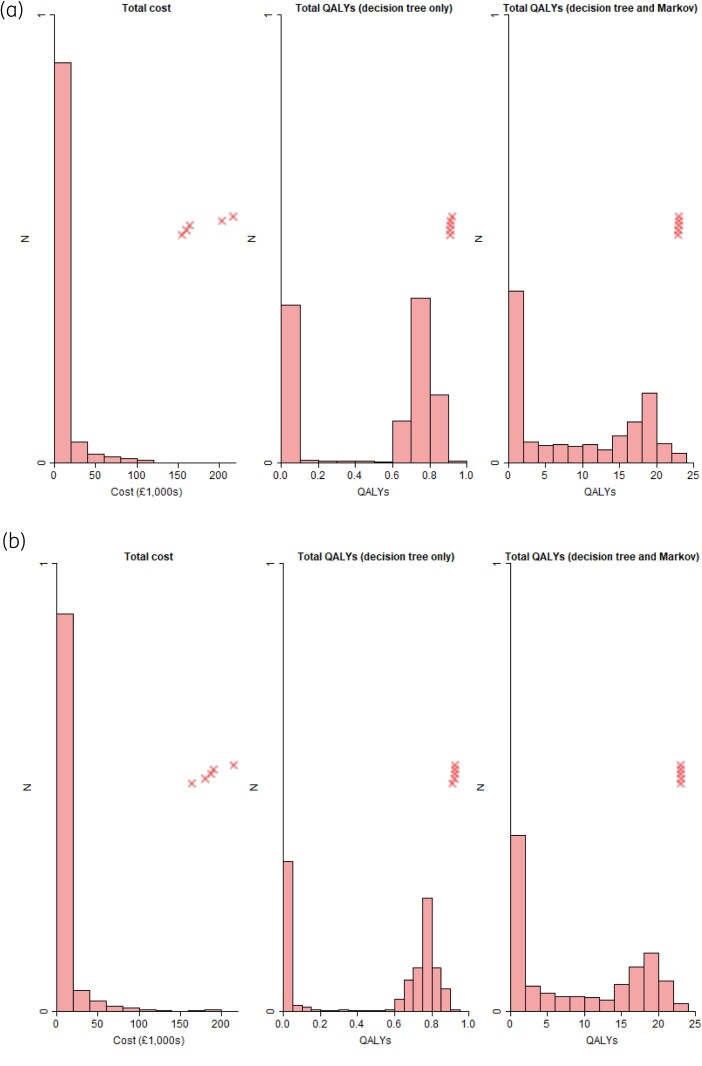
In Table 5, the average survival time (capped at 365.25 days) was 2 days higher for people who had PCT testing at baseline, at 234 days, though there was considerable overlap in 95% CIs. The baseline utility of each group was similar (0.767 versus 0.769), but people who had PCT testing at baseline accrued more QALYs, both when considering the decision-tree phase alone (0.486 versus 0.479) and when also including the Markov phase (8.76 versus 8.62). The total cost was also lower when people had a PCT test performed at baseline (£9830 versus £10 700). Figure 4 shows the distributions of total costs and QALYs, both including and excluding the Markov phase. For total costs, there was a long tail, with 90% of patients’ costs less than £15 000, yet at the same time, over 50 patients had costs in excess of £100 000, and the very highest costs were over £200 000. The distributions of QALYs were bimodal, with peaks for the decision tree alone occurring between 0 and 0.1 QALYs, and between 0.7 and 0.8 QALYs. When including the Markov phase as well, peaks were observed between 0 and 2 QALYs, and between 18 and 20 QALYs.
Table 5.
Survival time, total QALYs and costs for patients who had a PCT performed at baseline and those who did not
| Baseline PCT | No baseline PCT | |||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Survival time (days) | 234 | (227–241) | 232 | (222–241) |
| Probability of 1 year survival | 0.615 | (0.596–0.634) | 0.605 | (0.578–0.630) |
| Baseline utility | 0.767 | (0.765–0.769) | 0.769 | (0.766–0.772) |
| Total QALYs (decision-tree phase only) | 0.486 | (0.472–0.501) | 0.479 | (0.460–0.498) |
| Total QALYs (decision-tree and Markov phases) | 8.76 | (8.44–9.09) | 8.62 | (8.15–9.08) |
| Total cost (£) | 9830 | (9040–10 600) | 10 700 | (8830–12 300) |
| ICER (decision-tree phase only) | −117 000 | (−1 300 000 to 1 180 000) | ||
| ICER (decision-tree and Markov phases) | −5930 | (−58 300 to 55 300) | ||
Figure 4.
