Abstract
The bioenergetics of cellulose utilization by Clostridium thermocellum was investigated. Cell yield and maintenance parameters, 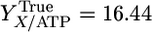 g cell/mol ATP and m = 3.27 mmol ATP/g cell per hour, were obtained from cellobiose-grown chemostats, and it was shown that one ATP is required per glucan transported. Experimentally determined values for
g cell/mol ATP and m = 3.27 mmol ATP/g cell per hour, were obtained from cellobiose-grown chemostats, and it was shown that one ATP is required per glucan transported. Experimentally determined values for 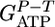 (ATP from phosphorolytic β-glucan cleavage minus ATP for substrate transport, mol ATP/mol hexose) from chemostats fed β-glucans with degree of polymerization (DP) 2-6 agreed well with the predicted value of (n-1)/n (n = mean cellodextrin DP assimilated). A mean
(ATP from phosphorolytic β-glucan cleavage minus ATP for substrate transport, mol ATP/mol hexose) from chemostats fed β-glucans with degree of polymerization (DP) 2-6 agreed well with the predicted value of (n-1)/n (n = mean cellodextrin DP assimilated). A mean 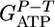 value of 0.52 ± 0.06 was calculated for cellulose-grown chemostat cultures, corresponding to n = 4.20 ± 0.46. Determination of intracellular β-glucan radioactivity resulting from 14C-labeled substrates showed that uptake is different for cellulose and cellobiose (G2). For 14C-cellobiose, radioactivity was greatest for G2; substantially smaller but measurable for G1, G3, and G4; undetectable for G5 and G6; and n was ≈2. For 14C-cellulose, radioactivity was greatest for G5; lower but substantial for G6, G2, and G1; very low for G3 and G4; and n was ≈4. These results indicate that: (i) C. thermocellum hydrolyzes cellulose by a different mode of action from the classical mechanism involving solubilization by cellobiohydrolase; (ii) bioenergetic benefits specific to growth on cellulose are realized, resulting from the efficiency of oligosaccharide uptake combined with intracellular phosphorolytic cleavage of β-glucosidic bonds; and (iii) these benefits exceed the bioenergetic cost of cellulase synthesis, supporting the feasibility of anaerobic biotechnological processing of cellulosic biomass without added saccharolytic enzymes.
value of 0.52 ± 0.06 was calculated for cellulose-grown chemostat cultures, corresponding to n = 4.20 ± 0.46. Determination of intracellular β-glucan radioactivity resulting from 14C-labeled substrates showed that uptake is different for cellulose and cellobiose (G2). For 14C-cellobiose, radioactivity was greatest for G2; substantially smaller but measurable for G1, G3, and G4; undetectable for G5 and G6; and n was ≈2. For 14C-cellulose, radioactivity was greatest for G5; lower but substantial for G6, G2, and G1; very low for G3 and G4; and n was ≈4. These results indicate that: (i) C. thermocellum hydrolyzes cellulose by a different mode of action from the classical mechanism involving solubilization by cellobiohydrolase; (ii) bioenergetic benefits specific to growth on cellulose are realized, resulting from the efficiency of oligosaccharide uptake combined with intracellular phosphorolytic cleavage of β-glucosidic bonds; and (iii) these benefits exceed the bioenergetic cost of cellulase synthesis, supporting the feasibility of anaerobic biotechnological processing of cellulosic biomass without added saccharolytic enzymes.
Keywords: cellulose hydrolysis, cellulase, cellulosome, anaerobic, thermophilic, ABC transport
Biologically mediated cellulose hydrolysis is a major flow of carbon in the biosphere (1, 2), important in several agricultural processes (3), and could be widely used to produce sustainable fuels and chemicals (3-7). Extensive evidence obtained with noncomplexed cellulase systems produced by aerobic microorganisms supports a mechanism involving synergistic action by endoglucanases and cellobiohydrolases with cellobiose the main product of cellulose solubilization (8-10). Anaerobic microorganisms possess complexed cellulase systems with architecture distinct from the noncomplexed systems of aerobes (11-13). In cell-free experiments involving complexed cellulase systems, cellobiose and glucose have been observed to accumulate (14, 15), consistent with a hydrolysis mechanism similar to that for noncomplexed systems. No evidence has been presented that the primary product of cellulose hydrolysis utilized by anaerobic microorganisms is other than cellobiose.
