Abstract
Detrusor underactivity, a condition in which the bladder muscle does not contract strongly or long enough to empty the bladder completely or within the normal time frame, is a common cause of lower urinary tract symptoms in older individuals of both sexes. Although aging is a known risk factor for detrusor underactivity, its pathophysiological mechanisms are not fully understood. Therefore, establishing animal models that closely mimic the pathophysiology of detrusor underactivity in humans is necessary to elucidate these mechanisms. Metabolic syndrome is a cluster of several risk factors, including obesity, hyperlipidemia, hyperglycemia, and hypertension, which are associated with the development of diabetes, cardiovascular disease, and lower urinary tract dysfunction in both sexes. Notably, bladder dysfunction resulting from detrusor underactivity is observed at an earlier age in animal models with diabetes mellitus than in those without. Recently, detrusor underactivity-like phenotypes have been observed at a relatively early age in animal models with metabolic syndrome, involving obesity, hyperlipidemia, and hypertension, compared with those without. Therefore, this review introduces the association of detrusor underactivity with aging and metabolic syndrome, as well as possible pathophysiological mechanisms for detrusor underactivity from reports of various animal models. Notably, metabolic syndrome may accelerate the onset of age-related detrusor underactivity, and further analysis of old animal models with metabolic syndrome may help elucidate the pathogenesis of detrusor underactivity in humans.
Keywords: aging, animal models, bladder, detrusor underactivity, metabolic syndrome
Introduction
Detrusor underactivity is a condition in which the bladder muscle does not contract strongly or long enough to empty the bladder completely or within the normal time frame. It is diagnosed using urodynamic tests that measure bladder pressure and urine flow. Detrusor underactivity is a common cause of lower urinary tract symptoms in older adults of both sexes (1,2,3,4,5,6). Clinical studies have reported that the incidence of detrusor underactivity increases with age, from 9–28% in males aged <50 years to 48% in those aged >70 years and from 12–45% in females (1,2,3). However, no effective treatment for detrusor underactivity remains available. Although aging is a known risk factor for detrusor underactivity, its pathophysiological mechanisms are not fully understood. Multiple factors, such as central or peripheral nerve damage, bladder outlet obstruction, and diabetes mellitus, are possible causes of detrusor underactivity (3, 5, 6). Cystometric studies in animal models of detrusor underactivity have revealed impaired bladder contractility, prolonged intercontraction intervals, decreased voiding efficiency, and reduced bladder sensation. Detrusor underactivity, from various causes, has been associated with histological features in the bladder, including increased bladder weight, bladder wall thickening, inflammation, and fibrosis (5,6,7,8,9). Additionally, decreased blood flow to the bladder (severe ischemia) and increased oxidative stress in the bladder have been observed in various animal models of detrusor underactivity, suggesting that these factors play important roles in the pathogenesis of detrusor underactivity (5,6,7,8,9).
Metabolic syndrome, a cluster of multiple metabolic abnormalities and risk factors including obesity, hyperlipidemia, hyperglycemia, and hypertension, is recognized as a metabolic dysfunction that is increasing worldwide. These risk factors are associated with the development of diabetes, cardiovascular diseases, and other diseases (10). Metabolic syndrome has also been associated with lower urinary tract dysfunction in both males and females (11,12,13). Recently, several animal models of metabolic syndrome have been developed based on the bladder dysfunctions observed in human patients with detrusor underactivity (14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29). Therefore, this review discusses the association between metabolic syndrome and detrusor underactivity in old animal models. These findings may be useful for understanding the pathophysiology of human detrusor underactivity.
Aging and Detrusor Underactivity
Age-related changes in the nervous system affect bladder function. Notably, nervous system control may weaken and detrusor contractility may be impaired with age, potentially leading to detrusor underactivity (5, 6, 30,31,32,33,34). Aging is a risk factor for atherosclerosis and ischemia, which are associated with a phenotype similar to that of detrusor overactivity (which causes increased non-voiding contraction and urinary frequency and decreased single voided volume) in the early stages of bladder dysfunction. Furthermore, detrusor underactivity may occur over time (5, 6, 30,31,32,33,34).
