Abstract
The transcription factor Pax6 is expressed by progenitors in the ventricular zone (VZ) of dorsal telencephalon (dTel), which generate all cortical glutamatergic neurons, but not by progenitors in the medial ganglionic eminence (MGE), which generate cortical GABAergic interneurons (GABA INs), or the lateral ganglionic eminence (LGE), which generate GABA INs that normally migrate to the olfactory bulb. We show that perinatally, Pax6sey/sey mice, which lack functional Pax6 protein, have large subpial ectopias in dTel and ventral telencephalon connected by cell streams arising from an aberrant paraventricular ectopia found throughout dTel. The subpial and paraventricular ectopias and connecting cell streams are comprised of postmitotic neurons expressing markers for GABA INs characteristic of a LGE fate. Marker analyses show that dTel VZ progenitors in Pax6 mutants are progressively ventralized, acquiring expression of regulatory genes normally limited to GE progenitors; by midneurogenesis, the entire dTel VZ exhibits ventralization. This ventralization of the dTel VZ is paralleled by the expression of markers for GABA INs superficial to it, suggesting that it ectopically produces GABA INs, leading to their ectopias and a thinner cortical plate due to diminished production of glutamatergic neurons. Genetic lineage tracing demonstrates that the GABA INs comprising the ectopias are from a cortical Emx1 lineage generated in the dTel VZ, definitively showing that dTel progenitors and progeny acquire a ventral, GE, fate in Pax6 mutants. Thus, Pax6 delimits the appropriate proliferative zone for GABA INs and regulates their numbers and distributions by repressing the ventral fates of dTel progenitors and progeny.
Keywords: cortical development, Emx1 lineage, forebrain patterning, medial ganglionic eminence, neuronal specification
The mammalian neocortex is assembled with neurons generated in different regions of the telencephalon (1, 2). Glutamatergic neurons, including all projection neurons, are generated in the neocortical ventricular zone (VZ) of dorsal telencephalon (dTel) and migrate radially to form the cortical plate (CP) (1). GABAergic interneurons (GABA INs) comprise a quarter of neocortical neurons (3). The majority of GABA INs are born in the medial ganglionic eminence (MGE) of the ventral telencephalon (vTel) and migrate tangentially throughout the cortical hemisphere (4-9); a smaller number are generated in caudal GE (10). The lateral GE (LGE) generates GABA INs that populate either the olfactory bulb (OB) (6, 11) or vTel (7). In mice, GABA INs migrating from the MGE enter cortex on embyronic day (E) 13.5 (4, 12) and take two tangential paths: (i) a superficial path in the marginal zone (MZ) and (ii) a deep path in the intermediate zone (IZ). Beginning on E15.5, INs leave their tangential paths and migrate radially into the CP (9, 13).
The paired-box transcription factor Pax6 is expressed by progenitors in the dTel VZ (14-17) and influences the development of cortical lamination (18, 19), projections (20, 21), and areal patterning (22-24). In small eye mutant mice (Pax6sey/sey), which lack functional Pax6 protein (25), progenitors in the dTel VZ have diminished expression of dorsal markers, such as the regulatory genes Ngn2 and Emx1, and acquire expression of markers expressed in the GE, such as the regulatory genes Mash1 and Gsh2 (26-28). An unanswered question is whether ventralization of dTel progenitors in Pax6 mutants results in a respecification of their neuronal progeny to a ventral fate exhibited by GABA INs generated in the GE. To address this issue, we analyzed Pax6sey/sey mice by using markers for region- and neuron type-specific identities and genetic fate-mapping of cells of an Emx1 lineage characteristic of dTel progenitors and their progeny. Our findings show that Pax6 represses ventral fates in the dTel VZ, delimits the proliferative zone for GABA INs, and regulates their numbers and distributions in the telencephalon.
Materials and Methods
Homozygous Pax6 mutants (sey/sey) were obtained from mating heterozygous small eye (sey/+) mice on a C57BL/6J-DBA/2J background and genotyped by eye morphology (25). Pax6sey/sey; Emx1-Cre;R26R mice were generated by breeding Pax6sey/+; Emx1-Cre knockin mice [K. Jones (29)] and Pax6sey/+;R26R mice [P. Soriano (30)]. Animal care was in accordance with institutional guidelines. X-Gal staining was performed as described in ref. 31. In situ hybridization (ISH) was done on Pax6 mutants and their WT littermates by using riboprobes as described in refs. 32 and 33: Emx1 (J. Chun, The Scripps Research Institute, La Jolla, CA); Ngn2 and Mash1 (D. Anderson, Weill Medical College of Cornell University, New York); ErbB4 (C. Lai, The Scripps Research Institute); Dlx1 and Dlx2 (J. Rubenstein, University of California, San Francisco); GAD67 (A. Tobin, University of California, Los Angeles); Gsh2 (M. Goulding, The Salk Institute); Sp8 (ImageClone 5751210); and Lhx6. For BrdUrd labeling, pregnant mice were given a single injection of BrdUrd (40 mg/kg i.p.) at E18.5. Two hours later, embryos were processed for immunohistochemistry (28).