Utilization of cellulose by anaerobic microorganisms is a challenging proposition from a bioenergetic perspective, because the modest ATP available from anaerobic catabolism needs to support both microbial growth and cellulase production (16). Because reaction rates catalyzed by cellulases are lower than for most other enzymes, typically by at least 2 orders of magnitude on a protein-specific basis (9, 10), large amounts of cellulase and hence ATP are required to achieve significant cellulose hydrolysis rates (17, 18). Existing bioenergetic models of microbial cellulose utilization are not sufficient to definitively evaluate the extent of the “metabolic burden” associated with cellulase synthesis for either naturally occurring cellulolytic microorganisms or biotechnological processes.
Were such an evaluation available, it would provide fundamental insights into the physiology of anaerobic cellulolytic microorganisms, the ecological communities in which these organisms occur, and the evolutionary strategies they embody. It would also address a central factor determining the feasibility of industrial processes in which anaerobic microorganisms convert cellulosic biomass to a desired product in the absence of added saccharolytic enzymes. Such “consolidated bioprocessing” is a potential breakthrough for low-cost production of ethanol or other fermentation products from cellulosic biomass (16, 19, 20).
In this study, we validate a comprehensive bioenergetic model of cellulose utilization by Clostridium thermocellum, a thermophilic cellulolytic anaerobic bacterium that exhibits one of the highest growth rates on crystalline cellulose among described microorganisms (16). In so doing, insights are gained with respect to substrate assimilation during cellulose utilization, the mechanism of cellulose hydrolysis, evaluation of the bioenergetic cost associated with growth on cellulose as compared with soluble substrates, and the feasibility of consolidated bioprocessing.
Materials and Methods
Chemicals and Microbial Cultures. All chemicals were reagent grade and were obtained from Sigma unless indicated otherwise. C. thermocellum ATCC 27405 has been maintained in our lab since 1983, as described (21-23). Chemically defined, MTC medium was prepared by combining six sterile solutions under a nitrogen atmosphere, as described (21-23). Cellodextrins were prepared by mixed acid hydrolysis and separated chromatographically, as reported (24).
Continuous Fermentation. Continuous fermentations on cellobiose (≈5g/liter) and microcrystilline cellulose (Avicel PH 105, FMC, Philadelphia) Avicel (≈5 g/liter) were carried out in 2.5-liter round-bottom reactors (Applikon Dependable Instruments, Foster City, CA) with agitation via a marine impeller at 300 rpm. Continuous fermentation on soluble cellodextrins with degree of polymerization from 2 to 6 was also carried out in a 60-ml working volume jacketed glass fermentor (NDS Glass, Vineland, NJ), as described (23). Cultures were considered to be at steady state when the samples exhibited <5% variation and no consistent increasing or decreasing trend over time. Each reported steady-state value is based on the average of at least four data points.
Analysis of Fermentation Products. The mass concentrations of cellulase and cells growing on Avicel and cellodextrins were calculated based on an indirect ELISA by using antibody raised against a sequence from the C. thermocellum scaffoldin protein, as described (21, 23). Noncellulase extracellular protein is defined as supernatant protein concentration minus the supernatant cellulase concentration as determined by ELISA. The dry weight, cellulose conversion, and fermentation product concentration of culture samples were measured as described (21, 23).
Source of 14C-Labeled Substrates. Radiolabeled cellulose (50 μCi; 1 Ci = 37 GBq), purchased from American Radiolabeled Chemicals (St. Louis), was extracted by using 90% ethanol followed by boiling, dilution using 40 mg of Avicel PH105, and then treated with 83% phosphoric acid to obtain a radiolabeled amorphous cellulose suspension (21). Radiolabeled cellobiose was prepared by using cellobiose phosphorylase in conjunction with radiolabeled glucose (Sigma) and glucose-1-phosphate, as described (25, 26). Labeled cellobiose was separated by TLC (Whatman LK6DF), as described (26, 27), and the specific activity of cellobiose was calibrated by the modified BCA method (28). Silica gel from TLC plates was scraped at a location indicated by unlabeled cellobiose controls, added to distilled water, incubated at 80°C for 30 min with repeated vortex mixing, and removed by centrifugation.
