Abstract
Two nasopharyngeal carcinoma (NPC) cell lines and one keratinocyte cell line could be infected with Epstein-Barr virus (EBV) by cocultivation with virus-producing cells but not by cell-free virus. Using porous culture inserts to manipulate the cell-to-cell contact, we demonstrated that contact between EBV donor B cells and EBV recipient epithelial cells was required for the infection. Cell-to-cell contact not only provided a CR2-independent route of infection but also enhanced CR2-mediated infection in a synergistic manner. Activity of two EBV promoters (Cp and Wp) and expression of EBNA2 were detected in the infected population. A small proportion of the infected cells spontaneously entered an EBV lytic state, which could be induced prominently by chemical treatment. This study provides information on how EBV may infect epithelial cells in vivo, such as at the onset of NPC development.
Epstein-Barr virus (EBV) is associated, both serologically and histologically, with nasopharyngeal carcinoma (NPC), a human malignant tumor derived from nasopharyngeal epithelial cells (19). EBV is present in NPC tumor cells and preinvasive lesions but is absent from normal nasopharyngeal epithelial cells, which suggests that EBV infects epithelial cells at an early stage of NPC development (5, 17, 22). However, the infection mechanism remains unclear and is difficult to study in vitro because most established NPC cell lines are EBV negative and were not susceptible to EBV infection in previous studies.
In contrast, EBV-positive cell lines can be established readily by culturing cells from Burkitt’s lymphoma (BL), another EBV-associated tumor originating from B-lymphoid cells (19). Furthermore, EBV can infect B cells efficiently in vitro, using CD21, also called CR2, as its receptor (12). On the other hand, CR2 expression in epithelial cells remains controversial, and it has been proposed that EBV may infect epithelial cells via a CR2-independent process (24).
Recently, a recombinant EBV carrying a neomycin-resistant marker (designated rAkata EBV) has been generated from wild-type EBV strain Akata (designated wtAkata EBV) (23). This system is a useful model for studying EBV infection of epithelial cells because the rAkata EBV-infected cells can be selected with G418, even when the infection efficiency is low (35). It has been reported that rAkata EBV could infect some epithelial cells which expressed little or no CR2 (11, 35). Of note, higher infection efficiency was observed when target epithelial cells were cocultivated with virus-producing B cells, and it has been implied indirectly that cell-to-cell contact may facilitate efficient infection (11). On the other hand, cell-free EBV particles could infect epithelial cells exhibiting endogenous CR2 expression or following transfection of CR2 cDNA, representing a cell contact-independent route of infection (4, 7, 14).
In this study, we examined the roles of cell-to-cell contact and CR2 in EBV infection of NPC cells and keratinocytes. It was demonstrated directly that cell-to-cell contact is required for infection with various EBV strains, virus donor B cells, or virus recipient epithelial cells. Cell-to-cell contact not only provided a CR2-independent route of infection but also enhanced CR2-mediated infection in a synergistic manner. In addition, we observed a unique pattern of EBV gene expression and an inducible viral lytic state in the infected population.
EBV infection of epithelial cells requires cell-to-cell contact.
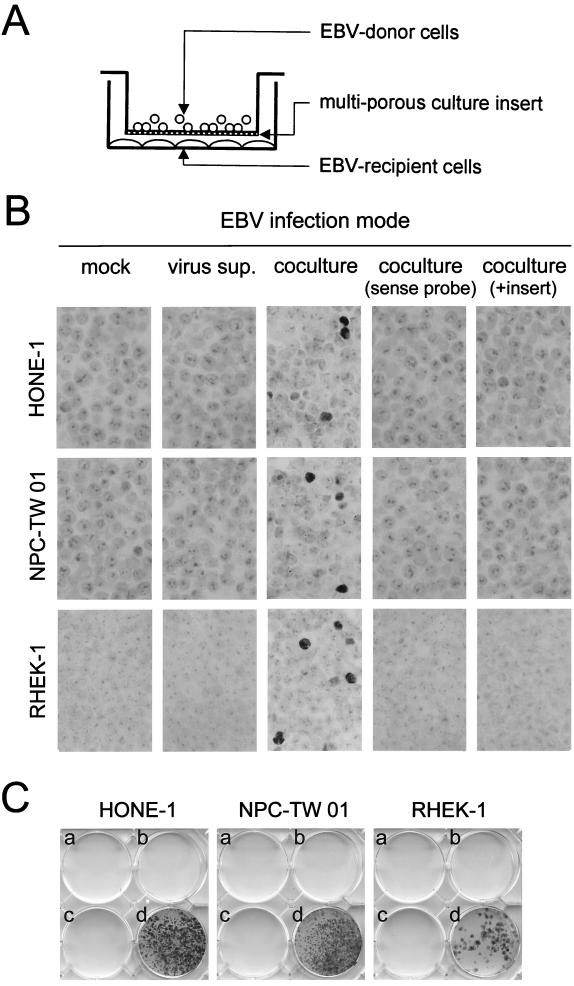
Two NPC cell lines, HONE-1 (10) and NPC-TW 01 (15), and a human keratinocyte cell line, RHEK-1 (18), were used as target epithelial cells for EBV infection (the three cell lines are EBV negative in culture). One day before infection, the target epithelial cells were seeded into six-well culture plates (2 × 105 cells/well). For preparation of virus donors, rAkata EBV-harboring BL cells (with an initial culture density of 2.5 × 105 cells/ml) were activated with 0.8% (vol/vol) rabbit anti-human immunoglobulin G (IgG) (Cappel, Aurora, Ohio) to induce lytic virus production. We carried out EBV infection using two procedures modified from a previous study (11). For infection by cell-free virus particles, virus-containing supernatants from 2-day cultures of activated rAkata BL cells were filtered through a 0.45-μm-pore-size filter and then applied (8 ml/well) to the epithelial cells at 37°C for 24 h. For infection by cocultivation, suspensions of 1-day-activated rAkata BL cells (2.5 × 105 cells/ml) were applied directly (8 ml/well) to the epithelial cells and incubated at 37°C for 24 h. After infection, the epithelial cells were washed thoroughly four or five times with phosphate-buffered saline (PBS), to remove residual virus or BL cells, and then refed with fresh medium.