Weighted histograms of total cost and QALYs. Crosses indicate the five highest values. (a) Participants with PCT tested at baseline. (b) Participants without PCT tested at baseline. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
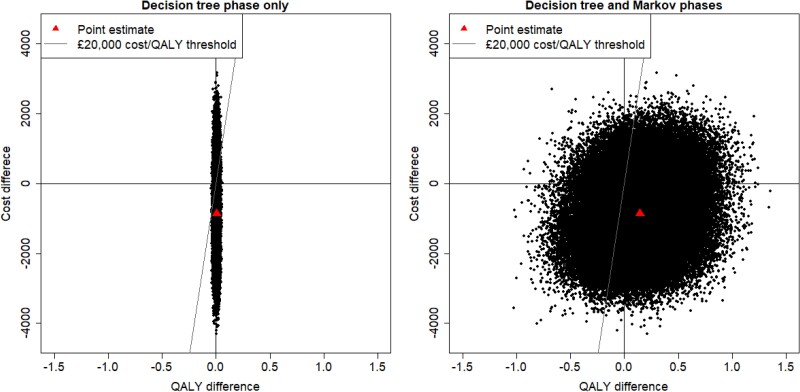
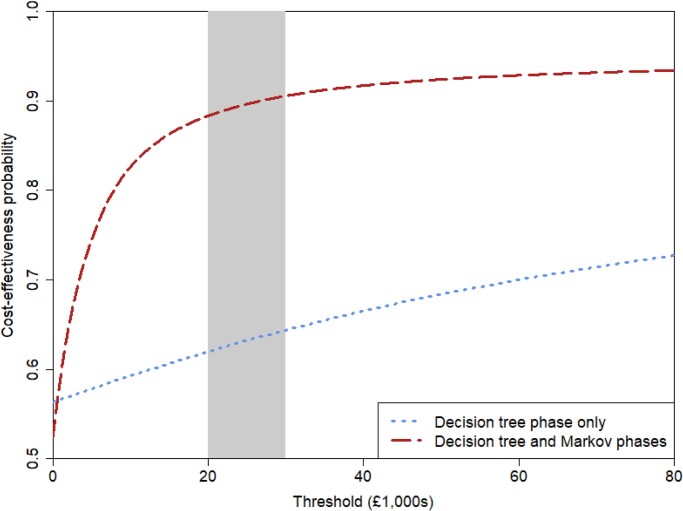
As baseline PCT testing resulted in more QALYs and lower costs, the point estimate of our analysis implies that baseline PCT testing is a dominant strategy against no baseline PCT testing. However, Figure 5 puts the point estimate in context by illustrating the results of the bootstrap analysis, demonstrating that there is a large amount of uncertainty around that conclusion. Figure 6 shows the CEACs, and with a cost-per-QALY threshold of £20 000 the probability of cost-effectiveness is 0.579 when considering only the decision-tree phase, which rises to 0.872 when considering both decision-tree and Markov phases.
Figure 5.
Cost-effectiveness planes. The x-axis and y-axis show, respectively, the QALY and cost differences between patients given and not given PCT tests at baseline with a 1 year (left) and lifetime (right) horizon. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 6.
Cost-effectiveness acceptability curve. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
There were 13 patients who spent the entire year of the decision-tree phase in a general ward in the ‘no baseline PCT’ group. After repeating the analysis with these outliers removed, the probability of baseline PCT testing being cost-effective rose to 0.753 when considering a 1 year horizon, and to 0.862 when considering a lifetime horizon. Full results are provided in Tables S1 and S2 and Figures S1 and S2 (available as Supplementary data at JAC Online).
Discussion
The results of this cost-effectiveness analysis demonstrate that using PCT to guide antibiotic decisions in individuals hospitalized with COVID-19 is more likely to be cost-effective than not, based on a cost-per-QALY threshold of £20 000, with a considerable amount of uncertainty in this conclusion, however. Baseline PCT testing resulted in more QALYs and lower costs overall both in the short (1 year post-admission) and long-term (lifetime analysis). However, a key driver of uncertainty in this analysis is the estimated QALYs; as an analysis largely based on matched retrospective observational data, we did not have quality-of-life data directly available and had to rely on estimates from the literature for this crucial component of the model.