Uptake of 14C-Labeled Substrates. Prewarmed oxygen-free labeled substrate (either cellulose or cellobiose) prepared as above was injected into 100-ml serum vials containing 50 ml of Avicel-grown cell cultures in late stationary phase (substrate recently exhausted) as well as a cell-free control containing C. thermocellum cellulase purified by affinity digestion (21, 29) at the same concentration present in the supernatant of the Avicel-grown culture. Samples were withdrawn at indicated intervals by using an 18-gauge needle connected to a 60-ml syringe containing 40 ml of ultracold (-70°C) methanol-Hepes buffer to quench metabolism immediately (30). Intracellular cellodextrins were extracted by boiling in hot ethanol (30) and then treated by using 13% perchloric acid (final concentration) at 40°C for 1 h to convert glucose phosphate to glucose. The acidified metabolite solution was neutralized by a solution containing2MK2CO3 and 2 M KOH (26). The concentrated metabolite extracts were applied to a TLC plate and run twice by using a developing solution, as described (26). The separated radiolabeled cellodextrins were scraped from the TLC plate according to the locations of cold cellodextrin standards (26, 27), and the radio activities of individual cellodextrins were measured by using a Beckman LS7500 liquid scintillation counter. The radioactivity of intracellular cellodextrins was calculated by a procedure involving subtraction of noncell-associated cellulose-adhered labeled cellodextrins inferred from a cell-free control experiment (see Supporting Text, which is published on the PNAS web site, for details).
Results
ATP-producing metabolic processes available to C. thermocellum include intracellular phosphorolytic cleavage of β-glucosidic bonds by cellodextrin and cellobiose phosphorylases (16, 21), glycolysis via the Emden-Meyerhoff pathway, and the action of acetate kinase. ATP-consuming metabolic processes include substrate transport via an adenosine-binding cassette system (16, 31), cell synthesis, cellulase synthesis, and nonbiosynthetic “maintenance” functions. For coupled ATP-limited metabolism, the rate of ATP production can be set equal to the rate of ATP consumption, resulting in Eq. 1 (ref. 16; see Justification and explanation for Eq. 1 in Supporting Text for additional details):
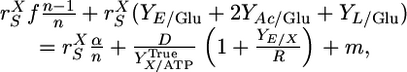 |
[1] |
with f the fraction of β-glucosidic bonds cleaved phosphorolytically; n, mean degree of polymerization of assimilated cellodextrins; YE/Glu, YAc/Glu, and YL/Glu, molar fermentation product yields (ethanol, acetate, and lactate, mol product/mol glucose); α, ATP expenditure/cellodextrin transported; 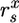 , cell-specific carbohydrate uptake rate (mol glucose equivalent per g cell per h); D, the dilution rate (1/h); YE/X, cell-specific yield of and additional supernatant protein cellulase (g protein/g cell);
, cell-specific carbohydrate uptake rate (mol glucose equivalent per g cell per h); D, the dilution rate (1/h); YE/X, cell-specific yield of and additional supernatant protein cellulase (g protein/g cell); 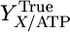 , true cell yield ATP (g cell/mol ATP used for anabolism); R, ratio of the true protein yield to the true cell yield; and m, maintenance (mol ATP/g cell per h). The literature supports a value of R = 0.82 for bacterial growth (32, 33), and f = 1 for C. thermocellum (22). We conclude that α = 1 for cellobiose and cellodextrin transport by C. thermocellum based on statistical analysis of
, true cell yield ATP (g cell/mol ATP used for anabolism); R, ratio of the true protein yield to the true cell yield; and m, maintenance (mol ATP/g cell per h). The literature supports a value of R = 0.82 for bacterial growth (32, 33), and f = 1 for C. thermocellum (22). We conclude that α = 1 for cellobiose and cellodextrin transport by C. thermocellum based on statistical analysis of 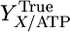 values (Table 2, which is published as supporting information on the PNAS web site). Several studies of adenosine-binding cassette transport systems under energy-limited conditions have also found that α = 1 (34-36).
values (Table 2, which is published as supporting information on the PNAS web site). Several studies of adenosine-binding cassette transport systems under energy-limited conditions have also found that α = 1 (34-36).