On day 3 postinfection, the infection efficiency was determined by in situ hybridization to detect the EBV-encoded small RNA EBER1 (5). Figure 1B shows that no EBER1 signal was detected in HONE-1, NPC-TW 01, or RHEK-1 cells when they were mock infected or incubated with cell-free virus, and the absence of EBV DNA from these cells was confirmed by a PCR assay (data not shown). In contrast, EBER1 was detected clearly in the epithelial cells following cocultivation with rAkata EBV-producing BL cells (Fig. 1B). The three cell lines could be infected with similar efficiencies (1 to 5%) on day 3 postinfection. Since no positive signal was detected when sense probes were applied, the EBER1 signal could not be attributable to hybridization to transcriptionally inactive EBV DNA (Fig. 1B). The epithelial characteristics of these EBER1-positive cells were confirmed by double staining of cytokeratins (data not shown). It was unlikely that EBER1-positive cells resulted from fusion between BL cells and epithelial cells because, using a PCR-based analysis of genomic polymorphism, no BL cell-specific allele was detected in the infected cells (data not shown).
FIG. 1.
Requirement for cell-to-cell contact in EBV infection of epithelial cells. (A) Schematic representation of EBV infection by cocultivation with culture inserts. Virus donor B cells were cocultivated with virus recipient epithelial cells in a ratio of 10:1. In the experiment to test the requirement for cell-to-cell contact, a multiporous culture insert was used to prevent contact between the two cell types. (B) Cell contact-dependent EBV infection of epithelial cells. Two NPC cell lines (HONE-1 and NPC-TW 01) and a keratinocyte cell line (RHEK-1) were infected with rAkata EBV by different modes: mock infection (medium only), infection by cell-free virus supernatants (sup.), or infection by cocultivation with or without culture inserts. The details of infection procedures are given in the text. On day 3 postinfection, infection efficiency was determined by EBER1 in situ hybridization modified from a previous study (5). Briefly, epithelial cells were pelleted and processed into a paraffin block. Block sections were subjected to digestion with 20 μg of proteinase K per ml at 50°C for 10 min and hybridized with digoxigenin-labeled oligonucleotide probes (antisense probe, 5′-CTCCTCCCTAGCAAAACCTCTAGGGCAGCG-3′; sense probe, 5′-CGCTGCCCTAGAGGTTTTGCTAGGGAGGAG-3′). The hybridized signals (dark staining in nuclei) were detected with a chromogenic detection kit (Boehringer Mannheim, Mannheim, Germany). EBER1 sense probes were used to confirm the hybridization specificity. (C) Selection of rAkata EBV-infected epithelial cells. Three epithelial cell lines were mock infected (a), infected by cell-free virus (b), infected by cocultivation with culture inserts (c), or infected by cocultivation without culture inserts (d). On day 2 postinfection, G418 (600 μg/ml) was added into the cultures and changed every 2 to 3 days. After treatment for 3 weeks, drug-resistant cell colonies were fixed with 10% acetic acid–10% methanol and then stained with 0.4% crystal violet.
EBV infection of these epithelial cells was achieved by cocultivation but not by cell-free virus, suggesting that cell-to-cell contact may be required for infection. Alternatively, the failure of infection by cell-free virus may simply reflect the fact that some essential factors are labile and are inactivated after virus preparation. To test the necessity for cell-to-cell contact, we included a multiporous culture insert during infection by cocultivation. The culture insert (Becton Dickinson, Franklin Lakes, N.J.) provides a porous membrane (108 pores/cm2, 0.4-μm pore size) which is permeable to virus particles and soluble factors but which prevents contact between EBV donor B cells and EBV recipient epithelial cells on opposite sides of the membrane (Fig. 1A). Figure 1B shows that blocking cell-to-cell contact with culture inserts prevented rAkata EBV infection of HONE-1, NPC-TW 01, and RHEK-1 cells completely. The blocking effect of culture inserts was observed even when the infection was performed for a longer period or when inserts with a larger pore size (1 μm) were used (data not shown).
Furthermore, we examined infection efficiency by selecting rAkata EBV-infected epithelial cells subjected to G418 treatment (35). No drug-resistant cells were selected following mock infection or infection by cell-free virus; G418-resistant epithelial cells emerged only following infection by cocultivation without culture inserts (Fig. 1C). All drug-resistant cells were positive for EBER1 (see below). Although the initial infection efficiencies were similar (Fig. 1B), the numbers of drug-resistant colonies differed among the three epithelial cell lines (Fig. 1C), probably reflecting differences in sensitivity to G418 or in ability to support persistent EBV infection. It was unlikely that some drug-resistant colonies were attributable to secondary virus spread, because similar numbers of colonies were recovered when the cells were split and plated into 96-well plates by limiting dilution immediately after infection (data not shown). Consistent with the results in Fig. 1B, use of culture inserts blocked EBV infection by cocultivation completely, indicating the importance of cell-to-cell contact in the infection process (Fig. 1C).
Cell-to-cell contact is a general mechanism for EBV infection of epithelial cells.