Mouse [22–26 (31) or 27–30 (32) months old C57BL/6 mice] and rat [24 months old F344 (33), and Sprague–Dawley (34) rats] models of aging reproduce some of the characteristics of detrusor underactivity, with age-related loss of bladder sensation manifested as prolonged intercontraction intervals (5, 6, 30,31,32,33,34).
Diabetes Mellitus and Detrusor Underactivity
Diabetes mellitus causes lower urinary tract dysfunction in humans through peripheral neuropathy and muscle injury (5, 6, 21, 22). This is characterized by impaired bladder sensation and emptying, increased bladder capacity and postvoiding residual urine volume, decreased detrusor contractility, and a detrusor underactivity-like urodynamic phenotype (5, 6, 21, 22). The pathophysiology of diabetic bladder dysfunction is multifactorial and includes detrusor muscle and urothelial dysfunction, as well as neuronal damage (5, 6, 21, 22). Altered glucose metabolism, oxidative stress, and autonomic neuropathy have been implicated in the development of diabetes mellitus associated with bladder dysfunctions such as detrusor underactivity (5, 6, 14, 15, 21). Animal models of types 1 and 2 diabetes mellitus exhibit detrusor underactivity. These features in animal models correspond to some in human patients with detrusor underactivity who have reduced bladder sensation and increased postvoiding residual urine volume (5, 6, 14,15,16,17,18,19,20,21,22). These models also show increased bladder weight, inflammatory cell infiltration, and collagen deposition in the bladder (5, 6, 14,15,16,17,18,19,20,21,22).
The early and late phases of diabetes mellitus result in compensated (such as detrusor overactivity) and decompensated (such as detrusor underactivity) bladder functions, respectively (5, 6, 14,15,16,17,18,19,20,21,22). The timing of the onset of detrusor underactivity and abnormalities in urinary parameters varies among different diabetic models. However, most diabetic models develop detrusor underactivity at an earlier age than normal rodent models (5, 6, 14,15,16,17,18,19,20,21,22). A systemic injection of streptozotocin was used to induce type 1 diabetes mellitus in rats and mice, as revealed based on the increased blood glucose levels and urine output (6, 14, 15, 17). Other reports have shown detrusor underactivity-like phenotypes occurring at 4 or 12 weeks in Sprague–Dawley rats (6, 14, 17) and at 3 or 20 weeks in C57BL/6 mice following streptozotocin injection (15). A 20-week-old male Akita mouse model, a model of type 1 diabetes mellitus, exhibited lower urinary tract dysfunction with increased micturition volume, residual urine volume, bladder capacity, and contraction duration (18). Female Akita mice with mildly elevated blood glucose levels showed signs of bladder overactivity at 15 weeks of age, progressing to an underactivity/decompensated state at 30 weeks (19,20,21). Another mouse model that harbors hepatic-specific insulin receptor substrate 1 and 2 deletions (double knockout) developed type 2 diabetes mellitus (16). Furthermore, clear signs of detrusor overactivity were observed in younger (6- or 12-week-old) double-knockout mice, whereas detrusor underactivity was observed in older (20-week-old) double-knockout animals (16).
Hyperlipidemia and Detrusor Underactivity
Old myocardial infarction-prone watanabe heritable hyperlipidemic rabbits are considered appropriate animal models for studying hyperlipidemia, atherosclerosis, and related ischemic diseases (23, 24). In a cystometric study, young (6–12 months old) and old (20–24 months old) myocardial infarction-prone watanabe heritable hyperlipidemic rabbits showed decreased micturition intervals compared with controls. However, unlike the young rabbits, the old myocardial infarction-prone watanabe heritable hyperlipidemic rabbits showed decreased voiding pressure and contractile responses to carbachol and electric field stimulation. Therefore, old myocardial infarction-prone watanabe heritable hyperlipidemic rabbits have detrusor hyperactivity with impaired contractions (24).