For cellobiose (n = 2) and substituting values for α, R, and f, Eq. 1 can be rewritten as
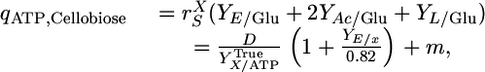 |
[2] |
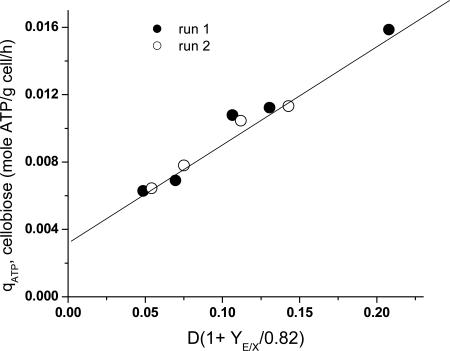
where qATP,Cellobiose denotes the net rate of ATP synthesis. A plot of qATP,Cellobiose vs. the biosynthesis rate from cellobiose chemostat data exhibits a linear trend (Fig. 1), consistent with Eq. 2. From the slope and intercept of this line, YTrue = 16.44 g cell/mol ATP and m = 3.27 mmol/g cell per h.
Fig. 1.
Plot for determination of 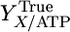 and m based on Eq. 2. Data are calculated from steady-state continuous cultures of C. thermocellum growing on cellobiose with a feed concentration of ≈5 g/liter (see Table 3 which is published as supporting information on the PNAS web site, for details).
and m based on Eq. 2. Data are calculated from steady-state continuous cultures of C. thermocellum growing on cellobiose with a feed concentration of ≈5 g/liter (see Table 3 which is published as supporting information on the PNAS web site, for details).
For cellulose, with the mean cellodextrin chain length assimilated, n, unknown, Eq. 1 can be rewritten as
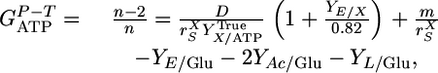 |
[3] |
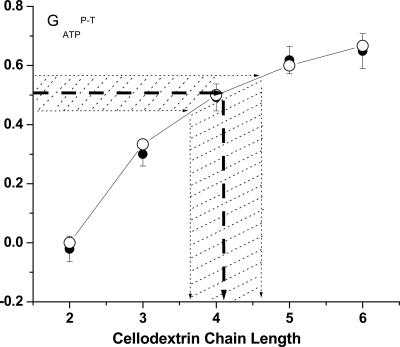
where 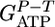 corresponds to the ATP gained by phosphorolytic cleavage minus ATP expended on substrate transport (mol ATP/mol hexose). A plot of
corresponds to the ATP gained by phosphorolytic cleavage minus ATP expended on substrate transport (mol ATP/mol hexose). A plot of 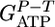 vs. n (Fig. 2) shows excellent agreement between the model prediction,
vs. n (Fig. 2) shows excellent agreement between the model prediction, 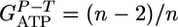 , and experimental values from calculated steady-state chemostat cultures grown on purified cellodextrins of length 2-6 at D = 0.167 h-1. For growth on cellulose, an average
, and experimental values from calculated steady-state chemostat cultures grown on purified cellodextrins of length 2-6 at D = 0.167 h-1. For growth on cellulose, an average 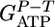 value of ± 0.06 was calculated for chemostat cultures with D from 0.0333 to 0.167 h-1. Solving Eq. 3 for n gives n = 4.20 ± 0.46.
value of ± 0.06 was calculated for chemostat cultures with D from 0.0333 to 0.167 h-1. Solving Eq. 3 for n gives n = 4.20 ± 0.46.
Fig. 2.
Predicted (○) and measured 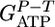 (•) values as a function of n. Experimental points are calculated from steady-state continuous cultures of C. thermocellum growing on cellodextrins of length 2-6 as well as cellulose (Avicel). The dashed line denotes the mean value of
(•) values as a function of n. Experimental points are calculated from steady-state continuous cultures of C. thermocellum growing on cellodextrins of length 2-6 as well as cellulose (Avicel). The dashed line denotes the mean value of 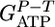 for cellulose fermentation, with the shaded region denoting the standard deviation for independent steady-state fermentation runs carried out at various dilution rates (see Tables 4 and 5, which are published as supporting information on the PNAS web site, for details).
for cellulose fermentation, with the shaded region denoting the standard deviation for independent steady-state fermentation runs carried out at various dilution rates (see Tables 4 and 5, which are published as supporting information on the PNAS web site, for details).