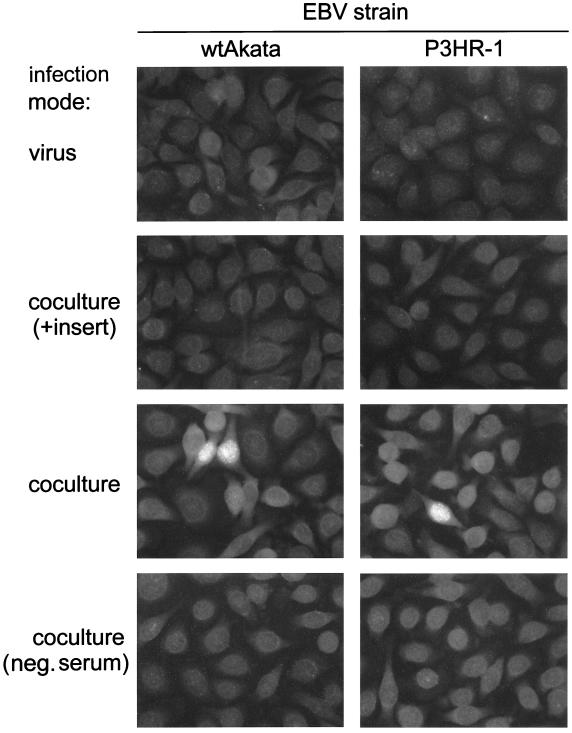
To rule out the possibility that the cell contact-dependent EBV infection may be exceptional, requiring a particular recombinant virus or a unique virus donor B cell, we examined other EBV strains and virus donor cells. BL cells harboring wtAkata or P3HR-1 EBV were chosen as virus donors. The infection efficiency was determined by an anticomplement immunofluorescence (IF) assay to detect expression of EBV nuclear antigen (EBNA) with an NPC patient’s serum containing anti-EBNA antibodies (4). Consistent with rAkata EBV infection, HONE-1 cells could be infected with wtAkata or P3HR-1 EBV only in the cocultivation mode, and use of culture inserts blocked the infection (Fig. 2). Similar results were also obtained when NPC-TW 01 and RHEK-1 cells were used as virus recipients and when rAkata EBV-transformed lymphoblastoid cells were used as virus donors (data not shown). This suggests that cell contact-dependent EBV infection is a general phenomenon of various virus strains, virus donor B cells, and virus recipient epithelial cells.
FIG. 2.
Cell contact-dependent infection of HONE-1 cells with wtAkata and P3HR-1 EBV. HONE-1 cells were infected with these two EBV strains by different modes: infection by cell-free virus or infection by cocultivation with or without culture inserts. Infection with wtAkata EBV was performed in the same way as infection with rAkata EBV. P3HR-1 EBV-harboring BL cells were activated with 40 ng of TPA per ml–3 mM sodium butyrate. For cell-free infection, virus particles were harvested from 3-day culture supernatants of activated P3HR-1 cells by ultracentrifugation and filtration as described previously (4). For infection by cocultivation, 2-day-activated P3HR-1 cells were washed with PBS to remove TPA-sodium butyrate and then used as virus donors. On day 3 postinfection, the infection efficiency was determined by an anticomplement IF assay, as described previously (4). Briefly, cells cultured on glass slides were fixed with 1:1 mixture of methanol and acetone and then sequentially reacted with an NPC patient’s serum containing anti-EBNA antibodies, guinea pig complement, and fluorescein isothiocyanate-labeled goat anti-guinea pig C3 (Cappel). The bright fluorescence in nuclei represents EBNA protein expression. A serum sample from an EBV-free donor (designated neg. serum) was used as an antibody-negative control.
Roles of cell-to-cell contact in CR2-independent and CR2-mediated EBV infections.
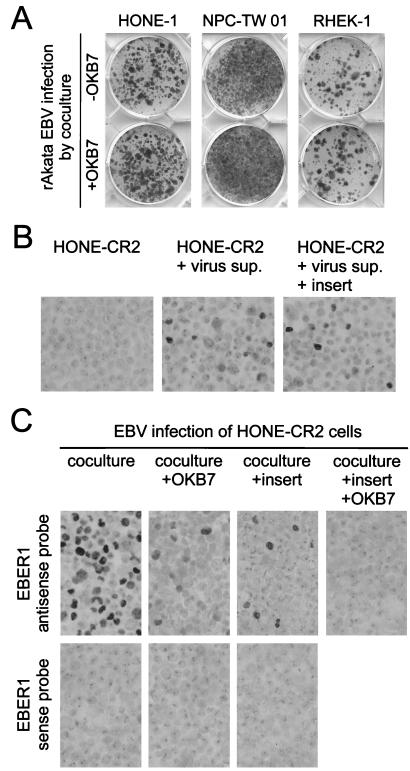
Although CR2 expression could not be detected in HONE-1, NPC-TW 01, or RHEK-1 cells by a flow cytometric assay (reference 4 and data not shown), low-level expression of CR2 cannot be ruled out completely (1, 7). To examine the involvement of CR2 in EBV infection, we used an anti-CR2 monoclonal antibody, OKB7 (Ortho Diagnostics, Raritan, N.J.), which can neutralize CR2-mediated infection (7, 35). Figure 3A shows that OKB7 did not reduce EBV infection of HONE-1, NPC-TW 01, and RHEK-1 cells by cocultivation, indicating a CR2-independent, cell contact-dependent mechanism of infection.
FIG. 3.
Mutually independent and synergistically cooperative effects of cell-to-cell contact and CR2 expression on EBV infection of epithelial cells. (A) CR2-independent EBV infection of epithelial cells. An anti-CR2 monoclonal antibody, OKB7, was used to neutralize CR2-mediated EBV infection (7, 35). Epithelial cells were preincubated with 100 μg of OKB7 per ml at 4°C for 1 h before infection, and medium contained 50 μg of OKB7 per ml throughout the infection-cocultivation procedure. Three epithelial cells were infected with rAkata EBV by cocultivation in the presence or absence of OKB7, and the infection efficiency was examined after G418 selection and crystal violet staining, as described in the legend to Fig. 1C. (B) Cell contact-independent, CR2-mediated EBV infection of HONE-CR2 cells. HONE-CR2 cells were infected with cell-free virus supernatants (sup.) of rAkata EBV while culture inserts were used or not. The infection efficiency on day 3 postinfection was determined by EBER1 in situ hybridization, as described in the legend to Fig. 1B. (C) CR2-mediated EBV infection of HONE-CR2 cells was enhanced significantly by cell-to-cell contact. HONE-CR2 cells were infected with rAkata EBV by cocultivation with or without culture inserts and with or without the addition of OKB7. The infection efficiency on day 3 postinfection was determined by EBER1 in situ hybridization. EBER1 sense probes were used to confirm the hybridization specificity.