Results
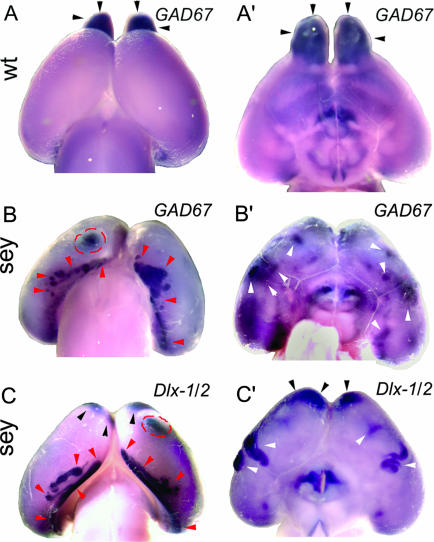
GABA INs Form Ectopias on the Surface of Pax6sey/sey Telencephalon. At E18.5, GABA INs are distributed throughout the cortex (5, 7, 34, 35). We analyzed by whole-mount ISH, E18.5 WT and Pax6 mutants by using markers for GABA INs, GAD67, or a mix of Dlx-1 and Dlx-2 probes (36, 37), the latter of which in WT selectively labels cortical GABA INs generated in the MGE, as well as progenitors throughout the GE (5). In WT, GAD67 (Fig. 1 A and A′) and Dlx-1/2-positive (not shown) cells are not evident from the surface because most are dispersed beneath it. However, GAD67 (Fig. 1 A and A′) and Dlx-1/2 (not shown) expression is seen in the OBs, marking GABA INs that are generated in the LGE and migrate through the rostral migratory stream (6, 11). In contrast with WT, in E18.5 Pax6 mutants, ectopic domains of strong GAD67 and Dlx-1/2 expression are evident on the dorsal (Fig. 1 B and C) and ventrolateral (Fig. 1 B′ and C′) cortical surfaces.
Fig. 1.
Markers specific for GABA INs reveal ectopias on the dorsal and ventral telencephalic surface in E18.5 Pax6sey/sey mice. Whole-mount E18.5 WT and Pax6sey/sey brains were processed for ISH by using digoxigenin-labeled riboprobes for GAD67 or Dlx-1/2. Dorsal (A) and ventral (A′) views of WT brains show GAD67 expression over the neocortex and OBs (black arrowheads). Dorsal (B and C) and ventral (B′ and C′) views of Pax6 mutants show ectopias containing GABA INs marked with GAD67 (B and B′) or Dlx-1/2 (C and C′). Dorsomedial ectopias (red arrowheads), ventrolateral ectopias (white arrowheads), and dorsal neocortical ectopias (red dashed circles) are marked. Black arrowheads mark INs attempting to migrate to the absent OBs.
The most striking ectopias are found caudally along the medial/lateral extent of the cortical hemisphere, near the inflection between posterior neocortex and parahippocampal structures (Fig. 1 B and C), and elsewhere (Fig. 1 B and C). Ectopic domains of strong GAD67 and Dlx-1/2 expression also are seen on the ventrolateral cortical surface, below the nascent rhinal sulcus, typically in piriform cortex (Fig. 1 B′ and C′). GABA INs also are evident rostromedially where the OBs would normally protrude but are absent in Pax6 mutants (Fig. 1 B′ and C).
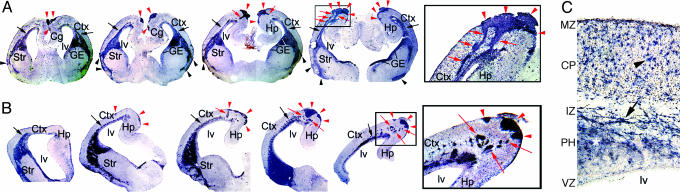
Subpial Ectopias of GABA INs in Pax6 Mutants Are Connected to a Paraventricular Ectopia. Ectopias are observed in each of the 21 E18.5 Pax6 mutants analyzed. To further characterize them, we used an ErbB4 probe, which selectively marks postmitotic GABA INs (38), hybridized to coronal (Fig. 2A) and sagittal (Fig. 2B) sections of E18.5 Pax6sey/sey brains. In WT, ErbB4 marks cells scattered across the CP (data not shown and Fig. 3A). Subpial ectopias of ErbB4 cells are found along the inflection between neocortex and medial cortex and become progressively larger caudally (Fig. 2 A and B). High-power examination indicates that the vast majority of cells in the subpial ectopias express ErbB4 (data not shown). Caudomedially, the ectopias are connected by streams of ErbB4 cells to a dense ectopic accumulation of ErbB4 cells deep in the cortical wall adjacent to the lateral ventricle (Fig. 2 A Inset). This paraventricular ectopia of ErbB4 cells lines the entire lateral ventricle of the neocortex, offset from the ventricular surface (Fig. 2).
Fig. 2.
Surface ectopias in E18.5 Pax6 mutants are subpial and connected to a paraventricular ectopia of GABA INs. Sections from E18.5 Pax6 mutants were processed for ISH by using digoxigenin-labeled riboprobes for ErbB4. (A) Coronal sections show the dorsomedial (red arrowheads) and ventrolateral (black arrowheads) ectopias. Streams of INs (red arrows) connect the paraventricular (black arrows) and dorsomedial ectopias, shown at higher magnification (Inset). (B) Sagittal sections (rostral faces left) show the dorsomedial ectopias. INs positioned between the paraventricular and dorsomedial ectopias are shown at higher magnification (Inset). (C) Higher-power view of cortical wall in an E18.5 Pax6 mutant shows ErbB4-expressing cells (arrowhead) in the CP. The paraventricular ectopia is marked with an asterisk. ErbB4-expressing cells in the IZ have a morphology resembling migrating INs (arrow). Str, striatum; Ctx, cortex; Cg, cingulate cortex; lv, lateral ventricle.
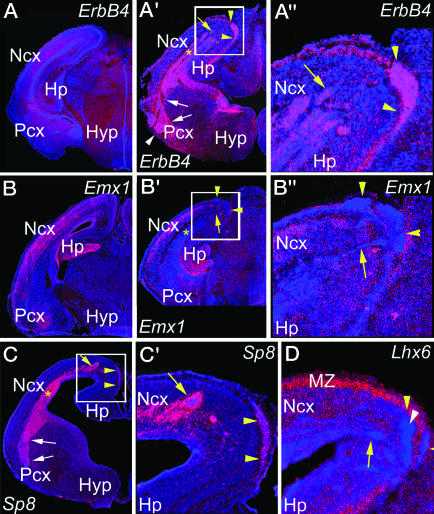
Fig. 3.