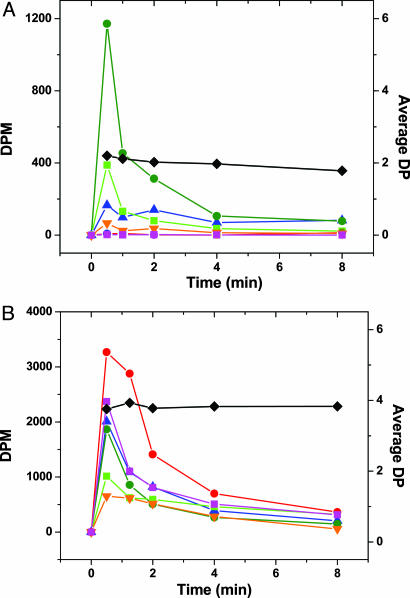
If the mean chain length of cellulose hydrolysis products taken up by C. thermocellum is substantially greater than 2, as indicated by the results presented thus far, then one would expect to see significantly different distributions of labeled intracellular cellodextrins accompanying growth on 14C-cellulose as compared with 14C-labeled cellobiose. On the other hand, if cellobiose were the primary hydrolysis product, as per the classical mechanism, then little or no difference between cellobiose- and cellulose-labeled substrates should be observed. With these observations in mind, 14C-labeled substrates, either cellobiose or cellulose, were added to substrate-exhausted cellulose-grown cultures, and the radioactivity of intracellular labeled cellodextrins was monitored. For labeled cellobiose added (Fig. 3A), the greatest amount of radioactivity is measured for cellobiose (G2) with lesser amounts for G1, G3, and G4. Radioactive G5 and G6 are not detected, and the mean cellodextrin degree of polymerization (DP) is ≈2. For labeled cellulose, the greatest amount of radioactivity is detected for G5, with somewhat lesser amounts for G6, G2, and G1, and the lowest amounts for G3 and G4. The mean cellodextrin DP is ≈4. Although the cellodextrin pool sizes measured cannot be equated to fluxes through these pools, the data indicate that G5 and G6 are assimilated in significant amounts during growth on cellulose, whereas this is not the case for growth on cellobiose. The results in Fig. 3 provide independent confirmation of our prediction based on bioenergetic analysis that n is substantially greater than 2 during growth on cellulose. The appearance of G3 and G4 during growth on cellobiose is not unexpected, because cellobiose and cellodextrin phosphorylases are readily reversible (20).
Fig. 3.
Radioactivity of intracellular cellodextrins following addition of [14C]cellobiose (0.05 μCi/ml broth, (A) and phosphoric acid-swollen [14C]cellulose (0.5 μCi/ml broth, B). ▴, glucose; •, cellobiose; ▪, cellotriose; ▾, cellotetraose; •, cellopentaose; ▪, cellohexaose; and ♦, number average degree of polymerization of intracellular cellodextrin.
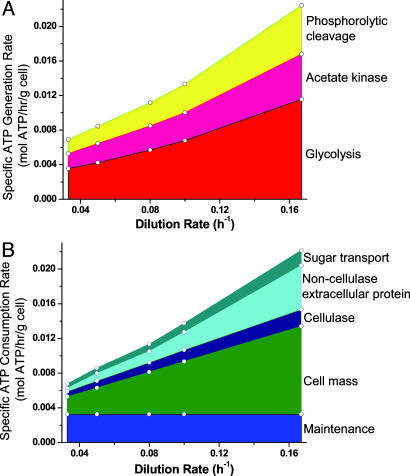
Fig. 4 presents the rates of ATP generation (Fig. 4A) and consumption (Fig. 4B) as a function of growth rate for C. thermocellum grown on cellulose. These data are calculated by using 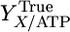 and m determined from Fig. 1 with n = 4.2. Glycolysis is responsible for slightly over half of the ATP supply with the remainder split roughly equally between phosphorolysis and acetate kinase. Cell synthesis accounts for an increasing share of ATP consumption with increasing growth rate up to a maximum of 45%. ATP demands for cellulase synthesis and transport are relatively small (≤17%). Because significant quantities of noncellulase protein were measured in culture supernatants, perhaps due to cell lysis, corresponding ATP consumption is shown in Fig. 4B.
and m determined from Fig. 1 with n = 4.2. Glycolysis is responsible for slightly over half of the ATP supply with the remainder split roughly equally between phosphorolysis and acetate kinase. Cell synthesis accounts for an increasing share of ATP consumption with increasing growth rate up to a maximum of 45%. ATP demands for cellulase synthesis and transport are relatively small (≤17%). Because significant quantities of noncellulase protein were measured in culture supernatants, perhaps due to cell lysis, corresponding ATP consumption is shown in Fig. 4B.