In a previous study, HONE-CR2 cells were established by transfecting HONE-1 cells with a CR2-expressing plasmid and were shown to be susceptible to infection with purified EBV particles, representing a CR2-mediated, cell contact-independent mechanism of infection (4). About 5 to 10% of HONE-CR2 cells could be infected with cell-free rAkata EBV on day 3 postinfection (Fig. 3B), and similar infection efficiencies were observed when HONE-CR2 cells were infected with cell-free virus of other EBV strains (4). Use of culture inserts did not affect cell-free EBV infection of HONE-CR2 cells (Fig. 3B), indicating that the inserts did not interfere with virus diffusion and cell contact-independent infection. Of note, cocultivation without culture inserts enhanced EBV infection of HONE-CR2 cells significantly, and around 50 to 60% of the cells were infected (Fig. 3C). Use of OKB7 antibody alone or culture inserts alone reduced the infection efficiency to 5 to 10%, and use of both OKB7 and culture inserts blocked the infection almost completely (Fig. 3C). CR2 and cell-to-cell contact contributed to the high efficiency of EBV infection in a synergistic manner. Therefore, cell-to-cell contact not only may be an alternative CR2-independent infection pathway but also may enhance CR2-mediated infection.
Examination of viral DNA and gene expression in EBV-infected epithelial cells.
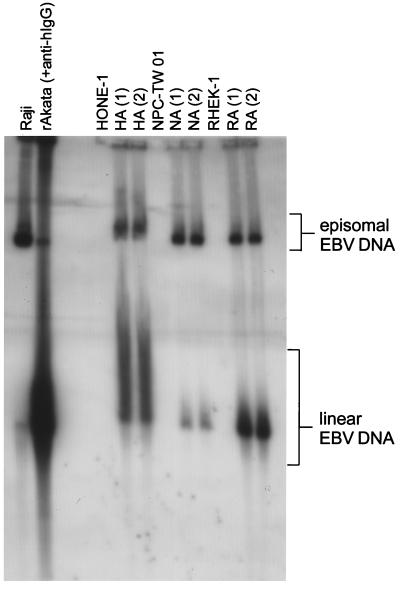
After G418 treatment, pools of rAkata EBV-infected HONE-1, NPC-TW 01, and RHEK-1 cells were selected and designated HA, NA, and RA cells, respectively. We examined the configuration of viral DNA in these cells using Gardella gel analysis, through which episomal and linear EBV DNAs can be distinguished according to their mobilities in the gel (8). Episomal EBV DNA was detected in Raji, a BL cell line latently infected with EBV, and linear EBV DNA was detected in activated rAkata cells in the viral lytic state (Fig. 4). Figure 4 shows that HA, NA, and RA cells contained both episomal and linear EBV DNA, suggesting a mixed state of latent and lytic infection.
FIG. 4.
Configuration of EBV DNA in virus-infected epithelial cells. Gardella gel analysis was carried out to detect episomal and linear EBV DNA in the cells. Raji cells were used as controls for episomal EBV DNA, and activated rAkata BL cells were used to show linear EBV DNA. HA, NA, and RA cells, as well as the uninfected parental cells, were examined. Two pools from two independent infection experiments for each cell line (numbered above the lanes) were used. Gardella gel analysis was carried out as described previously (8). Briefly, 106 intact cells were loaded into wells surrounded with 2% sodium dodecyl sulfate plus 1 mg of proteinase K per ml in a 0.8% agarose gel and electrophoresed at 4°C for 40 h. DNA in the gel was transferred onto a Hybond-N membrane (Amersham, Little Chalfont, Buckinghamshire, United Kingdom). The blot was hybridized with the random-primed, 32P-labeled EBV BamHI W fragments.
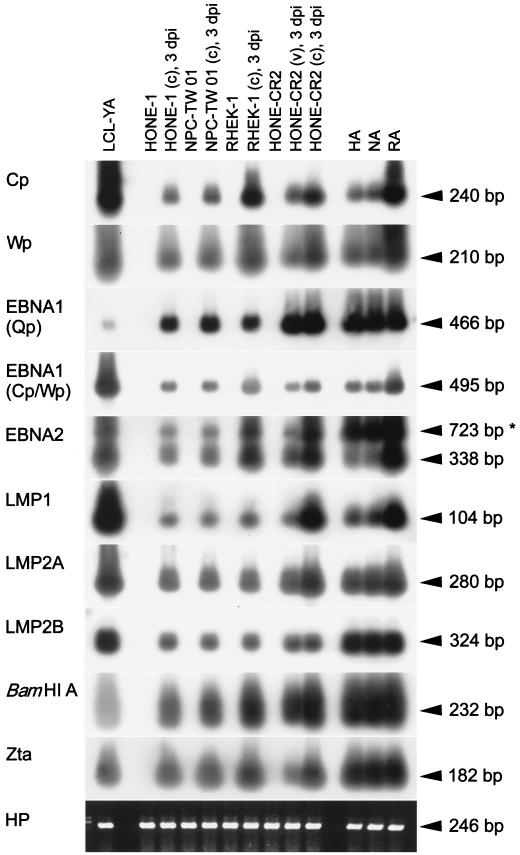
We also investigated EBV promoter usage and viral gene expression in the infected epithelial cells using a reverse transcription-PCR (RT-PCR) assay (4, 28). It is understood that activation of either of two viral promoters (Cp and Wp) results in expression of six EBNA genes (those for EBNA1 to -6), while activation of the Q promoter (Qp) results in expression of the EBNA1 gene only (12, 19). Our detection targets included 5′ common regions of all Cp- or Wp-initiated EBNA mRNAs, Qp-initiated EBNA1, Cp- or Wp-initiated EBNA1, Cp- or Wp-initiated EBNA2, latent membrane protein 1 (LMP1), LMP2A, LMP2B, viral rightward transcripts in the BamHI A region, a viral lytic gene (the Zta gene), and the cellular hypoxanthine phosphoryltransferase gene as an internal control (4, 16, 28). Figure 5 shows that identical patterns of viral gene expression were detected in EBV-infected HONE-1, NPC-TW 01, and RHEK-1 cells on day 3 postinfection or after G418 selection. Furthermore, EBV infection of HONE-CR2 cells by cell-free virus or by cocultivation also revealed the same expression pattern, indicating that different infection routes may lead to identical patterns of viral gene expression (Fig. 5). As has been found frequently for NPC biopsy specimens, EBNA1 (Qp initiated), LMP1, LMP2A, LMP2B, BamHI A RNA, and Zta were detected in EBV-infected epithelial cells (Fig. 5) (2, 3, 6). In addition, not only 5′ regions of Cp- or Wp-initiated mRNAs but also the mRNAs of Cp- or Wp-initiated EBNA1 and EBNA2 were detected (Fig. 5). In a previous study, a similar pattern of viral gene expression, including Cp- or Wp-initiated transcription, was also detected in some NPC biopsy specimens and in HONE-CR2 cells infected with other EBV strains, B95-8 and P3HR-1 (4).