Neurons that comprise the ectopias in Pax6 mutants selectively express a marker for GABA INs of a LGE fate but not markers for a MGE fate or for cortical glutamatergic neurons. Coronal sections from E18.5 WT and Pax6 mutants were processed for ISH by using S35-labeled riboprobes, using the indicated markers and were DAPI-stained. Ectopias containing ErbB4-expressing cells (A′ and A″) are seen in Pax6 mutants but not in WT (A). Emx1 labels CP glutamatergic neurons in WT (B) and Pax6 mutants (B′ and B″) but does not mark these ectopias (B′ and B″). Sp8 marks the ectopias (C and C′), whereas Lhx6 is dramatically excluded from the ectopias (D) but is present in the CP and MZ. Boxed regions in the Pax6 mutants are shown at higher magnification (A″, B″, and C′). Streams of GABA INs (yellow arrows) connect the paraventricular ectopia to the dorsomedial ectopias (yellow arrowheads). Streams (white arrows) from the deep paraventricular ectopia (asterisk) also are continuous with the ventrolateral ectopias (white arrowheads). Ncx, neocortex; Pcx, piriform cortex; Hyp, hypothalamus.
In addition to ectopias, ErbB4 cells are distributed in the CP of Pax6 mutants (Fig. 2C) at a density similar to that of WT (data not shown). ErbB4 cells in the CP and the ectopias both have appearances similar to postmigratory INs, whereas ErbB4 cells in the IZ (Fig. 2C), the MZ, and the aberrant streams (data not shown) have elongated morphologies that resemble GABA INs migrating through the rostral migratory stream from the LGE to the OB in WT (5, 7, 9, 13).
The subpial ectopias on the ventrolateral cortical surface are connected to the dTel paraventricular ectopia of ErbB4 cells by a stream of ErbB4 cells originating from the paraventricular ectopia near the junction of neocortex with LGE (Fig. 2 A and B). This aberrant stream reaches the surface of piriform cortex and extends ventrally at or just beneath the surface. Fingers of ErbB4 cells extend superficially from this stream to the surface.
Ectopias Express Markers Characteristic of GABA INs Derived from the LGE. To assess further the ventral vs. dorsal fate of neurons forming the ectopias, we analyzed E18.5 WT and Pax6sey/sey brains by using ErbB4 and Dlx-1/2 as markers for GABA INs normally generated in the GE (5, 38) and Emx1, which marks all glutamatergic neurons of a dTel origin and all progenitors in the dTel VZ (which are depleted by E18.5) (29, 39). In WT, ErbB4 (38) and Dlx-1/2 (5) mark INs distributed across the cortex (Fig. 3A). In Pax6 mutants, the subpial dorsomedial/caudal and ventrolateral ectopias, their connecting streams, and the paraventricular ectopia strongly express ErbB4 (Fig. 3 A′ and A″) and Dlx-1/2 (data not shown, but see Fig. 4). The signal density suggests that the great majority of cells in the ectopias express each of these markers. In contrast, Emx1 is not expressed in the ectopias or their connecting streams (Fig. 3 B′ and B″), although it is robustly expressed in the CP, the parahippocampal formation, and the piriform cortex of Pax6 mutants (Fig. 3 B′ and B″), as in WT (Fig. 3B). Thus, the subpial ectopias, their connecting streams, and the paraventricular ectopia express markers for GABA INs generated in the GE but not a marker for glutamatergic neurons generated in the dTel VZ.
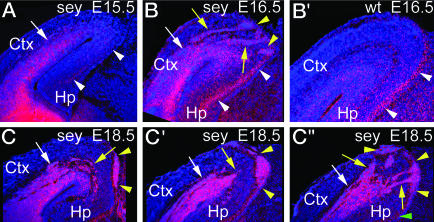
Fig. 4.
Ectopias of GABA INs develop in mid- to late cortical neurogenesis in Pax6 mutants. Coronal sections from E15.5, E16.5 Pax6sey/sey, and E16.5 WT brains were processed for ISH by using S35-labeled Dlx-1/2 riboprobes and were DAPI-stained. Only the paraventricular ectopia of Dlx-1/2-expressing cells is evident at E15.5 (A). By E16.5, Pax6 mutants (B) show large streams (yellow arrows) of Dlx-1/2-positive cells between the paraventricular ectopia and their entry point into the MZ (yellow arrowheads), which are not seen in WT (B′). Tangentially migrating INs in the MZ of the Hp (white arrowheads) are seen in both E16.5 Pax6 mutant (B) and WT (C). Serial coronal sections through an E18.5 Pax6 mutant (C, C′, and C″) show streams of INs that appear to migrate from the neocortex to the pial surface. The green arrowhead marks the location of INs in the Hp MZ (C″).
To determine whether the ectopias are formed by GABA INs of an LGE or MGE fate, we used the zinc finger transcription factor Sp8 (40) as a marker for LGE-derived INs (S. Sahara and D.D.M.O., unpublished observation) and Lhx6 as a marker for MGE-derived INs (4, 41). The expression patterns of Sp8 and Lhx6 in Pax6 mutants strongly complement one another (Fig. 3 C, C′, and D and data not shown). All ectopias and their connecting streams express Sp8, but Sp8 expression is low or nondetectable in the CP and MZ (Fig. 3 C and C′). In contrast, the ectopias and connecting streams do not express Lhx6, although Lhx6-expressing INs are distributed in the CP and MZ of Pax6 mutants in a pattern resembling WT, although at an increased density (Fig. 3D), possibly reflecting an increased migration of MGE-derived INs into Pax6 mutant cortex (42). These analyses indicate that the GABA INs forming the ectopias are of a LGE, rather than a MGE, fate.