There have been some published studies that have looked at the impact of using PCT to guide antibiotic therapy in patients hospitalized with COVID-19, all consistently concluding that PCT is a safe and simple way to reduce antibiotic use in patients hospitalized with COVID-19.28–31 On the other hand, the usefulness of PCT has been questioned when applied to patients treated with contemporary state-of-the-art immunomodulators.32 To our knowledge, this is the first study to examine whether the use of PCT to guide antibiotic prescribing in patients hospitalized with COVID-19 is a cost-effective strategy. Van der Pol et al.33 conducted a systematic review of economic analyses of diagnostics for respiratory tract infections and found three studies that evaluated the cost-effectiveness of PCT testing in a hospital setting (two studies focused on hospital in general and one on intensive care).33 All three of these studies concluded that PCT was likely to be cost-effective, but none of the studies were from a UK perspective.34–36 There are currently two randomized controlled trials in the UK in adults evaluating the use of PCT in individuals with sepsis: ADAPT-Sepsis in hospitalized adults (nearing completion); and PRONTO in adults with suspected sepsis presenting to the Emergency Department (recruitment completed).37,38 Both of these studies include planned health economics analysis, and it will be useful to compare their results with the findings from the current study.
Comparing the findings reported here with the companion paper of Sandoe et al.,9 they are consistent, as should be expected given that they both use the same data. Their main result was significantly fewer antibiotic days for those who had a PCT test at baseline compared with those who did not, which is also shown here. Sandoe et al. do not report statistically significant differences in length of stay and mortality (at 30 and 60 days) whereas here differences in those variables are key drivers in the central estimate that baseline PCT testing is cost-effective. The results are not contradictory, and in both analyses, there are similar differences in the point estimates of the variables, but we do not test for statistical significance between those variables, as such tests are not relevant for our analytical approach. It is also possible for small and non-significant differences in a variables such as ICU length of stay to lead to greater differences in costs and QALYs due to the associated large unit costs and disutilities.
A key strength of our study is that it is based on ‘end-to-end’ individual-level data for a large, multiregional cohort of patients rather than having to rely heavily on a linked evidence approach, which is common for the economic evaluation of diagnostic tests. This limited the number of modelling assumptions that needed to be made to build the model, and means that the evidence on which we have based the model is reflective of real-world clinical practice. As an observational study, however, there is a risk that some unknown confounding factors may have influenced effectiveness estimates, which could not be adjusted for in the matched analysis. A sensitivity analysis conducted as part of the main statistical analysis indicated that this possibility of residual confounding cannot be ruled out.9 The retrospective nature of the data collection also meant that we did not have individual-level quality-of-life data available, and we had to rely on generic utility decrements associated with being in general or ICU wards, and being on antibiotics, leading to high uncertainty in the incremental QALYs. This study was conducted in England and Wales, and judged cost-effectiveness according to the relevant Health Technology Assessment body for those countries, i.e. NICE. Thus its findings will not necessarily translate to other contexts where not only may the patient population have different characteristics, but where different standards may be used for assessing cost-effectiveness.
This economic evaluation, based on a large cohort of retrospective matched observational individual-level data from 11 NHS Trusts and Health Boards in England and Wales, provides real-world evidence that using PCT to guide antibiotic therapy in patients hospitalized with COVID-19 is more likely to be cost-effective than not, albeit with considerable uncertainty.
Future work could usefully look at other respiratory diseases. Also, given that the mean ICU stay was relatively short, it could be that baseline PCT testing is more cost-effective in diseases with longer average ICU stays, and future research could address this. This study focused specifically on PCT testing at baseline, i.e. at the time when a positive COVID-19 result was returned. This means that many people in the control group had a PCT test at some point, and may well have ceased/not initiated antibiotic treatment on the basis of the result. The reason for choosing baseline testing as the treatment/control criteria is that baseline PCT testing represents a clear, implementable protocol for hospitals. Future work could usefully explore the (cost-) effectiveness of PCT testing later in the treatment pathway.
Supplementary Material
Acknowledgements
Members of The PEACH Study Group
Co-chief investigators: Jonathan Sandoe1,2, Enitan Carrol3 (1Department of Microbiology, The General Infirmary at Leeds, Leeds, UK; 2Healthcare Associated Infection Group, Leeds Institute of Medical Research, University of Leeds, Leeds, UK; 3Department of Clinical Infection, Microbiology and Immunology, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, UK). Coordinating Centre: Study lead, Emma Thomas-Jones1; Study manager, Joanne Euden1; Qualitative researchers, Lucy Brookes-Howell1, Josie Henley2; Data manager, Wakunyambo Maboshe1; Co-lead statistician, Philip Pallmann1; Statistician, Detelina Grozeva1; Database support, Marcin Bargiel1; Study administrator, Judith Evans1 (1Centre for Trials Research, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK; 2School of Social Sciences, Cardiff University, King Edward VII Avenue, Cardiff CF10 3WA). Research Team: Health economics, Edward Webb1, Rebecca Bestwick1, Daniel Howdon1, Natalie King1, Bethany Shinkins (lead)1,2; Co-lead statistician, Robert West1 (1Leeds Institute for Health Sciences, University of Leeds, UK; 2Division of Health Sciences, University of Warwick, Coventry, UK). Study Partners: RX Info, Colin Richman1; UK Health Security Agency (UKHSA), Sarah Gerver2, Russell Hope2, Susan Hopkins2; Public Health Wales, Margaret Heginbothom3; NHS England, Philip Howard4 (1Rx-Info Ltd, Exeter Science Park, Exeter EX5 2FN, UK; 2UK Health Security Agency, UK; 3Healthcare Associated Infection, Antimicrobial Resistance and Prescribing Programme, Public Health Wales, UK; 4NHS England and NHS Improvement, North-East and Yorkshire Region, UK).