FIG. 5.
RT-PCR analysis of EBV promoter usage and gene expression in virus-infected epithelial cells. We examined HONE-1, NPC-TW 01, RHEK-1, and HONE-CR2 cells infected with rAkata EBV by cocultivation (c) or by cell-free virus (v) on day 3 postinfection (3 dpi), as well as the virus-infected pools (HA, NA, and RA cells) selected with G418 treatment. An rAkata EBV-transformed lymphoblastoid cell line (LCL-YA) was used as a positive control. Detection of a housekeeping gene, the hypoxanthine phosphoryltransferase gene (HP), was carried out as an internal control (16). The expected sizes of PCR products representing spliced mRNAs are indicated, except that 723-bp PCR products for EBNA2 (asterisk) may be amplified from unspliced mRNA or viral DNA (4). The experimental procedures and details for primers and probes were described in previous studies (4, 16, 28). Briefly, cellular RNA was reverse transcribed with oligo(dT)15 as the primer. RT products were used for PCR amplification, and the PCR products were then detected by Southern hybridization with digoxigenin-labeled probes.
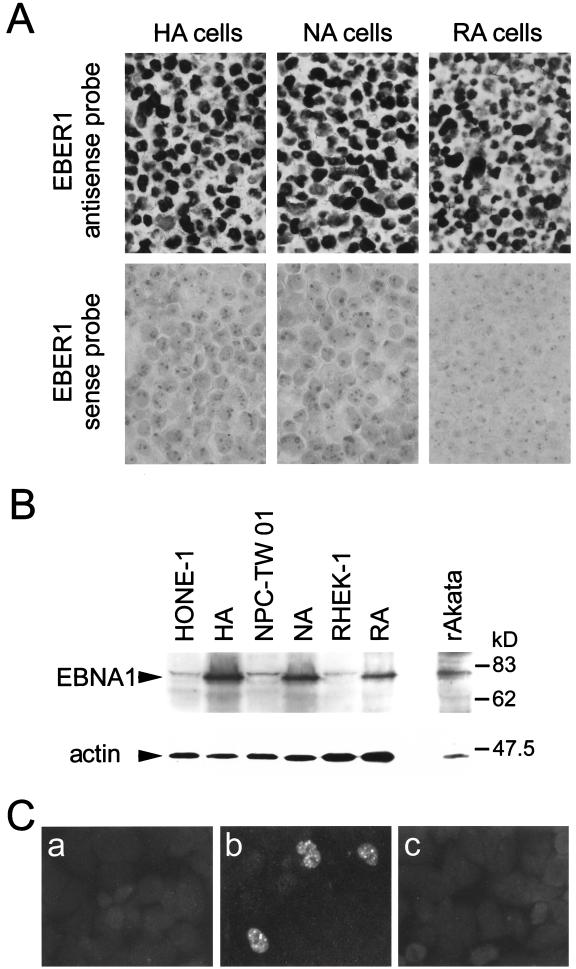
Viral gene expression in EBV-infected epithelial cells was examined further by EBER1 in situ hybridization, IF (4), and immunoblotting (32) assays. All HA, NA, and RA cells were EBER1 positive (Fig. 6A). Figure 6B and 6C show that EBNA1 proteins were detected in these infected epithelial cells, and EBNA2 proteins could be demonstrated in 1 to 2% of RA cells, in which EBNA2 mRNAs were found to be the most prominent by RT-PCR (Fig. 5). EBNA2 proteins could hardly be observed in HA and NA cells by the IF assay, probably because of the low expression levels or posttranslational regulation (4). In a small percentage of HA cells, we detected protein expression of LMP1 (1 to 2%), Zta (1 to 2%), viral capsid antigen (VCA) (0.1 to 0.5%), and viral membrane antigen (MA) (0.1 to 0.5%) (Fig. 7A). Expression of these proteins was detected in similar percentages of NA and RA cells (data not shown). Detection of lytic proteins (Zta, VCA, and MA) is consistent with detection of Zta mRNA (Fig. 5) and linear EBV DNA (Fig. 4), suggesting that the EBV lytic cycle may occur spontaneously in a small proportion of the infected cells. In the majority of infected cells, however, LMP1, EBNA2, and lytic proteins were not detected. Such heterogeneity of viral gene expression and latent or lytic states in the infected population is consistent with observations in NPC biopsy specimens and other EBV-infected epithelial cells (6, 11, 25).
FIG. 6.
Expression of EBER1 RNA and EBNA proteins in EBV-infected epithelial cells. (A) EBV-converted pools (HA, NA, and RA cells) were examined for EBER1 expression by in situ hybridization with antisense or sense probes, as described in the legend to Fig. 1B. (B) Detection of EBNA1 by the immunoblotting assay. The proteins with expected sizes are indicated. Detection of human actin was used as a control for protein loading. The immunoblotting assay was performed as described previously (32). Briefly, cellular proteins were applied to a sodium dodecyl sulfate–10% polyacrylamide gel, electrophoresed, and transferred onto a Hybond-C membrane (Amersham). The blot was reacted with an NPC patient’s serum (diluted 1:500) containing antibodies to EBNA1 or with a monoclonal antibody NH3 (antiactin; Serotec, Kidlington, United Kingdom). After incubation with peroxidase-labeled goat anti-human IgA or goat anti-mouse IgM (Amersham), final detection was achieved with a chemiluminescent detection kit (Amersham). (C) Detection of EBNA2 by the IF assay. Uninfected RHEK-1 cells (a) and EBV-infected RA cells (b and c) were stained with an anti-EBNA2 monoclonal antibody, PE2 (a and b) (36), or with PBS (c). The IF assay was modified from a previous study (4). Briefly, cells were cultured or smeared on glass slides, fixed in methanol at 4°C for 10 min, and reacted with primary monoclonal antibodies. After reaction with fluorescein isothiocyanate-labeled goat anti-mouse IgG (Sigma, St. Louis, Mo.), the slides were examined by fluorescence microscopy.