Development of GABA IN Ectopias. To determine when and how the ectopias form, we analyzed a developmental progression of WT and Pax6sey/sey brains by using Dlx-1/2 as a probe, because it strongly labels the ectopias (Fig. 1 C and C′). We do not detect subpial ectopias before E17.5. However, at E15.5, a high density of Dlx-1/2-expressing cells is evident in the SVZ and IZ throughout the neocortex in Pax6 mutants (Fig. 4A) and appears to be a nascent form of the paraventricular ectopia. By E16.5, the paraventricular ectopia is clearly defined in Pax6 mutants, and distinct streams of Dlx-1/2 cells extend from it and pass through the CP to the pial surface (Fig. 4B); the ectopia and streams are not evident in WT (Fig. 4B′). The density of Dlx-1/2 labeling in the MZ of the hippocampal formation in Pax6 mutants (Fig. 4B) is similar to that in WT (Fig. 4B′), suggesting that MGE-derived INs distribute normally in Pax6 mutants. At E18.5, streams of Dlx-1/2 cells connecting the paraventricular ectopia to the dorsomedial/caudal subpial ectopias are present over a more limited rostrocaudal extent than the subpial ectopias (Fig. 4 C, C′, and C″ and data not shown). These findings indicate that GABA INs take a limited migration path from the paraventricular ectopia to the subpial ectopias, which expand subpially as more cells feed into them. BrdUrd pulse-labeling confirms that cells expressing markers for postmitotic GABA INs in the ectopias are nonproliferative (data not shown).
Relating Progressive Ventralization of the dTel VZ in Pax6 Mutants to the Ectopias. Our marker analyses and the development of the ectopias suggest that progenitors in the dTel VZ become respecified in Pax6 mutants leading to a fate change in their progeny from glutamatergic CP neurons to GABA INs. Previous studies report that the dTel VZ in Pax6 mutants exhibits marker changes consistent with this model (16, 26, 27) but have not effectively related it to a progressive fate change in its progeny. We addressed this issue by using a marker analysis to relate a potential respecification of progenitors in the dTel VZ to the fate of their progeny, focusing on ages ranging from E12.5, when the generation of CP neurons begins, to E14.5, the day before we detect a dense paraventricular accumulation of GABA INs reflecting a nascent paraventricular ectopia. Our findings at E12.5 and E13.5 are similar; therefore, we mainly present E13.5 data and compare it with E14.5 data.
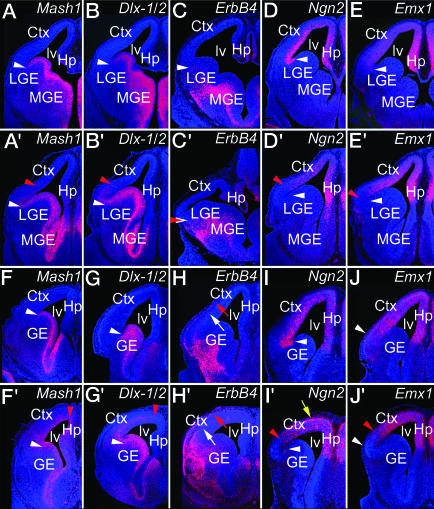
Progenitors in the GE express Mash1 (Fig. 5A), Dlx-1/2 (Fig. 5B) (43), and Gsh2 (data not shown) (27) with a sharp border on the lateral edge of the LGE and low or nondetectable expression in the dTel VZ (44). In E13.5 WT, the GABA IN markers, Dlx-1/2 (Fig. 5B), GAD67 (data not shown), and ErbB4 (E12.5, Fig. 5C), are restricted to the GE or its progeny but show differences in laminar distributions: GAD67 (not shown) and ErbB4 (Fig. 5C) are limited to the mantle zone of vTel that contains postmitotic neurons generated in the GE, whereas Dlx-1/2 is expressed in both the mantle zone and GE (Fig. 5B). In E13.5 Pax6 mutants, the expression border between the LGE and dTel VZ is blurred because expression of Mash1 (Fig. 5A′) and Gsh2 (data not shown) extends a modest distance dorsally into the dTel VZ. Expression of Dlx-1/2 (Fig. 5B′) and GAD67 (data not shown) also exhibits a modest dorsal extension in Pax6 mutants that parallels but is mainly superficial to that of Mash1 and Gsh2. In contrast, no difference is evident in ErbB4 expression between E12.5 WT and Pax6 mutants (Fig. 5 C and C′).
Fig. 5.
Progenitors in the dTel VZ of Pax6 mutants undergo rapid ventralization between E13.5 and E14.5. Coronal sections of E13.5 and E14.5 WT and Pax6 mutant brains were processed for ISH by using S35-labeled riboprobes for the indicated markers and were DAPI-stained. A, B, D, and E and A′, B′, D′, and E′ are from E13.5 WT and Pax6 mutants, respectively; C and C′ are from E12.5 WT and Pax6 mutants; and F-J and F′-J′ are from E14.5 WT and Pax6 mutants, respectively. White arrowheads mark the expression boundary between WT vTel and dTel, whereas red arrowheads mark the shifted marker expression boundary in Pax6 mutants. Yellow arrow in I′ marks aberrant superficial expression of Ngn2. The two normal migratory streams of ErbB4-expressing cells, through the MZ (red arrow) and IZ (white arrow), are seen at E14.5 in both WT and Pax6 mutants (H′).
In E13.5 WT, the dTel markers Ngn2 and Emx1 are expressed in the dTel VZ (Fig. 5 D and E) and form a sharp border with the expression of Mash1 and Dlx-1/2 in the GE (Fig. 5 A and B). In contrast, in E13.5 Pax6 mutants, Ngn2 and Emx1 expression (Fig. 5 D′ and E′) in ventrolateral dTel is coincident with the dorsal extension in Mash1 and Dlx-1/2 expression (Fig. 5 A′ and B′), resulting in an overlap in the two sets of markers and a blurring of the normally sharp expression border.