Obesity and Detrusor Underactivity
Twenty-four-week-old obese-prone rats fed a high-fat diet showed increased body weight, triglyceride level, and serum insulin level and decreased serum high-density lipoprotein level compared with obese-resistant rats. Obese-prone rats exhibited an increased volume threshold and decreased peak micturition pressure compared with obese-resistant rats, indicating urinary retention and detrusor underactivity (25).
Metabolic Syndrome and Detrusor Underactivity
Female Ossabaw miniature pigs fed with a metabolic syndrome diet for 10 months (metabolic syndrome pigs) exhibited increased body weight, blood pressure, fasting blood glucose, and total cholesterol compared with lean-fed pigs (lean pigs) (26). Pigs with metabolic syndrome showed lower full bladder pressure than lean pigs, suggesting the presence of detrusor underactivity. Additionally, they had significantly more collagen in the muscularis layer. In another study, 56-week-old metabolic syndrome pigs showed an increase in the relative levels of urinary thiobarbituric acid reactive substances (TBARS-lipid peroxidation byproducts) and interleukin-17a (IL17a-profibrotic activity). Moreover, the Ossabaw pig model of diet-induced metabolic syndrome was associated with oxidative stress and profibrotic activity in the bladder, leading to detrusor underactivity (26).
Rhesus macaques are well-suited for translational research studies associated with aging and age-related diseases because they exhibit approximately 95% genetic similarities and share a closely related evolutionary lineage with humans (27). Older rhesus macaques (20.0–31.8 years of age) exhibited detrusor underactivity with increased bladder capacity and compliance compared with adult rhesus macaques (3.9–14.9 years). Furthermore, older rhesus macaques exhibited increased body weight, triglyceride, lactate dehydrogenase, alanine aminotransferase, and high-sensitivity C-reactive protein levels. Moreover, the analysis data showed a strong correlation between detrusor underactivity and metabolic syndrome markers in older primates with detrusor underactivity rather than in those without (27).
Hypertension and Detrusor Underactivity
Hypertension causes bladder dysfunction and atherosclerosis in bladder blood flow (35,36,37,38,39). Spontaneously hypertensive rats, a model of essential hypertension, exhibit bladder dysfunction or frequent urination, with an increased number of voids and decreased bladder blood flow (35,36,37,38,39). Moreover, our previous studies showed that aging (54 or 72-week-old) caused detrusor underactivity (increased postvoiding residual urine volume, elevated intercontraction interval, and decreased voiding efficiency), higher blood pressure, lower bladder blood flow, bladder hypertrophy, and bladder wall thickening in old spontaneously hypertensive rats than in young spontaneously hypertensive rats. However, this phenomenon was not observed in Wistar–Kyoto rats, which are normotensive control rats (28, 29). Therefore, age-related severe hypertension causes detrusor underactivity by decreasing bladder blood flow (28, 29).
After high-salt loading for 6 weeks, Dahl salt-sensitive rats exhibited increased blood pressure, urine output, water intake, intercontraction interval, and micturition volume compared with normal diet-treated rats. Additionally, collagen content was higher in the lamina propria of the high-salt-loaded group (40). These results suggest that hypertension accelerates the onset of age-related detrusor underactivity. Although no clear reports exist on an association between hypertension and detrusor underactivity in humans, this evidence from basic research may help understand the pathophysiology of detrusor underactivity in humans.
Discussion
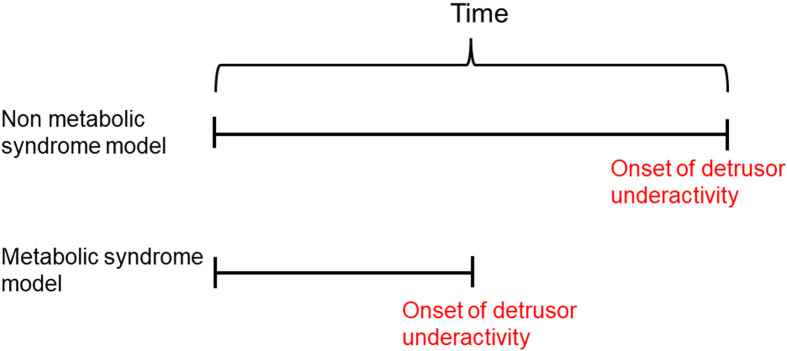
Detrusor underactivity was observed at an earlier age in metabolic syndrome models than in the non-metabolic syndrome models (Fig. 1). Metabolic syndrome conditions may be associated with aging, with a shift from detrusor overactivity to detrusor underactivity (14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29).