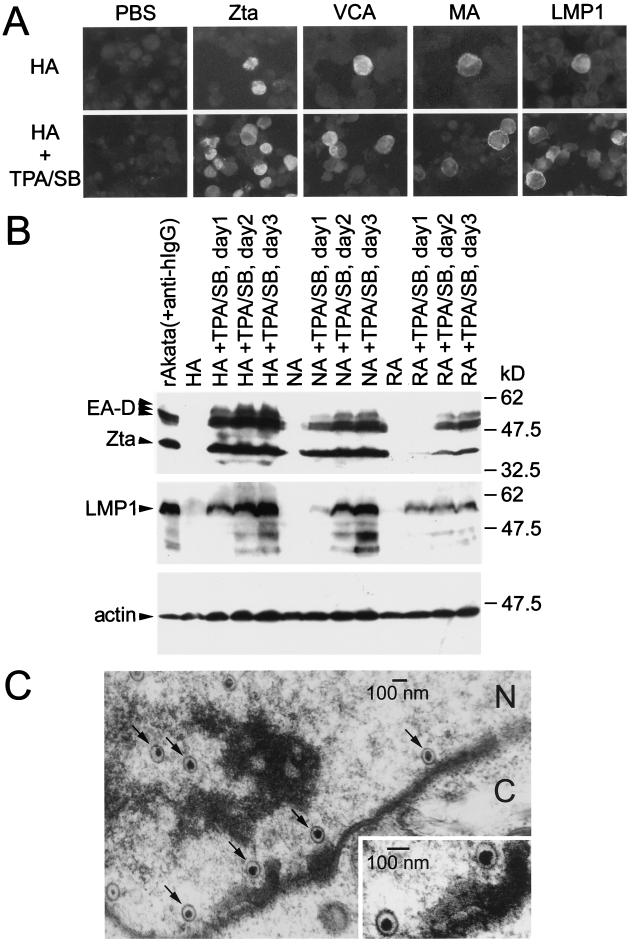
FIG. 7.
Induction of the viral lytic cycle in EBV-infected epithelial cells. (A) IF analysis of viral protein expression before and after chemical induction. Untreated HA cells and HA cells treated with TPA and sodium butyrate (SB) for 2 days were stained with monoclonal antibodies 4F10 (anti-Zta) (32), 343D12 (anti-VCA) (31), 201D6 (anti-MA) (31), CS1-4 (anti-LMP1) (21), or PBS. (B) Immunoblotting analysis of viral protein expression after chemical induction. HA, NA, and RA cells were chemically treated for 1 to 3 days; rAkata BL cells activated with anti-human IgG were used as positive controls. The protein blots were reacted with monoclonal antibodies 88A9 (anti-EA-D) (33), 4F10 (anti-Zta), CS1-4 (anti-LMP1), and NH3 (antiactin) and then with peroxidase-labeled goat anti-mouse IgG (Amersham). The proteins with expected sizes are indicated. (C) Electron photomicrograph of HA cells chemically treated for 2 days. The typical herpesvirus-like particles in the nucleus are indicated by arrows. N, nucleus. C, cytoplasm. To prepare samples for electron microscopy, cell pellets were fixed in 2.5% glutaraldehyde–0.5% tannic acid at 4°C for 15 h and postfixed in 1% OsO4 at 4°C for 4 h. After dehydration with ethanol, the cells were embedded in Spurr’s resin. Ultrathin sections (80-nm thickness) were stained with uranyl acetate and lead citrate and then examined with a transmission electron microscope (Zeiss EM-109) at 75 kV.
Induction of the viral lytic cycle in EBV-infected epithelial cells.
The EBV lytic cycle has been considered abortive or tightly restricted in NPC and some epithelial cells (7, 13, 16), but productive EBV infection has been detected in other epithelial cells (26, 35). We examined whether HA, NA, and RA cells can support complete viral lytic progression following chemical induction. After treatment with 40 ng of 12-O-tetradecanoylphorbol-13-acetate (TPA) per ml and 3 mM sodium butyrate, expression of EBV lytic antigens, including Zta, the 52- or 50-kDa early antigen (EA-D), VCA, and MA, was induced significantly in these EBV-infected epithelial cells (Fig. 7A and B). Expression of LMP1 also increased after chemical treatment (Fig. 7A and B), consistent with a previous study of lytic induction in Akata BL cells (30). On the other hand, EBNA2 expression did not increase following lytic induction (data not shown). An electron microscopic method was used to examine virus particles in the cells. Typical, electron-dense, herpesvirus-like particles were observed in the nuclei of chemically treated HA cells (Fig. 7C). Moreover, EBV bioactivity, i.e., transforming ability of infectious virus, was detectable in the culture supernatants of chemically treated HA, NA, and RA cells by a cord blood lymphocyte transformation assay (data not shown).
In summary, we demonstrated cell contact-dependent EBV infection of epithelial cells and characterized a unique viral expression pattern as well as an inducible lytic infection state in these cells. Cell-to-cell contact provides a general mechanism for EBV to infect epithelial cells in vitro, and perhaps also in vivo, especially in the nasopharynx, where NPC emerges. Lytic EBV infection has been detected in some intraepithelial lymphocytes of normal nasopharyngeal tissues, so virus-producing lymphocytes may serve as virus donors for infection of epithelial cells (5, 27). Close contact between epithelial cells and lymphocytes has been observed through electron microscopic analysis of normal nasopharyngeal mucosa, consistent with the requirement for infection (9). The importance of cell-to-cell contact for EBV infection may be explained by several mechanisms. (i) Interaction of surface molecules between virus donors and recipients may trigger some signaling events essential for infection (20). (ii) Close contact may transiently generate a local bridge for the intercellular transport of EBV (9). (iii) Cell-to-cell contact may facilitate the accessibility of virus particles to a novel low-affinity receptor on the target cells (11).