E14.5 Pax6 mutants exhibit substantial differences in expression of vTel markers, compared with both E13.5 mutants and E14.5 WT. In E14.5 WT, Mash1 expression is limited to the GE (Fig. 5F), whereas in Pax6 mutants, it is robustly expressed throughout the dTel VZ (Fig. 5F′). Dlx-1/2 also shows a prominent expansion. In E14.5 WT, Dlx-1/2 expression is limited to the GE and deeper parts of the mantle zone (Fig. 5G), whereas in Pax6 mutants, Dlx-1/2 expression extends throughout most of dTel (Fig. 5G′). In both vTel and dTel of Pax6 mutants, Mash1 expression is limited to the proliferative zones, the GE and VZ, respectively (Fig. 5F′). In contrast, in vTel of Pax6 mutants, Dlx-1/2 is expressed strongest in GE, but in dTel, its expression is strongest superficial to the VZ (Fig. 5G′), in a distribution resembling the nascent paraventricular ectopia at E15.5 (Fig. 4A). At E14.5, ErbB4 expression is similar in WT and Pax6 mutants (Fig. 5 H and H′). In both, ErbB4 cells emanate from the vTel mantle zone and migrate into the dTel along their normal paths in the MZ and IZ, again suggesting the generation of a normal population of GABA INs in the MGE that distribute to the CP (Fig. 2C).
In E14.5 Pax6 mutants, the expression of dTel markers Ngn2 (Fig. 5 I and I′) and Emx1 (Fig. 5 J and J′) in ventrolateral dTel exhibits a modest dorsal shift. However, Ngn2 has an aberrant laminar pattern: In E14.5 WT, Ngn2 is most highly expressed in the VZ, whereas in Pax6, mutant cortex Ngn2 expression is strongest superficially.
These findings show that the dTel VZ undergoes a progressive ventralization: Only the most ventrolateral aspect exhibits ventral characteristics at E12.5 and E13.5, whereas most if not all of dTel VZ expresses ventral markers at E14.5. The dorsal shift in Dlx-1/2 expression from E12.5 to E14.5 in Pax6 mutants and its ectopic domain of enhanced paraventricular expression at E14.5 parallel the dorsal extension in the dTel VZ of markers normally expressed by progenitors in the GE (Mash1,Gsh2), whereas ErbB4 expression resembles that observed in WT. These findings suggest that as the dTel VZ progressively becomes ventralized, it begins to generate cells that express markers for GABA INs, with the possible exception of ErbB4, which might have a late onset.
dTel VZ Progenitors in Pax6 Mutants Are Respecified to Generate GABA INs. To demonstrate definitively that the ectopias of GABA INs are generated by progenitors in the dTel VZ, we determined whether the ectopias are of an Emx1 lineage that characterizes all dTel progenitors and their glutamatergic neuronal progeny (29, 39). Neurons of an Emx1 lineage are selectively marked in Emx1-Cre; R26R heterozygous mice, in which all dTel progenitors and their CP progeny are permanently labeled by a β-gal reporter, whereas progenitors in the GE and their progeny, including GABA INs, are not labeled (29). Therefore, we performed a triple cross using Pax6sey/+, Emx1-Cre knock-in, and R26R mice (30).
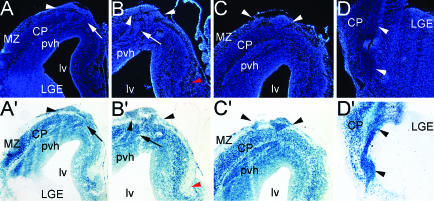
Three E18.5 brains homozygous for the sey allele (Pax6sey/sey) and heterozygous for Emx1-Cre and R26R were analyzed; all exhibited the same phenotype and pattern of reporter expression. During processing of whole mounts for X-Gal histochemistry (n = 2; data not shown), the subpial ectopias become selectively marked on the surface of hemispheres before they are masked by intense staining of the deeper CP. Three additional hemispheres were sectioned and then processed for X-Gal histochemistry. In addition to the CP, all three types of ectopias described above to express markers for GABA INs (Figs. 1, 2, 3, 4) but not the CP marker Emx1 (Fig. 3) are strongly β-gal-positive, with essentially all DAPI-stained cells expressing the β-gal reporter (Fig. 6). In contrast, as in WT (data not shown) (29), the LGE (Fig. 6) and MGE (data not shown) are β-gal-negative in the triple-crossed brains, indicating that LGE and MGE progenitors and their progeny remain Emx1-negative in these mutants. Consistent with this finding, in Pax6 mutants, as in WT, the MZ contains significant numbers of cells that express markers for MGE-derived GABA INs but a relatively low density of β-gal-positive cells (Fig. 6). These findings conclusively demonstrate that the GABA IN ectopias are derived from progenitors in the dTel VZ of an Emx1 lineage that normally produce Emx1-expressing glutamatergic CP neurons, but during mid-stages of cortical neurogenesis, these dTel progenitors undergo a fate change, become ventralized, and produce GABA INs.
Fig. 6.
GABA INs that form ectopias in Pax6 mutants are of an Emx1 lineage and generated by dTel progenitors. Coronal sections from an E18.5 Pax6sey/sey;Emx1-Cre;R26R brain processed by X-Gal histochemistry to reveal β-gal-labeled cells of an Emx1 lineage and DAPI-stained. DAPI reveals the paraventricular heterotopia (pvh), dorsal ectopia (arrowheads, A-C), and cell stream migrating toward the ventrolateral ectopia (D). β-gal reporter densely labels cells in the CP and in each of the ectopias (A′-D′). The LGE, which never expresses Emx1, does not contain an appreciable number of β-gal cells (A′ and D′). (A-C and A′-C′) Arrows mark cell streams connecting the pvh to the dorsal subpial ectopia. (D and D′) Arrowheads mark the cell streams extending from the pvh to the ventrolateral ectopias. Red arrowheads (B and B′) denote the MZ of the Hp.