Fig. 4.
ATP generation (A) and demand (B) for C. thermocellum growing on cellulose (Avicel). Data are calculated from steady-state continuous cultures of C. thermocellum growing on Avicel (see Table 5 for details).
Discussion
We conclude that C. thermocellum assimilates cellodextrins with n ≈ 4 during growth on cellulose, implying that the immediate products of cellulose solubilization have an average degree of polymerization of at least this value. The mechanism of cellulose hydrolysis by C. thermocellum thus is different from the accepted mechanism for aerobic microorganisms such as Trichoderma reesei, in which cellobiose is the primary direct product of cellulose hydrolysis. Although this result contradicts current understanding of cellulose utilization by C. thermocellum, as well as other cellulolytc microbes, it is consistent with a number of observations from prior studies. C. thermocellum is known to have an uptake system capable of assimilating cellodextrins from G2 to G6 (26). Of the 16 catalytically active cellulosome components whose mode of action has been determined (11-13), 12 are endoglucanases, and only 2 are strict cellobiohydrolases. Prior data do not contradict the hypothesis that the initial products of cellulose hydrolysis produced by the C. thermocellum cellulase system are higher molecular weight cellodextrins that are rapidly cleaved in the absence of cells and thus do not appear in significant concentrations in cell-free experiments but are available for microbial uptake (16). Recent results involving rapid sampling of several purified cellulase components support this interpretation (ref. 37; W. Schwartz, personal communication). The distance between two adjacent catalytic subunits in the C. thermocellum cellulosome has been estimated at eight glucosidic bonds (38). Thus, simultaneous catalytic events mediated by adjacent catalytic components would be expected to result in an insoluble G8 fragment, and any subsequent cleavage of this G8 fragment would result in two soluble products with mean chain length 4.
C. thermocellum utilizes a relatively narrow range of substrates and appears to have evolved to function as a cellulose-using “specialist” (16, 31). Cellodextrin transport using an adenosine-binding cassette system, which typically features very high affinity [e.g., Km = 52 μM for cellobiose (26)], puts the organism in a strong position to compete for products of cellulose hydrolysis and is responsive to demands associated with growth under high-temperature and nutrient-limited conditions (39, 40). By assimilating cellodextrins with length ≈4 during growth on cellulose, the organism avoids an otherwise rather high ATP requirement for an anaerobe to expend on substrate transport. An equally large additional benefit of such assimilation is realized by phosphorolytic cleavage of β-glucan bonds. C. thermocellum does not grow easily or well on glucose (16) and uses cellobiose instead of glucose if presented with both substrates (16, 31). This can readily be understood in light of the fact that no benefit is derived from phosphorolytic cleavage during growth on glucose, whereas the cost of transport per hexose, by either an adenosine-binding cassette system or any other active mechanism, is higher for glucose than for oligosaccharides. As shown in Table 1, the benefits of efficient transport and phosphorolytic cleavage of cellodextrins more than compensate for the higher ATP expended on cellulase synthesis when the organism is grown on cellulose as compared with cellobiose. In addition to cellobiose and cellodextrin phosphorylases, found in a variety of cellulolytic microorganisms as well as C. thermocellum (22), several other intracellular phosphorylases have been reported to cleave soluble oligosaccharides such as maltose and maltodextrins in the presence of inorganic phosphate to glucose-1-phosphate and Gn-1 (35, 41, 42). Although this suggests that substrate level phosphorylation linked to phosphorolytic glucosidic bond cleavage may be widespread, the quantitative significance of these enzyme activities remains to be evaluated in the context of overall ATP supply in the organisms that produce them.