Participating NHS Trusts
Leeds Teaching Hospitals NHS Trust (lead Trust): Principal investigator, Jonathan Sandoe1,2; Research group (data collection—alphabetical order), Claire Berry3, Georgina Davis3, Vikki Wilkinson3 (1Department of Microbiology, The General Infirmary at Leeds, Leeds, UK; 2Healthcare Associated Infection Group, Leeds Institute of Medical Research, University of Leeds, Leeds, UK; 3Leeds Teaching Hospitals NHS Trust, Leeds, UK). Liverpool University Hospitals NHS Foundation Trust: Principal investigator, Stacy Todd1; Research Group (data collection—alphabetical order), Eleanor Taylor-Barr1, Mary Brodsky1, Jo Brown1, Jenni Burns1, Sharon Glynn1, Alvyda Gureviciute1, Megan Howard1, Jennifer Kirkpatrick1, Hannah Murphy1, Emma Richardson1, Deborah Scanlon1, Claire Small1, Graham Sweeney1, Lisa Williams1 (1Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK). Aneurin Bevan University Health Board: Principal investigator, Tamas Szakmany1,2; Research group (data collection—alphabetical order), Evelyn Baker3, Yusuf Cheema3, Jill Dunhill3, Charlotte Killick3, Charlie King3, Simran Kooner3, Swyn Lewis3, Maxine Nash3, Owen Richardson3, Jemma Tuffney3, Clare Westacott3, Sarah Williams3 (1Critical Care Directorate, Aneurin Bevan University Health Board, Cwmbran, UK; 2Department of Anaesthesia, Intensive Care and Pain Medicine, Division of Population Medicine, Cardiff University, Cardiff, UK; 3Aneurin Bevan University Health Board, Cwmbran, UK). Sheffield Teaching Hospital NHS Foundation Trust: Co-principal investigators, David Partridge1, Helena Parsons1; Research group (data collection—alphabetical order), Kay Cawthron1, Yuen Kiu Tai1, Thomas Newman1, Megan Plowright1, Helen Shulver1, Anna Sivakova1 (1Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK). Royal Cornwall Hospitals NHS Trust: Principal investigator, Neil Powell1; Research group (data collection—alphabetical order), Freddie Ayliffe1, Emma Darke1, Eve Fletcher1, Fiona Hammonds1, Gladys Marquez1, Leanne Welch1 (1Royal Cornwall Hospitals NHS Foundation Trust, Truro, UK). Mid Yorkshire Teaching NHS Trust: Principal investigator, Stuart Bond1; Research group (data collection—alphabetical order), Jade Lee-Milner2, Joseph Spencer2 (1Medicines Optimisation and Pharmacy Services, Pinderfields Hospital, Mid Yorkshire Teaching NHS Trust, Wakefield, UK; 2Mid Yorkshire Teaching NHS Trust, Wakefield, UK). North Bristol NHS Trust, Bristol: Principal investigator, Mahableshwar Albur1; Research group (data collection—alphabetical order), Rodrigo Brandao1, Joshua Hrycaiczuk1, Jack Stanley1 (1North Bristol NHS Trust, Bristol, UK). University Hospital Sussex NHS Foundation Trust: Principal investigator, Martin Llewelyn1; Research group (data collection—alphabetical order), Elizabeth Cross2, Daniel Hansen2, Ethan Redmore2, Abigail Whyte2 (1Brighton and Sussex Medical School, University of Sussex and University Hospitals Sussex NHS Foundation Trust, Brighton UK; 2University Hospitals Sussex NHS Foundation Trust, Brighton, UK). Newcastle-upon-Tyne Hospitals NHS Foundation Trust: Principal investigators, Tom Hellyer1,2, Iain McCullagh1,2; Research group (data collection—alphabetical order), Benjamin Brown3, Michele Calabrese3, Cameron Cole3, Jessica DeSousa3, Leigh Dunn3, Stephanie Grieveson3, Arti Gulati3, Elizabeth Issac3, Ruaridh Mackay3, Fatima Simoes3 (1Critical Care Department, Royal Victoria Infirmary, The Newcastle-upon-Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK; 2Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK; 3Newcastle-upon-Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK). Salford Royal NHS Foundation Trust: Principal investigator: Paul Dark1; Research group (data collection—alphabetical order), Elena Apatri2, Bethan Charles2, Helen Christensen2, Alice Harvey2, Diane Lomas2, Melanie Taylor2, Vicky Thomas2, Danielle Walker2 (1Division of Immunology, Immunity to Infection and Respiratory Medicine, University of Manchester, Manchester, UK; 2Salford Royal NHS Foundation Trust, Salford, UK). Nottingham University Hospitals NHS Trust: Principal investigator, Dominick Shaw1; Research group (data collection), Lucy Howard2, Amelia Joseph2, Saheer Sultan2 (1Leicester NIHR Biomedical Research Centre and Department of Respiratory Sciences, University of Leicester, Leicester, UK; 2Nottingham University Hospitals NHS Trust, Nottingham, UK). Patient and public representatives: Chikezie Knox-Macaulay1, Margaret Ogden1, Graham Prestwich1, Ryan Hamilton2,3 (1Centre for Trials Research, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK; 2Antibiotic Research UK, York, UK; 3School of Pharmacy, De Montfort University, Leicester, UK).