Fig. 1.
Metabolic syndrome may accelerate the onset of age-related detrusor underactivity.
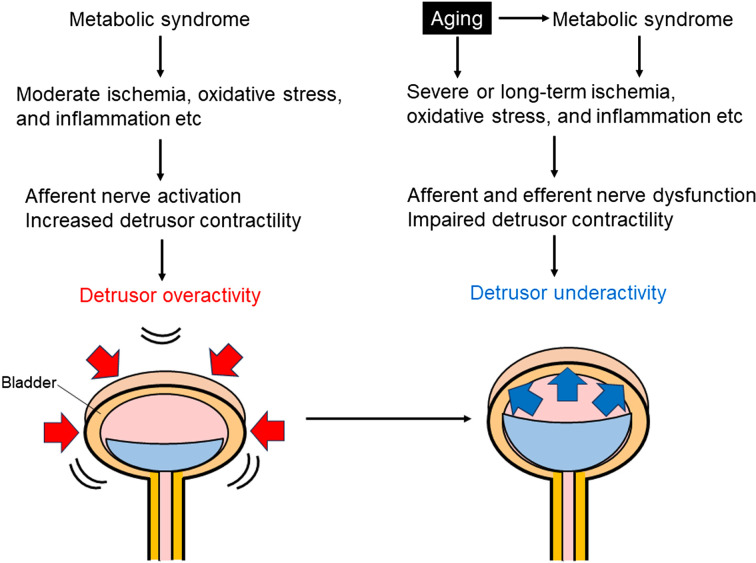
Previous reports show that moderate ischemia, oxidative stress, and inflammation in the bladder can sensitize the bladder afferent pathway and increase detrusor smooth muscle contraction, leading to detrusor overactivity (41,42,43,44). Moreover, prolonged severe ischemia impairs bladder contractile activity and provokes degenerative changes in smooth muscle cells and nerve fibers (41,42,43,44). Severe or age-related long-term ischemia, increased oxidative stress, inflammation, fibrosis, and bladder wall thickening were observed in metabolic syndrome models with detrusor underactivity phenotypes (5,6,7,8,9, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29). Age is also an independent risk factor for metabolic syndrome (45). Therefore, age and metabolic syndrome-related prolonged severe ischemia, oxidative stress, and fibrosis in the lower urinary tract can cause bladder afferent and efferent nerve dysfunction, as well as impaired detrusor contractility, leading to detrusor underactivity (Fig. 2). Consequently, further analysis involving older animal models with metabolic syndrome may help elucidate the pathogenesis of detrusor underactivity in humans.
Fig. 2.
Possible mechanisms by which metabolic syndrome causes a shift from detrusor overactivity to detrusor underactivity with aging. This bladder drawing was reproduced from Shimizu et al. (37).
Conclusion
This review shows that metabolic syndrome may accelerate the onset of age-related detrusor underactivity.
Conflict of Interest
The author declares that I have no competing financial interests or personal relationships that may have influenced the work reported in this study.
Acknowledgments
This study was supported by JSPS KAKENHI (grant number: 22K09450 [SS]).