Table 1. Net ATP availability associated with metabolic processes impacted by substrate chain length.
| ATP/mol glucose
|
||
|---|---|---|
| Process | Cellobiose | Cellulose |
| Phosphorolytic cleavage | +.5 | +.75 |
| Transport | −0.5 | −0.25 |
| Cellulase synthesis | −0.1 to −0.2 | −0.26 to −0.32 |
| Net | −0.1 to −0.2 | +0.18 to +0.24 |
Values for cellulose are based on n = 4.2, with ATP requirements for cellulase synthesis based on the range of values observed for cellulose-grown chemostats with D = 0.033 to 0.167 h−1.
From a biotechnological perspective, our results establish that consolidated bioprocessing of cellulose is bioenergetically feasible for fermentative production of ethanol or other products that do not require ATP for synthesis from intermediates of central metabolism. The strategy of assimilating oligosaccharides combined with phosphorolytic cleavage may be of value for biotechnological application beyond cellulose conversion. Engineering these features into microbial biocatalysts and using widely available feedstocks containing soluble oligosaccharies would provide extra ATP for microbial growth, product synthesis, and/or transport. Although such extra ATP represents a small fraction of the total available from aerobic respiration, it could make a significant and potentially enabling difference for applications involving high yields of reduced products and for processes based on anaerobic fermentation in particular.
Supplementary Material
Acknowledgments
We thank P. J. Weimer, W. Schwarz, and H. J. Strobel for useful discussions, and we are grateful to Michael Campbell for editorial support. We also acknowledge support from the Department of Energy (DE-FG02-02ER15350) and the National Institute of Standards and Technology (60NANB1D0064).
Author contributions: Y.-H.P.Z. and L.R.L. designed research; Y.-H.P.Z. performed research; Y.-H.P.Z. and L.R.L. analyzed data; and Y.-H.P.Z. and L.R.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berner, R. A. (2003) Nature 426, 323-326. [DOI] [PubMed] [Google Scholar]
- 2.Melillo, J. M., Steudler, P. A., Aber, J. D., Newkirk, K., Lux, H., Bowles, F. P., Catricala, C., Magill, A., Ahrens, T. & Morrisseau, S. (2002) Science 298, 2173-2176. [DOI] [PubMed] [Google Scholar]
- 3.Russell, J. B. & Rychlik, J. L. (2001) Science 292, 1119-1122. [DOI] [PubMed] [Google Scholar]
- 4.Kamm, B. & Kamm, M. (2004) Appl. Microbiol. Biotechnol. 64, 137-145. [DOI] [PubMed] [Google Scholar]
- 5.Wahlaud, B., Yan, J. & Westermark, M. (2004) Biomass Bioenerg. 26, 531-544. [Google Scholar]
- 6.Wirth, T. E., Gray, C. B. & Podesta, J. D. (2003) Foreign Aff. 82, 125-144. [Google Scholar]
- 7.Wyman, C. E. (2003) Biotechnol. Prog. 19, 254-262. [DOI] [PubMed] [Google Scholar]
- 8.Divne, C., Stahlberg, J., Reinikainen, T., Ruohonen, L., Pettersson, G., Knowles, J. K., Teeri, T. T. & Jones, T. A. (1994) Science 265, 524-528. [DOI] [PubMed] [Google Scholar]
- 9.Teeri, T. T., Koivula, A., Linder, M., Wohlfahrt, G., Divne, C. & Jones, T. A. (1998) Biochem. Soc. Trans. 26, 173-178. [DOI] [PubMed] [Google Scholar]
- 10.Zhang, Y.-H. P. & Lynd, L. R. (2004) Biotechnol. Bioeng. 88, 797-824. [DOI] [PubMed] [Google Scholar]
- 11.Bayer, E. A., Belaich, J.-P., Shoham, Y. & Lamed, R. (2004) Annu. Rev. Microbiol. 58, 521-554. [DOI] [PubMed] [Google Scholar]
- 12.Doi, R. H. & Kosugi, A. (2004) Nat. Rev. Microbiol. 2, 541-551. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz, W. H. (2001) Appl. Microbiol. Biotechnol. 56, 634-649. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, E. A., Reese, E. T. & Demain, A. L. (1982) J. Appl. Biochem. 4, 64-71. [Google Scholar]
- 15.Hom-nami, K., Coughlan, M. P., Hon-nami, H. & Ljungdahl, L. G. (1986) Arch. Microbiol. 145, 13-19. [Google Scholar]
- 16.Lynd, L. R., Weimer, P. J., van Zyl, W. H. & Pretorius, I. S. (2002) Microbiol. Mol. Biol. Rev. 66, 506-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou, S. D., Davis, F. C. & Ingram, L. O. (2001) Appl. Environ. Microbiol. 67, 6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Walsum, G. P. & Lynd, L. R. (1998) Biotechnol. Bioeng. 58, 316-320. [PubMed] [Google Scholar]
- 19.Office of the Biomass Program of U.S. Department of Energy (2003) Biomass Program: Multi-Year Technical Plan FY04-FY08, www.nrel.gov/docs/fy05osti/36999.pdf.