Contributor Information
Edward J D Webb, Leeds Institute for Health Sciences, University of Leeds, Leeds, UK.
Daniel Howdon, Leeds Institute for Health Sciences, University of Leeds, Leeds, UK.
Rebecca Bestwick, Leeds Institute for Health Sciences, University of Leeds, Leeds, UK.
Natalie King, Leeds Institute for Health Sciences, University of Leeds, Leeds, UK.
Jonathan A T Sandoe, Healthcare Associated Infection Group, Leeds Institute of Medical Research, University of Leeds, Leeds, UK; Department of Microbiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Joanne Euden, Centre for Trials Research, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK.
Detelina Grozeva, Centre for Trials Research, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK.
Robert West, Leeds Institute for Health Sciences, University of Leeds, Leeds, UK.
Philip Howard, Healthcare Associated Infection Group, Leeds Institute of Medical Research, University of Leeds, Leeds, UK; NHS England North-East & Yorkshire, Leeds, UK.
Neil Powell, Pharmacy Department, Royal Cornwall Hospital, Royal Cornwall Hospitals NHS Foundation Trust, Truro TR1 3LJ, UK.
Mahableshwar Albur, Severn Infectious Sciences, Southmead Hospital, North Bristol NHS Trust, Bristol BS10 5NB, UK.
Stuart Bond, Medicines Optimisation and Pharmacy Services, Pinderfields Hospital, Mid Yorkshire Teaching NHS Trust, Wakefield WF1 4DG, UK.
Lucy Brookes-Howell, Centre for Trials Research, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK.
Paul Dark, Division of Immunology, Faculty of Biology, Medicine and Health, Immunity to Infection and Respiratory Medicine, School of Biological Sciences, University of Manchester, Manchester M13 9PL, UK.
Thomas Hellyer, Perioperative and Critical Care Department, Institute of Transplantation, Freeman Hospital, Newcastle upon Tyne Hospital NHS Foundation Trust, Newcastle upon Tyne NE7 7DN, UK; Faculty of Medical Sciences, Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne NE2 4HH, UK.
Martin Llewelyn, Global Health and Infection, Brighton and Sussex Medical School, University of Sussex, Brighton BN1 9PS, UK; Department of Infection Medicine, University Hospitals Sussex NHS Foundation Trust, Brighton, UK.
Iain J McCullagh, Perioperative and Critical Care Department, Institute of Transplantation, Freeman Hospital, Newcastle upon Tyne Hospital NHS Foundation Trust, Newcastle upon Tyne NE7 7DN, UK; Faculty of Medical Sciences, Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne NE2 4HH, UK.
Margaret Ogden, Public and Patient Involvement Representative, NIHR, London SW1A 2NS, UK.
Philip Pallmann, Centre for Trials Research, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK.
Helena Parsons, Department of Microbiology, Laboratory Medicine, Northern General Hospital, Sheffield Teaching Hospital NHS Foundation Trust, Sheffield S5 7AU, UK.
David Partridge, Department of Microbiology, Laboratory Medicine, Northern General Hospital, Sheffield Teaching Hospital NHS Foundation Trust, Sheffield S5 7AU, UK.
Dominick Shaw, Department of Respiratory Sciences, University of Leicester, Leicester, UK.
Tamas Szakmany, Royal Gwent Hospital, Aneurin Bevan University Health Board, Newport, UK.
Stacy Todd, Tropical and Infectious Disease Unit, The Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool, UK.
Emma Thomas-Jones, Centre for Trials Research, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK.