References
- 1.Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014; 65(2): 389–98. doi: 10.1016/j.eururo.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 2.Osman NI, Esperto F, Chapple CR. Detrusor underactivity and the underactive bladder: a systematic review of preclinical and clinical studies. Eur Urol. 2018; 74(5): 633–43. doi: 10.1016/j.eururo.2018.07.037 [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Ren L, Liu X, Liu J, Ling Q. Underactive bladder and detrusor underactivity: new advances and prospectives. Int J Mol Sci. 2023; 24(21): 15517. doi: 10.3390/ijms242115517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida M, Sekido N, Matsukawa Y, Yono M, Yamaguchi O. Clinical diagnostic criteria for detrusor underactivity: a report from the Japanese Continence Society working group on underactive bladder. Low Urin Tract Symptoms. 2021; 13(1): 13–6. doi: 10.1111/luts.12356 [DOI] [PubMed] [Google Scholar]
- 5.Tyagi P, Smith PP, Kuchel GA, de Groat WC, Birder LA, Chermansky CJ, et al. Pathophysiology and animal modeling of underactive bladder. Int Urol Nephrol. 2014; 46(0 1 Suppl 1): S11–21. doi: 10.1007/s11255-014-0808-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jhang JF, Jiang YH, Hsu YH, Ho HC, Kuo HC. Pathogenesis evidence from human and animal models of detrusor underactivity. Tzu Chi Med J. 2021; 34(3): 287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azadzoi KM, Tarcan T, Siroky MB, Krane RJ. Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J Urol. 1999; 161(5): 1626–35. doi: 10.1016/S0022-5347(05)68995-1 [DOI] [PubMed] [Google Scholar]
- 8.Nomiya M, Yamaguchi O, Akaihata H, Hata J, Sawada N, Kojima Y, et al. Progressive vascular damage may lead to bladder underactivity in rats. J Urol. 2014; 191(5): 1462–9. doi: 10.1016/j.juro.2013.10.097 [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Yu HY, Ju H, Shin JH, Kim A, Lee J, et al. Induction of detrusor underactivity by extensive vascular endothelial damages of iliac arteries in a rat model and its pathophysiology in the genetic levels. Sci Rep. 2019; 9(1): 16328. doi: 10.1038/s41598-019-52811-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden MR. Overview and new insights into the metabolic syndrome: risk factors and emerging variables in the development of type 2 diabetes and cerebrocardiovascular disease. Medicina (Kaunas). 2023; 59(3): 561. doi: 10.3390/medicina59030561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WC, Chuang YC, Chiang PH, Chien CT, Yu HJ, Wu CC. Pathophysiological studies of overactive bladder and bladder motor dysfunction in a rat model of metabolic syndrome. J Urol. 2011; 186(1): 318–25. doi: 10.1016/j.juro.2011.03.037 [DOI] [PubMed] [Google Scholar]
- 12.Uzun H, Zorba OÜ. Metabolic syndrome in female patients with overactive bladder. Urology. 2012; 79(1): 72–5. doi: 10.1016/j.urology.2011.08.050 [DOI] [PubMed] [Google Scholar]
- 13.Hsu LN, Hu JC, Chen PY, Lee WC, Chuang YC. Metabolic syndrome and overactive bladder syndrome may share common pathophysiologies. Biomedicines. 2022; 10(8): 1957. doi: 10.3390/biomedicines10081957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006; 176(1): 380–6. doi: 10.1016/S0022-5347(06)00582-9 [DOI] [PubMed] [Google Scholar]