Discussion
Our findings indicate that in the telencephalon, Pax6 represses the generation of GABA INs in the dTel VZ and influences genetic mechanisms that restrict the appropriate proliferative zone for GABA INs to the GE. Our marker analyses with region- and neuron type-specific markers show that the telencephalon of perinatal Pax6sey/sey mutants has substantially more postmitotic cells that express markers for GABA INs: GAD67, ErbB4, and Dlx1/2. These markers also reveal large, subpial ectopias of GABA INs at reproducible locations in dTel and vTel, connected by streams of GABA INs to a paraventricular ectopia of GABA INs adjacent to the lateral ventricle throughout the cortex. These ectopias express a marker specific to GABA INs generated in the LGE (Sp8) but not a marker for INs originating in the MGE (Lhx6). Although the ectopias do not express the dTel marker Emx1, genetic fate-mapping reveals that they originate from the Emx1 dTel lineage rather than an overproduction and aberrant migration of GABA INs derived from the LGE (or MGE). Our findings show that the ectopias of GABA INs are due to a progressive ventralization of the dTel VZ and a concurrent change in the specification of dTel progenitors neurons from a dTel fate to a vTel fate, resulting in their generation of GABA INs normally generated in the LGE.
Pax6 has been implicated in regulating dorsal/ventral regional identity of proliferative zones in the telencephalon (26-28, 45). In contrast with our study, these studies have focused on earlier developmental stages and have interpreted a dorsal shift in GABA IN markers in Pax6 mutants at E14 as being due to an exuberant migration from an expanded MGE (26, 28), possibly due in part to a breakdown in the dTel/vTel boundary (46), although Torreson et al. (27) suggest the contribution of a fate change in dTel progenitors. The generation of glutamatergic CP neurons in the dTel VZ occurs mainly from E12.5 to E16.5 (47), as does the generation of cortical GABA INs in the MGE (1, 48-50). Early in cortical neurogenesis, E12.5 and E13.5, the pattern of marker expression in Pax6 mutants shows a modest dorsal extension of GE markers into the dTel VZ (present study and refs. 26 and 27). However, the dorsal extension of ventral markers increases substantially by E14.5, such that essentially the entire dTel VZ expresses GE markers, including Mash1 and Dlx-1/2 (present study and ref. 27), and produces GABA INs of a vTel fate (present study). These findings are consistent with data showing that ectopic Mash1 expression in the dTel can drive production of cells with a vTel fate (51). Interestingly, in the human neocortex, a subset of progenitors in the dTel VZ normally expresses Mash1 and produces GABA INs that are Mash1- and Dlx-1/2-positive (52).
At E14.5 and earlier, the expression pattern of ErbB4 in Pax6 mutants resembles WT and suggests that a normal population of GABA INs are generated in the MGE of Pax6 mutants and migrate tangentially into the cortex along their normal paths. At later ages in Pax6 mutants, we find GABA INs that express ErbB4, Dlx-1/2, and Lhx6 in the CP and MZ in a WT-like distribution and are likely those generated in the MGE, suggesting that the breakdown of the dTel/vTel boundary does not grossly affect the tangential migration of GABA INs generated in the MGE.
Our findings indicate that at early stages of cortical neurogenesis, E12.5 and E13.5, only the ventrolateral dTel VZ, which abuts the LGE, is ventralized and ectopically produces GABA INs. However, by E14.5 and later, the dTel VZ ectopically generates GABA INs in substantial numbers throughout its extent and has a diminished production of glutamatergic CP neurons, an interpretation consistent with complementary lines of data. First, the aberrant distributions of GABA INs develops progressively after the dTel VZ broadly expresses vTel markers: A dense paraventricular ectopia is first clearly evident on E15.5 (although Dlx-1/2 expression suggests that it begins to form as early as E14.5), the aberrant streams leading from the paraventricular ectopia to the pial surface are not evident until E16.5, and the subpial ectopias subsequently develop from the streams. Second, as we show in Pax6 mutants, the subpial ectopias, the paraventricular ectopia, and the streams that connect them, express markers for GABA INs but not for glutamatergic CP neurons, although a CP develops and expresses markers for glutamatergic neurons of a dTel origin. Third, all of the ectopias and their connecting streams strongly express a β-gal reporter in E18.5 Pax6sey/sey;Emx1-Cre;R26R mutants, providing definitive confirmation that the GABA INs that comprise them are derived from an Emx1 lineage and are generated by dTel progenitors rather than by GE progenitors, which are not of an Emx1 lineage in either WT or Pax6 mutants. GABA INs of an Emx1 lineage are clearly the predominant cell type that form the ectopias, but we cannot exclude that a small number of other cell types are present.
Our conclusions are consistent with the findings of Caric et al. (18) in Pax6 mutants and provide an explanation. They describe that the thinner CP in Pax6 mutants is largely due to a failure of most neurons generated after E13.5 to migrate into the CP; instead, they accumulate deep in the cortical wall in a layer that they define as an aberrantly thick VZ/SVZ (18). Our findings indicate that the thinner CP and defective migration of the later-generated neurons in Pax6 mutants are due to a change in the fate of these neurons from a glutamatergic CP neuron to a GABA IN, reflecting the progressive and late change from a dorsal to a ventral fate of their dTel progenitors; we conclude that the late-generated CP neurons, defined by BrdUrd birthdating by Caric et al. (18) as defective in their migration and “stuck” in the VZ/SVZ in Pax6 mutants, are the GABA INs that we have identified to form the paraventricular ectopia.