Enitan D Carrol, Department of Clinical Infection, Microbiology and Immunology, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, UK.
Bethany Shinkins, Leeds Institute for Health Sciences, University of Leeds, Leeds, UK; Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK.
PEACH Study Group:
Jonathan Sandoe, Enitan Carrol, Emma Thomas-Jones, Lucy Brookes-Howell, Josie Henley, Wakunyambo Maboshe, Philip Pallmann, Detelina Grozeva, Marcin Bargiel, Judith Evans, Edward Webb, Rebecca Bestwick, Daniel Howdon, Robert West, Colin Richman, Sarah Gerver, Russell Hope, Susan Hopkins, Margaret Heginbothom, Philip Howard, Jonathan Sandoe, Claire Berry, Georgina Davis, Vikki Wilkinson, Stacy Todd, Eleanor Taylor-Barr, Mary Brodsky, Jo Brown, Jenni Burns, Sharon Glynn, Alvyda Gureviciute, Megan Howard, Jennifer Kirkpatrick, Hannah Murphy, Emma Richardson, Deborah Scanlon, Claire Small, Graham Sweeney, Lisa Williams, Tamas Szakmany, Evelyn Baker, Yusuf Cheema, Jill Dunhill, Charlotte Killick, Charlie King, Simran Kooner, Swyn Lewis, Maxine Nash, Owen Richardson, Jemma Tuffney, Clare Westacott, Sarah Williams, David Partridge, Helena Parsons, Kay Cawthron, Yuen Kiu Tai, Thomas Newman, Megan Plowright, Helen Shulver, Anna Sivakova, Neil Powell, Freddie Ayliffe, Emma Darke, Eve Fletcher, Fiona Hammonds, Gladys Marquez, Leanne Welch, Stuart Bond, Jade Lee-Milner, Joseph Spencer, Mahableshwar Albur, Rodrigo Brandao, Joshua Hrycaiczuk, Jack Stanley, Martin Llewelyn, Elizabeth Cross, Daniel Hansen, Ethan Redmore, Abigail Whyte, Tom Hellyer, Iain McCullagh, Benjamin Brown, Michele Calabrese, Cameron Cole, Jessica DeSousa, Leigh Dunn, Stephanie Grieveson, Arti Gulati, Elizabeth Issac, Ruaridh Mackay, Fatima Simoes, Paul Dark, Elena Apatri, Bethan Charles, Helen Christensen, Alice Harvey, Diane Lomas, Melanie Taylor, Vicky Thomas, Danielle Walker, Dominick Shaw, Lucy Howard, Amelia Joseph, Saheer Sultan, Chikezie Knox-Macaulay, Margaret Ogden, Graham Prestwich, and Ryan Hamilton
Funding
This research was funded by the National Institute for Health and Care Research (NIHR) COVID Recovery & Learning call (NIHR132254). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The Centre for Trials Research (CTR), Cardiff University receives infrastructure funding from Health and Care Research Wales. The funder of the study had no role in the design, data collection, analysis, interpretation or writing of this report. The corresponding author had full access to all the data and final responsibility for the decision to submit for publication.
Transparency declarations
J.A.T.S. has received research funding from MRC, EPSRC, NIHR, Lumos diagnostics, and Jon Moulton Charity Trust in relation to development of infection diagnostics and is a council member of the British Society for Antimicrobial Chemotherapy (BSAC); is co-investigator (co-I) for PROTECT (NIHR-HTA 156664); has received honoraria from Medtronic and Tillotts Pharma. M.A. has received honoraria payments for lectures and research from Shionogi, Merck and Pfizer and received research funding from The Showering Fund. P.D. is Chief investigator (CI) for the ADAPT-Sepsis Trial (NIHR-HTA 15/99/02) and co-I for PROTECT (NIHR-HTA 156664). J.E. is co-I for PROTECT (NIHR-HTA 156664). T.H. is CI for the RISC-Sepsis Study (NIHR EME 128374) and CI for the SHORTER trial (NIHR HTA 134101). I.J.M. is co-I on the RISC-Sepsis Study (NIHR EME 128374). D.P. is British Infection Association President. N.P. has received honoraria payments for lectures from Thermo Fisher. D.S. is co-I on RISC-sepsis (NIHR EME 128374). T.S. has received fees from Thermo Fisher Ltd in relation to PCT testing in the ICU and is CI for VAMp-SEPSIS (HCRW 1951/2023) and co-I on the EXTEND trial (NIHR 131784). S.T. is co-CI for PRONTO (NIHR HTA 17/136/13) and co-I for PROTECT (NIHR-HTA 156664). E.T.J. is co-I for PRONTO (NIHR HTA 17/136/13), BATCH (NIHR-HTA 15/188/42) and PROTECT (NIHR-HTA 156664). E.D.C. is CI for the BATCH Trial (NIHR HTA 15/188/42), co-CI for the PROTECT study (NIHR HTA 156664), co-I for the PRONTO Trial (NIHR HTA 17/136/13), a former member of the NICE diagnostic advisory committee (2014–20) and NICE sepsis guideline development committee (2014–16). P.P. is co-I for PRONTO (NIHR HTA 17/136/13) and co-CI for PROTECT (NIHR HTA 156664). L.B.H. is co-I for PRONTO (NIHR HTA 17/136/13) and BATCH (NIHR HTA 15/188/42). All other authors declare no competing interests.