- 15.Daneshgari F, Huang X, Liu G, Bena J, Saffore L, Powell CT. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol. 2006; 290(6): R1728–35. doi: 10.1152/ajpregu.00654.2005 [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Cheng Z, Cristofaro V, Li J, Xiao X, Gomez P, et al. Inhibition of TNF-α improves the bladder dysfunction that is associated with type 2 diabetes. Diabetes. 2012; 61(8): 2134–45. doi: 10.2337/db11-1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nirmal J, Tyagi P, Chuang YC, Lee WC, Yoshimura N, Huang CC, et al. Functional and molecular characterization of hyposensitive underactive bladder tissue and urine in streptozotocin-induced diabetic rat. PLoS One. 2014; 9(7): e102644. doi: 10.1371/journal.pone.0102644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolber PC, Jin H, Nassar R, Coffman TM, Gurley SB, Fraser MO. The effects of Ins2(Akita) diabetes and chronic angiotensin II infusion on cystometric properties in mice. Neurourol Urodyn. 2015; 34(1): 72–8. doi: 10.1002/nau.22511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes FM, Jr , Hirshman NA, Inouye BM, Jin H, Stanton EW, Yun CE, et al. Nlrp3 promotes diabetic bladder dysfunction and changes in symptom-specific bladder innervation. Diabetes. 2019; 68(2): 430–40. doi: 10.2337/db18-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes FM, Jr , Allkanjari A, Odom MR, Jin H, Purves JT. Diabetic bladder dysfunction progresses from an overactive to an underactive phenotype in a type-1 diabetic mouse model (Akita female mouse) and is dependent on NLRP3. Life Sci. 2022; 299: 120528. doi: 10.1016/j.lfs.2022.120528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes FM, Jr , Odom MR, Cervantes A, Purves JT. Inflammation triggered by the NLRP3 inflammasome is a critical driver of diabetic bladder dysfunction. Front Physiol. 2022; 13: 920487. doi: 10.3389/fphys.2022.920487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell CR, Gehring V. Mechanisms of action for diabetic bladder dysfunction—state of the art. Curr Bladder Dysfunct Rep. 2023; 18: 173–82. doi: 10.1007/s11884-023-00691-w [DOI] [Google Scholar]
- 23.Yoshida M, Masunaga K, Nagata T, Satoji Y, Shiomi M. The effects of chronic hyperlipidemia on bladder function in myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits. Neurourol Urodyn. 2010; 29(7): 1350–4. doi: 10.1002/nau.20843 [DOI] [PubMed] [Google Scholar]
- 24.Yoshida M, Kudoh J, Masunaga K, Nagata T, Shiomi M. Effects of chronic hyperlipidemia on lower urinary tract function-bladder dysfunction in myocardial infarction-prone watanabe heritable hyperlipidemic rabbits. Low Urin Tract Symptoms. 2012; 4(Suppl 1): 21–6. doi: 10.1111/j.1757-5672.2011.00138.x [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez EJ, Grill WM. The effects of neuromodulation in a novel obese-prone rat model of detrusor underactivity. Am J Physiol Renal Physiol. 2017; 313(3): F815–25. doi: 10.1152/ajprenal.00242.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell CR, Kim A, Roth J, Byrd JP, Mohammad K, Alloosh M, et al. Ossabaw pig demonstrates detrusor fibrosis and detrusor underactivity associated with oxidative stress in metabolic syndrome. Comp Med. 2020; 70(5): 329–34. doi: 10.30802/AALAS-CM-20-000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biscola NP, Bartmeyer PM, Christe KL, Colman RJ, Havton LA. Detrusor underactivity is associated with metabolic syndrome in aged primates. Sci Rep. 2023; 13(1): 6716. doi: 10.1038/s41598-023-33112-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu S, Nagao Y, Kurabayashi A, Shimizu T, Higashi Y, Karashima T, et al. Aging-related severe hypertension induces detrusor underactivity in rats. Life Sci. 2021; 283: 119855. doi: 10.1016/j.lfs.2021.119855 [DOI] [PubMed] [Google Scholar]
- 29.Shimizu S, Nagao Y, Kurabayashi A, Shimizu T, Higashi Y, Karashima T, et al. Effects of losartan on bladder dysfunction due to aging-related severe hypertension in rats. Eur J Pharmacol. 2022; 922: 174911. doi: 10.1016/j.ejphar.2022.174911 [DOI] [PubMed] [Google Scholar]