It has not been concluded whether epithelial cells express CR2 generally in vivo and whether CR2 is involved in natural EBV infection of epithelial cells. In previous in vitro studies, EBV could infect gastric carcinoma cells in a CR2-independent manner (35) but CR2-dependent infection was observed in 293 cells that expressed CR2 endogenously (7). Possibly, more than one mechanism can be utilized for EBV infection of epithelial cells. In our study, cell-to-cell contact and ectopic expression of CR2 provided mutually independent routes for infection (Fig. 3A and B). Although either of the routes contributed to limited infection efficiency, cell-to-cell contact and CR2 enhanced infection efficiency synergistically (Fig. 3C). This suggests that cell-to-cell contact may further facilitate EBV binding to CR2 or other postbinding events, such as virus internalization and cellular signaling for proper viral gene expression. Since CR2 has been detected in some epithelial tissues, including the pharyngeal mucosa, these epithelial cells may be effective targets for EBV infection in vivo if they express CR2 and contact virus-producing lymphocytes (29, 37).
Detection of Cp- or Wp-initiated gene expression in our infected epithelial cells was notable because Cp and Wp had been considered inactive in EBV-infected epithelial cells (7, 11, 19). However, in recent studies, Cp- or Wp-initiated transcripts and/or EBNA2 expression has been detected in two EBV-harboring gastric epithelial cell lines (25, 26) and biopsy specimens of oral hairy leukoplakia, an EBV-associated epithelial lesion (34). Together with our observations, the accumulated evidence indicates that Cp or Wp can be active and EBNA2 can be expressed in some EBV-infected epithelial cells. In previous studies of EBV-infected epithelial cells, viral promoter usage and gene expression were examined mostly in infected clones rather than infected pools, so Cp- or Wp-initiated EBNA expression in the minority of infected populations might have been missed (7, 11). In our study, EBNA2 proteins were detected in 1 to 2% of the pooled EBV-infected keratinocytes, RA cells, reflecting the heterogeneity among infected cells in supporting Cp- or Wp-initiated EBNA expression (Fig. 6C). On the other hand, we could hardly detect EBNA2 proteins in EBV-infected NPC cells and biopsy specimens, even though Cp- or Wp-initiated transcripts and EBNA2 mRNA were detected in these cells by a sensitive RT-PCR assay (reference 4 and Fig. 5). It has been proposed that cells expressing Cp- or Wp-initiated EBNAs may be suppressed or eliminated in vivo under host immune surveillance because EBNA2 to -6 are attractive targets for cytotoxic T lymphocytes (19). As the result of immunologic selection, developed NPC cells may intrinsically have little ability to support Cp- or Wp-initiated EBNA expression, especially at the protein level (4).
We thank Kenzo Takada of Hokkaido University School of Medicine (Sapporo, Japan) for providing rAkata EBV-infected BL cells, Alan B. Rickinson of the University of Birmingham Medical School (Birmingham, United Kingdom) for providing monoclonal antibodies PE2 and CS1-4, Ortho Diagnostics for providing OKB7 antibody, Chi-Long Chen of the College of Medicine, National Taiwan University (Taipei, Taiwan), for technical support for in situ hybridization, and Yuh-Shan Jou of National Health Research Institutes (Taipei, Taiwan) for technical support for genomic polymorphism analysis. We are indebted to Tim J. Harrison of the Royal Free and University College Medical School of University College London (London, United Kingdom) for critically reading the manuscript.
This work was supported by the National Science Council (grants NSC88-2314-B002-152 and NSC89-2320-B002-046).
REFERENCES
- 1.Birkenbach M, Tong X, Bradbury L E, Tedder T F, Kieff E. Characterization of an Epstein-Barr virus receptor on human epithelial cells. J Exp Med. 1992;176:1405–1414. doi: 10.1084/jem.176.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks L, Yao Q Y, Rickinson A B, Young L S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks L A, Lear A L, Young L S, Rickinson A B. Transcripts from the Epstein-Barr virus BamHI A fragment are detectable in all three forms of virus latency. J Virol. 1993;67:3182–3190. doi: 10.1128/jvi.67.6.3182-3190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y, Sheen T S, Lu J, Huang Y T, Chen J Y, Yang C S, Tsai C H. Detection of transcripts initiated from two viral promoters (Cp and Wp) in Epstein-Barr virus-infected nasopharyngeal carcinoma cells and biopsies. Lab Investig. 1998;78:715–726. [PubMed] [Google Scholar]
- 5.Chen C L, Hsu M M, Hsu H C. Differential expression of EBER1 in nontumor nasopharyngeal biopsies and nontumor component of nasopharyngeal carcinoma. Intervirology. 1996;39:230–235. doi: 10.1159/000150522. [DOI] [PubMed] [Google Scholar]
- 6.Cochet C, Martel-Renoir D, Grunewald V, Bosq J, Cochet G, Schwaab G, Bernaudin J F, Joab I. Expression of the Epstein-Barr virus immediate early gene, BZLF1, in nasopharyngeal carcinoma tumor cells. Virology. 1993;197:358–365. doi: 10.1006/viro.1993.1597. [DOI] [PubMed] [Google Scholar]
- 7.Fingeroth J D, Diamond M E, Sage D R, Hayman J, Yates J L. CD21-dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J Virol. 1999;73:2115–2125. doi: 10.1128/jvi.73.3.2115-2125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardella T, Medveczky P, Sairenji T, Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984;50:248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzolo L, De-The G, Vuillaume M, Ho H C. Nasopharyngeal carcinoma. II. Ultrastructure of normal mucosa, tumor biopsies, and subsequent epithelial growth in vitro. J Natl Cancer Inst. 1972;48:73–86. [PubMed] [Google Scholar]