Supplementary data
Figures S1 and S2 and Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Russell CD, Fairfield CJ, Drake TMet al. . Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe 2021; 2: e354–65. 10.1016/S2666-5247(21)00090-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malik P, Patel U, Mehta Det al. . Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med 2021; 26: 107–8. 10.1136/bmjebm-2020-111536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schuetz P, Wirz Y, Sager Ret al. . Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18: 95–107. 10.1016/S1473-3099(17)30592-3 [DOI] [PubMed] [Google Scholar]
- 4. Powell N, Howard P, Llewelyn MJet al. . Use of procalcitonin during the first wave of COVID-19 in the acute NHS hospitals: a retrospective observational study. Antibiotics 2021; 10: 516. 10.3390/antibiotics10050516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NICE . COVID-19 Rapid Guideline: Antibiotics for Pneumonia in Adults in Hospital. NICE Guideline NG173. https://www.nice.org.uk/guidance/ng173. [PubMed]
- 6. Metlay JP, Waterer GW, Long ACet al. . Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–67. 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Euden J, Pallmann P, Grozeva Det al. . Procalcitonin evaluation of antibiotic use in COVID-19 hospitalised patients (PEACH): protocol for a retrospective observational study. Methods Protoc 2022; 5: 95. 10.3390/mps5060095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Llewelyn MJ, Grozeva D, Howard Pet al. . Impact of introducing procalcitonin testing on antibiotic usage in acute NHS hospitals during the first wave of COVID-19 in the UK: a controlled interrupted time series analysis of organization-level data. J Antimicrob Chemother 2022; 77: 1189–96. 10.1093/jac/dkac017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandoe JA, Grozeva D, Albur Met al. . A retrospective propensity-score-matched cohort study of the impact of procalcitonin testing on antibiotic use in hospitalised patients during the first wave of COVID-19. Unpublished manuscript 2024.
- 10. Jovanoski N, Kuznik A, Becker Uet al. . Cost-effectiveness of casirivimab/imdevimab in patients with COVID-19 in the ambulatory setting. J Manag Care Spec Pharm 2022; 28: 555–65. 10.18553/jmcp.2022.21469 [DOI] [PubMed] [Google Scholar]
- 11. Sheinson D, Dang J, Shah Aet al. . A cost-effectiveness framework for COVID-19 treatments for hospitalized patients in the United States. Adv Ther 2021; 38: 1811–31. 10.1007/s12325-021-01654-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelton K, Klein T, Murphy Det al. . Cost-effectiveness of combination of baricitinib and remdesivir in hospitalized patients with COVID-19 in the United States: a modelling study. Adv Ther 2022; 39: 562–82. 10.1007/s12325-021-01982-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Webb EJ, King N, Howdon Det al. . Evidence of quality of life for hospitalised patients with COVID-19: a scoping review. Health Technol Assess 2024. 10.3310/ATPR4281 [DOI] [PubMed] [Google Scholar]
- 14. McNamara S, Schneider PP, Love-Koh Jet al. . Quality-adjusted life expectancy norms for the English population. Value Health 2023; 26: 163–9. 10.1016/j.jval.2022.07.005 [DOI] [PubMed] [Google Scholar]
- 15. Westwood M, Ramaekers B, Whiting Pet al. . Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015; 19: 3–236. 10.3310/hta19960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilcox MH, Ahir H, Coia JEet al. . Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother 2017; 72: 2647–56. 10.1093/jac/dkx174 [DOI] [PubMed] [Google Scholar]
- 17. Hollmann M, Garin O, Galante Met al. . Impact of influenza on health-related quality of life among confirmed (H1N1) 2009 patients. PLoS One 2013; 8: e60477. 10.1371/journal.pone.0060477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oppong R, Kaambwa B, Nuttall Jet al. . The impact of using different tariffs to value EQ-5D health state descriptions: an example from a study of acute cough/lower respiratory tract infections in seven countries. Eur J Health Econ 2013; 14: 197–209. 10.1007/s10198-011-0360-9 [DOI] [PubMed] [Google Scholar]
- 19. Metry A, Pandor A, Ren Set al. Therapeutics for People with COVID-19 [ID4038]. https://www.nice.org.uk/guidance/ta878/documents/assessment-report.