- 30.Birder LA, Kullmann AF, Chapple CR. The aging bladder insights from animal models. Asian J Urol. 2018; 5(3): 135–40. doi: 10.1016/j.ajur.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith PP, DeAngelis A, Kuchel GA. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol. 2012; 302(5): R577–86. doi: 10.1152/ajpregu.00508.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamei J, Ito H, Aizawa N, Hotta H, Kojima T, Fujita Y, et al. Age-related changes in function and gene expression of the male and female mouse bladder. Sci Rep. 2018; 8(1): 2089. doi: 10.1038/s41598-018-20406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai TC, Andersson KE, Tuttle JB, Steers WD. Altered neural control of micturition in the aged F344 rat. Urol Res. 2000; 28(5): 348–54. doi: 10.1007/s002400000135 [DOI] [PubMed] [Google Scholar]
- 34.Lluel P, Deplanne V, Heudes D, Bruneval P, Palea S. Age-related changes in urethrovesical coordination in male rats: relationship with bladder instability? Am J Physiol Regul Integr Comp Physiol. 2003; 284(5): R1287–95. doi: 10.1152/ajpregu.00499.2001 [DOI] [PubMed] [Google Scholar]
- 35.Saito M, Ohmasa F, Tsounapi P, Inoue S, Dimitriadis F, Kinoshita Y, et al. Nicorandil ameliorates hypertension-related bladder dysfunction in the rat. Neurourol Urodyn. 2012; 31(5): 695–701. doi: 10.1002/nau.21213 [DOI] [PubMed] [Google Scholar]
- 36.Shimizu S, Tsounapi P, Shimizu T, Honda M, Inoue K, Dimitriadis F, et al. Lower urinary tract symptoms, benign prostatic hyperplasia/benign prostatic enlargement and erectile dysfunction: are these conditions related to vascular dysfunction? Int J Urol. 2014; 21(9): 856–64. doi: 10.1111/iju.12501 [DOI] [PubMed] [Google Scholar]
- 37.Shimizu S. Insights into the associative role of hypertension and angiotensin II receptor in lower urinary tract dysfunction. Hypertens Res. 2024; 47(4): 987–97. doi: 10.1038/s41440-024-01597-8 [DOI] [PubMed] [Google Scholar]
- 38.Shimizu S, Saito M, Oiwa H, Ohmasa F, Tsounapi P, Oikawa R, et al. Olmesartan ameliorates urinary dysfunction in the spontaneously hypertensive rat via recovering bladder blood flow and decreasing oxidative stress. Neurourol Urodyn. 2014; 33(3): 350–7. doi: 10.1002/nau.22405 [DOI] [PubMed] [Google Scholar]
- 39.Langdale CL, Degoski D, Milliken PH, Grill WM. Voiding behavior in awake unrestrained untethered spontaneously hypertensive and Wistar control rats. Am J Physiol Renal Physiol. 2021; 321(2): F195–206. doi: 10.1152/ajprenal.00564.2020 [DOI] [PubMed] [Google Scholar]
- 40.Velasquez Flores M, Mossa AH, Cammisotto P, Campeau L. Bladder overdistension with polyuria in a hypertensive rat model. Neurourol Urodyn. 2018; 37(6): 1904–12. doi: 10.1002/nau.23550 [DOI] [PubMed] [Google Scholar]
- 41.Nomiya M, Andersson KE, Yamaguchi O. Chronic bladder ischemia and oxidative stress: new pharmacotherapeutic targets for lower urinary tract symptoms. Int J Urol. 2015; 22(1): 40–6. doi: 10.1111/iju.12652 [DOI] [PubMed] [Google Scholar]
- 42.Andersson KE, Nomiya M, Sawada N, Yamaguchi O. Pharmacological treatment of chronic pelvic ischemia. Ther Adv Urol. 2014; 6(3): 105–14. doi: 10.1177/1756287214526768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang JH, Choi HP, Niu W, Azadzoi KM. Cellular stress and molecular responses in bladder ischemia. Int J Mol Sci. 2021; 22(21): 11862. doi: 10.3390/ijms222111862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu YH, Chueh KS, Chuang SM, Long CY, Lu JH, Juan YS. Bladder hyperactivity induced by oxidative stress and bladder ischemia: a review of treatment strategies with antioxidants. Int J Mol Sci. 2021; 22(11): 6014. doi: 10.3390/ijms22116014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spinelli R, Parrillo L, Longo M, Florese P, Desiderio A, Zatterale F, et al. Molecular basis of ageing in chronic metabolic diseases. J Endocrinol Invest. 2020; 43(10): 1373–89. doi: 10.1007/s40618-020-01255-z [DOI] [PMC free article] [PubMed] [Google Scholar]