- 10.Glaser R, Zhang H Y, Yao K T, Zhu H C, Wang F X, Li G Y, Wen D S, Li Y P. Two epithelial tumor cell lines (HNE-1 and HONE-1) latently infected with Epstein-Barr virus that were derived from nasopharyngeal carcinomas. Proc Natl Acad Sci USA. 1989;86:9524–9528. doi: 10.1073/pnas.86.23.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai S, Nishikawa J, Takada K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J Virol. 1998;72:4371–4378. doi: 10.1128/jvi.72.5.4371-4378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 13.Knox P G, Li Q X, Rickinson A B, Young L S. In vitro production of stable Epstein-Barr virus-positive epithelial cell clones which resemble the virus: cell interaction observed in nasopharyngeal carcinoma. Virology. 1996;215:40–50. doi: 10.1006/viro.1996.0005. [DOI] [PubMed] [Google Scholar]
- 14.Li Q X, Young L S, Niedobitek G, Dawson C W, Birkenbach M, Wang F, Rickinson A B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992;356:347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- 15.Lin C T, Chan W Y, Chen W, Huang H M, Wu H C, Hsu M M, Chuang S M, Wang C C. Characterization of seven newly established nasopharyngeal carcinoma cell lines. Lab Investig. 1993;68:716–727. [PubMed] [Google Scholar]
- 16.Martel-Renoir D, Grunewald V, Touitou R, Schwaab G, Joab I. Qualitative analysis of the expression of Epstein-Barr virus lytic genes in nasopharyngeal carcinoma biopsies. J Gen Virol. 1995;76:1401–1408. doi: 10.1099/0022-1317-76-6-1401. [DOI] [PubMed] [Google Scholar]
- 17.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- 18.Rhim J S. Neoplastic transformation of human epithelial cells in vitro. Anticancer Res. 1989;9:1345–1365. [PubMed] [Google Scholar]
- 19.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 20.Roberts M L, Luxembourg A T, Cooper N R. Epstein-Barr virus binding to CD21, the virus receptor, activates resting B cells via an intracellular pathway that is linked to B cell infection. J Gen Virol. 1996;77:3077–3085. doi: 10.1099/0022-1317-77-12-3077. [DOI] [PubMed] [Google Scholar]
- 21.Rowe M, Evans H S, Young L S, Hennessy K, Kieff E, Rickinson A B. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J Gen Virol. 1987;68:1575–1586. doi: 10.1099/0022-1317-68-6-1575. [DOI] [PubMed] [Google Scholar]
- 22.Sam C K, Brooks L A, Niedobitek G, Young L S, Prasad U, Rickinson A B. Analysis of Epstein-Barr virus infection in nasopharyngeal biopsies from a group at high risk of nasopharyngeal carcinoma. Int J Cancer. 1993;53:957–962. doi: 10.1002/ijc.2910530616. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu N, Yoshiyama H, Takada K. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J Virol. 1996;70:7260–7263. doi: 10.1128/jvi.70.10.7260-7263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sixbey J W, Yao Q Y. Immunoglobulin A-induced shift of Epstein-Barr virus tissue tropism. Science. 1992;255:1578–1580. doi: 10.1126/science.1312750. [DOI] [PubMed] [Google Scholar]
- 25.Tajima M, Komuro M, Okinaga K. Establishment of Epstein-Barr virus-positive human gastric epithelial cell lines. Jpn J Cancer Res. 1998;89:262–268. doi: 10.1111/j.1349-7006.1998.tb00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takasaka N, Tajima M, Okinaga K, Satoh Y, Hoshikawa Y, Katsumoto T, Kurata T, Sairenji T. Productive infection of Epstein-Barr virus (EBV) in EBV-genome-positive epithelial cell lines (GT38 and GT39) derived from gastric tissues. Virology. 1998;247:152–159. doi: 10.1006/viro.1998.9231. [DOI] [PubMed] [Google Scholar]
- 27.Tao Q, Srivastava G, Chan A C L, Chung L P, Loke S L, Ho F C S. Evidence for lytic infection by Epstein-Barr virus in mucosal lymphocytes instead of nasopharyngeal epithelial cells in normal individuals. J Med Virol. 1995;45:71–77. doi: 10.1002/jmv.1890450114. [DOI] [PubMed] [Google Scholar]
- 28.Tierney R J, Steven N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timens W, Boes A, Vos H, Poppema S. Tissue distribution of the C3d/EBV-receptor: CD21 monoclonal antibodies reactive with a variety of epithelial cells, medullary thymocytes, and peripheral T-cells. Histochemistry. 1991;95:605–611. doi: 10.1007/BF00266748. [DOI] [PubMed] [Google Scholar]
- 30.Torii T, Konishi K, Sample J, Takada K. The truncated form of the Epstein-Barr virus LMP-1 is dispensable or complimentable by the full-length form in virus infection and replication. Virology. 1998;251:273–278. doi: 10.1006/viro.1998.9411. [DOI] [PubMed] [Google Scholar]
- 31.Tsai C H, Glaser R. A comparison of Epstein-Barr virus specific proteins expressed by three Epstein-Barr virus isolates using specific monoclonal antibodies. Intervirology. 1991;32:376–382. doi: 10.1159/000150221. [DOI] [PubMed] [Google Scholar]
- 32.Tsai C H, Liu M T, Chen M R, Lu J, Yang H L, Chen J Y, Yang C S. Characterization of monoclonal antibodies to the Zta and DNase proteins of Epstein-Barr virus. J Biomed Sci. 1997;4:69–77. doi: 10.1007/BF02255596. [DOI] [PubMed] [Google Scholar]
- 33.Tsai C H A, Williams M V, Glaser R. Characterization of two monoclonal antibodies to Epstein-Barr virus diffuse early antigen which react to two different epitopes and have different biological function. J Virol Methods. 1991;33:47–52. doi: 10.1016/0166-0934(91)90006-l. [DOI] [PubMed] [Google Scholar]
- 34.Webster-Cyriaque J, Raab-Traub N. Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia. Virology. 1998;248:53–65. doi: 10.1006/viro.1998.9268. [DOI] [PubMed] [Google Scholar]
- 35.Yoshiyama H, Imai S, Shimizu N, Takada K. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J Virol. 1997;71:5688–5691. doi: 10.1128/jvi.71.7.5688-5691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young L, Alfieri C, Hennessy K, Evans H, O’Hara C, Anderson K C, Ritz J, Shapiro R S, Rickinson A, Kieff E, Cohen J I. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- 37.Young L S, Clark D, Sixbey J W, Rickinson A B. Epstein-Barr virus receptors on human pharyngeal epithelia. Lancet. 1986;i:240–242. doi: 10.1016/s0140-6736(86)90776-2. [DOI] [PubMed] [Google Scholar]