- 20. NHS England . 2020/21 National Cost Collection Data Publication. https://www.england.nhs.uk/publication/2020-21-national-cost-collection-data-publication/.
- 21. NICE . Procalcitonin Testing for Diagnosing and Monitoring Sepsis (ADVIA Centaur BRAHMS PCT Assay, BRAHMS PCT Sensitive Kryptor Assay, Elecsys BRAHMS PCT Assay, LIAISON BRAHMS PCT Assay and VIDAS BRAHMS PCT assay) Diagnostics Guidance [DG18]. https://www.nice.org.uk/guidance/dg18.
- 22. Department of Health and Social Care . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT). https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit.
- 23. NICE . British National Formulary (BNF). https://bnf.nice.org.uk/.
- 24. Oppong R, Smith RD, Little Pet al. . Cost effectiveness of amoxicillin for lower respiratory tract infections in primary care: an economic evaluation accounting for the cost of antimicrobial resistance. Br J Gen Pract 2016; 66: e633–9. 10.3399/bjgp16X686533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones K, Weatherly HLA, Birch Set al. Unit Costs of Health and Social Care 2022. https://kar.kent.ac.uk/100519/1/Unit%20Costs%20of%20health%20and%20Social%20Care%202022%20%28amended%2013%20July%202023%29.pdf.
- 26. Office for National Statistics . National life tables: England. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandreferencetables.
- 27. NICE . NICE Health Technology Evaluations: the Manual. Process and Methods [PMG36]. https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation.
- 28. Williams EJ, Mair L, de Silva TIet al. . Evaluation of procalcitonin as a contribution to antimicrobial stewardship in SARS-CoV-2 infection: a retrospective cohort study. J Hosp Infect 2021; 110: 103–7. 10.1016/j.jhin.2021.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heesom L, Rehnberg L, Nasim-Mohi Met al. . Procalcitonin as an antibiotic stewardship tool in COVID-19 patients in the intensive care unit. J Glob Antimicrob Resist 2020; 22: 782–4. 10.1016/j.jgar.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peters C, Williams K, Un EAet al. . Use of procalcitonin for antibiotic stewardship in patients with COVID-19: a quality improvement project in a district general hospital. Clinical Medicine 2021; 21: e71–6. 10.7861/clinmed.2020-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pulia MS, Wolf I, Schwei RJet al. . Antibiotic prescribing patterns for coronavirus disease 2019 (COVID-19) in two emergency departments with rapid procalcitonin. Infect Control Hosp Epidemiol 2021; 42: 359–61. 10.1017/ice.2020.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harte E, Kumarasamysarma S, Phillips Bet al. . Procalcitonin values fail to track the presence of secondary bacterial infections in COVID-19 ICU patients. Antibiotics 2023; 12: 709. 10.3390/antibiotics12040709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Pol S, Garcia PR, Postma MJet al. . Economic analyses of respiratory tract infection diagnostics: a systematic review. Pharmacoeconomics 2021; 39: 1411–27. 10.1007/s40273-021-01054-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Michaelidis CI, Zimmerman RK, Nowalk MPet al. . Cost-effectiveness of procalcitonin-guided antibiotic therapy for outpatient management of acute respiratory tract infections in adults. J Gen Intern Med 2014; 29: 579–86. 10.1007/s11606-013-2679-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stojanovic I, Schneider JE, Wei Let al. . Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a Chinese hospital system perspective. Clin Chem Lab Med 2017; 55: 561–70. 10.1515/cclm-2016-0349 [DOI] [PubMed] [Google Scholar]
- 36. Schuetz P, Balk R, Briel Met al. . Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a US health system perspective. Clin Chem Lab Med 2015; 53: 583–92. 10.1515/cclm-2014-1015 [DOI] [PubMed] [Google Scholar]
- 37. Euden J, Thomas-Jones E, Aston Set al. . PROcalcitonin and NEWS2 evaluation for timely identification of sepsis and optimal use of antibiotics in the emergency department (PRONTO): protocol for a multicentre, open-label, randomised controlled trial. BMJ Open 2022; 12: e063424. 10.1136/bmjopen-2022-063424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dark P, Perkins GD, McMullan Ret al. . biomArker-guided duration of antibiotic treatment in hospitalised patients with suspecTed sepsis (ADAPT-sepsis): a protocol for a multicentre randomised controlled trial. J Intensive Care Soc 2023; 24: 175114372311691. 10.1177/17511437231169193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.