The late onset of the extension of ventral markers into the dTel VZ of Pax6 mutants (16, 27, 53) indicates that Pax6 does not act alone in specifying the dTel or in maintaining its boundary with vTel (16, 27, 53). Our findings confirm and extend this interpretation and demonstrate that Pax6 is required to maintain the dorsal fate of progenitors in the dTel VZ and of their glutamatergic neuronal progeny that form the CP. However, we find that, as a population, progenitors in the dTel VZ of Pax6 mutants exhibit an incomplete ventralization, indicated by the persistent, albeit altered, expression of dorsal markers, such as Emx1 and Ngn2 at E14.5, coincident with the expression of ventral markers. Therefore, it is possible that individual progenitors in the dTel VZ express both dTel and vTel markers, perhaps resulting in the generation of aberrant cell types that do not properly migrate or differentiate. An alternative conclusion is that, in Pax6 mutants, distinct subsets of dTel progenitors either acquire a ventral fate or retain a dorsal fate, with the former respecified to generate GABA INs and the latter continuing to generate glutamatergic CP neurons. This mixed dTel/vTel phenotype of the dTel VZ is consistent with the continued but decreased number of superficial layer CP neurons generated after E13.5 in Pax6 mutants (18), and also is consistent with our findings that CP neurons in Pax6 mutants are of an Emx1 lineage and express Emx1, as in WT, whereas the GABA IN ectopias do not express Emx1 but are of an Emx1 dTel lineage and are generated by progenitors that transiently expressed Emx1 at an earlier stage before their ventralization.
Note Added in Proof. After the original submission of this paper, a study by Schuurmans et al. (54) provides evidence suggesting a late respecification of dTel progenitors in Pax6sey/sey mutants to produce Dlx1-positive progeny, consistent with our demonstration that they undergo a fate change and produce GABA INs.
Acknowledgments
We thank S.-J. Chou, T. Hamasaki, H. Li, and S. Sahara for advice; K. Jones for generously providing Emx1-Cre mice; and P. Soriano for generously providing R26R mice. This work was supported by National Institutes of Health Grant R37NS31558 (to D.D.M.O.) and a fellowship (to T.T.K.) from National Institutes of Health Grant T32AG00216.
Author contributions: T.T.K. and D.D.M.O. designed research; T.T.K. performed research; T.T.K. and D.D.M.O. analyzed data; and T.T.K. and D.D.M.O. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: VZ, ventricular zone; dTel, dorsal telencephalon; En, embryonic day n; GE, ganglionic eminence; MGE, medial GE; LGE, lateral GE; IN, interneuron; CP, cortical plate; OB, olfactory bulb; IZ, intermediate zone; MZ, marginal zone; ISH, in situ hybridization.
References
- 1.Nadarajah, B. & Parnavelas, J. G. (2002) Nat. Rev. Neurosci. 3, 423-432. [DOI] [PubMed] [Google Scholar]
- 2.Marin, O. & Rubenstein, J. L. R. (2003) Annu. Rev. Neurosci. 26, 441-483. [DOI] [PubMed] [Google Scholar]
- 3.Gonchar, Y. & Burkhalter, A. (1997) Cereb. Cortex 7, 347-358. [DOI] [PubMed] [Google Scholar]
- 4.Lavdas, A. A., Grigoriou, M., Pachnis, V. & Parnavelas, J. G. (1999) J. Neurosci. 19, 7881-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson, S. A., Eisenstat, D. D., Shi, L. & Rubenstein, J. L. R. (1997) Science 278, 474-476. [DOI] [PubMed] [Google Scholar]
- 6.Wichterle, H., Turnbull, D. H., Nery, S., Fishell, G. & Alvarez-Buylla, A. (2001) Development (Cambridge, U.K.) 128, 3759-3771. [DOI] [PubMed] [Google Scholar]
- 7.De Carlos, J. A., Lopez-Mascaraque, L. & Valverde, F. (1996) J. Neurosci. 16, 6146-6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamamaki, N., Yanagawa, Y., Tomioka, R., Miyazaki, J., Obata, K. & Kaneko, T. (2003) J. Comp. Neurol. 467, 60-79. [DOI] [PubMed] [Google Scholar]
- 9.Ang, E. S., Jr., Haydar, T. F., Gluncic, V. & Rakic, P. (2003) J. Neurosci. 23, 5805-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nery, S., Fishell, G. & Corbin, J. G. (2002) Nat. Neurosci. 5, 1279-1287. [DOI] [PubMed] [Google Scholar]
- 11.Corbin, J. G., Gaiano, N., Machold, R. P., Langston, A. & Fishell, G. (2000) Development (Cambridge, U.K) 127, 5007-5020. [DOI] [PubMed] [Google Scholar]
- 12.Stuhmer, T., Anderson, S. A., Ekker, M. & Rubenstein, J. L. R. (2002) Development (Cambridge, U.K.) 129, 245-252. [DOI] [PubMed] [Google Scholar]
- 13.Nadarajah, B., Alifragis, P., Wong, R. O. & Parnavelas, J. G. (2003) Cereb. Cortex 13, 607-611. [DOI] [PubMed] [Google Scholar]
- 14.Walther, C. & Gruss, P. (1991) Development (Cambridge, U.K.) 113, 1435-1449. [DOI] [PubMed] [Google Scholar]
- 15.Stoykova, A. & Gruss, P. (1994) J. Neurosci. 14, 1395-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoykova, A., Fritsch, R., Walther, C. & Gruss, P. (1996) Development (Cambridge, U.K.) 122, 3453-3465. [DOI] [PubMed] [Google Scholar]
- 17.Gotz, M., Stoykova, A. & Gruss, P. (1998) Neuron 21, 1031-1044. [DOI] [PubMed] [Google Scholar]