- 20.National Research Council (1999) Review of the Research Strategy for Biomass-Derived Transportation Fuels (Natl. Acad. Press, Washington, DC).
- 21.Zhang, Y & Lynd, L. R. (2003) Anal. Chem. 75, 219-227. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, Y.-H. P. & Lynd, L. R. (2004) Appl. Environ. Microbiol. 70, 1563-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, Y.-H. P. & Lynd, L. R. (2005) J. Bacteriol. 188, 99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, Y.-H. P. & Lynd, L. R. (2003) Anal. Biochem. 322, 225-332. [DOI] [PubMed] [Google Scholar]
- 25.Ng, T. K. & Zeikus, J. G. (1986) Appl. Environ. Microbiol. 52, 902-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strobel, H. J., Caldwell, F. C. & Dawson, K. A. (1995) Appl. Environ. Microbiol. 61, 4012-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chirico, W. J. & Brown, R. D., Jr. (1985) Anal. Biochem. 150, 264-272. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, Y.-H. P. & Lynd, L. R. (2005) Biomacromolecules 6, in press. [DOI] [PubMed]
- 29.Morag, E., Bayer, E. A. & Lamed, R. (1992) Enzyme Microb. Technol. 14, 289-295. [Google Scholar]
- 30.Raamsdonk, L. M., Teusink, B., Broadhurst, D., Zhang, N., Hayes, A., Walsh, M. C., Berden, J. A., Brindle, K. M., Kell, D. B., Rowland, J. J., et al. (2001) Nat. Biotechnol. 19, 45-50. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell, W. J. (1998) Adv. Microb. Physiol. 39, 31-130. [DOI] [PubMed] [Google Scholar]
- 32.Forrest, W. W. & Walker, D. J. (1971) Adv. Microb. Physiol. 5, 213-274. [DOI] [PubMed] [Google Scholar]
- 33.Russell, J. B. & Cook, G. M. (1995) Microbiol. Rev. 9, 48-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimmack, M. L., Gallagher, M. P., Pearce, S. R., Hyde, S. C., Booth, I. R. & Higgins, C. F. (1989) Proc. Natl. Acad. Sci. USA 86, 8257-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muir, M., Williams, L. & Ferenci, T. (1985) J. Bacteriol. 163, 1237-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro, A. B. & Ling, V. (1998) Eur. J. Biochem. 254, 189-193. [DOI] [PubMed] [Google Scholar]
- 37.Zverlov, V. V., Velikodvorskaya, G. A. & Schwarz, W. H. (2003) Microbiology 149, 515-524. [DOI] [PubMed] [Google Scholar]
- 38.Mayer, F., Coughlan, M. P., Mori, Y. & Ljungdahl, L. G. (1987) Appl. Environ. Microbiol. 53, 2785-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albers, S. V., Van de Vossenberg, J. L., Driessen, A. J. & Konings, W. N. (2001) Extremophiles 5, 285-294. [DOI] [PubMed] [Google Scholar]
- 40.Ferenci, T. (1999) Curr. Opin. Microbiol. 2, 208-213. [DOI] [PubMed] [Google Scholar]
- 41.Schinzel, R. & Nidetzky, B. (1999) FEMS Microbiol. Lett. 171, 73-79. [DOI] [PubMed] [Google Scholar]
- 42.Geremia, S., Campagnolo, M., Schinzel, R. & Johnson, L. N. (2002) J. Mol. Biol. 322, 413-423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.