- 18.Caric, D., Gooday, D., Hill, R. E., McConnell, S. K. & Price, D. J. (1997) Development (Cambridge, U.K.) 124, 5087-5096. [DOI] [PubMed] [Google Scholar]
- 19.Warren, N. & Price, D. J. (1997) Development (Cambridge, U.K.) 124, 1573-1582. [DOI] [PubMed] [Google Scholar]
- 20.Jones, L., Lopez-Bendito, G., Gruss, P., Stoykova, A. & Molnar, Z. (2002) Development (Cambridge, U.K.) 129, 5041-5052. [DOI] [PubMed] [Google Scholar]
- 21.Molnar, Z., Higashi, S. & Lopez-Bendito, G. (2003) Cereb. Cortex 13, 661-669. [DOI] [PubMed] [Google Scholar]
- 22.Bishop, K. M., Goudreau, G. & O'Leary, D. D. M. (2000) Science 288, 344-349. [DOI] [PubMed] [Google Scholar]
- 23.Bishop, K. M., Rubenstein, J. L. R. & O'Leary, D. D. M. (2002) J. Neurosci. 22, 7627-7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muzio, L. & Mallamaci, A. (2003) Cereb. Cortex 13, 641-647. [DOI] [PubMed] [Google Scholar]
- 25.Hill, R. E., Favor, J., Hogan, B. L., Ton, C. C., Saunders, G. F., Hanson, I. M., Prosser, J., Jordan, T., Hastie, N. D. & van Heyningen, V. (1991) Nature 354, 522-525. [DOI] [PubMed] [Google Scholar]
- 26.Stoykova, A., Treichel, D., Hallonet, M. & Gruss, P. (2000) J. Neurosci. 20, 8042-8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toresson, H., Potter, S. S. & Campbell, K. (2000) Development (Cambridge, U.K.) 127, 4361-4371. [DOI] [PubMed] [Google Scholar]
- 28.Yun, K., Potter, S. & Rubenstein, J. L. R. (2001) Development (Cambridge, U.K.) 128, 193-205. [DOI] [PubMed] [Google Scholar]
- 29.Gorski, J. A., Talley, T., Qiu, M., Puelles, L., Rubenstein, J. L. R. & Jones, K. R. (2002) J. Neurosci. 22, 6309-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soriano, P. (1999) Nat. Genet. 21, 70-71. [DOI] [PubMed] [Google Scholar]
- 31.Stuhmer, T., Puelles, L., Ekker, M. & Rubenstein, J. L. R. (2002) Cereb. Cortex 12, 75-85. [DOI] [PubMed] [Google Scholar]
- 32.Liu, Q., Dwyer, N. D. & O'Leary, D. D. M. (2000) J. Neurosci. 20, 7682-7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa, Y., Johnson, J. E. & O'Leary, D. D. M. (1999) J. Neurosci. 19, 10877-10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mione, M. C., Cavanagh, J. F., Harris, B. & Parnavelas, J. G. (1997) J. Neurosci. 17, 2018-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamamaki, N., Fujimori, K. E. & Takauji, R. (1997) J. Neurosci. 17, 8313-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman, D. L., Houser, C. R. & Tobin, A. J. (1991) J. Neurochem. 56, 720-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson, S. A., Qiu, M., Bulfone, A., Eisenstat, D. D., Meneses, J., Pedersen, R. & Rubenstein, J. L. R. (1997) Neuron 19, 27-37. [DOI] [PubMed] [Google Scholar]
- 38.Yau, H. J., Wang, H. F., Lai, C. & Liu, F. C. (2003) Cereb. Cortex 13, 252-264. [DOI] [PubMed] [Google Scholar]
- 39.Chan, C. H., Godinho, L. N., Thomaidou, D., Tan, S. S., Gulisano, M. & Parnavelas, J. G. (2001) Cereb. Cortex 11, 1191-1198. [DOI] [PubMed] [Google Scholar]
- 40.Bell, S. M., Schreiner, C. M., Waclaw, R. R., Campbell, K., Potter, S. S. & Scott, W. J. (2003) Proc. Natl. Acad. Sci. USA 100, 12195-12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grigoriou, M., Tucker, A. S., Sharpe, P. T. & Pachnis, V. (1998) Development (Cambridge, U.K.) 125, 2063-2074. [DOI] [PubMed] [Google Scholar]
- 42.Chapouton, P., Gartner, A. & Gotz, M. (1999) Development (Cambridge, U.K.) 126, 5569-5579. [DOI] [PubMed] [Google Scholar]
- 43.Guillemot, F. & Joyner, A. L. (1993) Mech. Dev. 42, 171-185. [DOI] [PubMed] [Google Scholar]
- 44.Fode, C., Ma, Q., Casarosa, S., Ang, S. L., Anderson, D. J. & Guillemot, F. (2000) Genes Dev. 14, 67-80. [PMC free article] [PubMed] [Google Scholar]
- 45.Muzio, L., DiBenedetto, B., Stoykova, A., Boncinelli, E., Gruss, P. & Mallamaci, A. (2002) Cereb. Cortex 12, 129-139. [DOI] [PubMed] [Google Scholar]
- 46.Stoykova, A., Gotz, M., Gruss, P. & Price, J. (1997) Development (Cambridge, U.K.) 124, 3765-3777. [DOI] [PubMed] [Google Scholar]
- 47.Gillies, K. & Price, D. J. (1993) Eur. J. Neurosci. 5, 73-84. [DOI] [PubMed] [Google Scholar]
- 48.Miller, M. W. (1985) Brain Res. 355, 187-192. [DOI] [PubMed] [Google Scholar]
- 49.Cavanagh, M. E. & Parnavelas, J. G. (1988) J. Comp. Neurol. 268, 1-12. [DOI] [PubMed] [Google Scholar]
- 50.DeDiego, I., Smith-Fernandez, A. & Fairen, A. (1994) Eur. J. Neurosci. 6, 983-997. [DOI] [PubMed] [Google Scholar]
- 51.Parras, C. M., Schuurmans, C., Scardigli, R., Kim, J., Anderson, D. J. & Guillemot, F. (2002) Genes Dev. 16, 324-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Letinic, K., Zoncu, R. & Rakic, P. (2002) Nature 417, 645-649. [DOI] [PubMed] [Google Scholar]
- 53.Stenman, J., Yu, R. T., Evans, R. M. & Campbell, K. (2003) Development (Cambridge, U.K.) 130, 1113-1122. [DOI] [PubMed] [Google Scholar]
- 54.Schuurmans, C., Armant, O., Nieto, M., Stenman, J. M., Britz, O., Klenin, N., Brown, C., Langevin, L. M., Seibt, J., Tang, H., et al. (2004) EMBO J. 23, 2892-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]