Abstract
In vivo passage of a simian-human immunodeficiency virus (SHIV-89.6) generated a virus, SHIV-89.6P, that exhibited increased resistance to some neutralizing antibodies (G. B. Karlsson et al., J. Exp. Med. 188:1159–1171, 1998). Here we examine the range of human immunodeficiency virus type 1 (HIV-1) neutralizing antibodies to which the passaged virus became resistant and identify envelope glycoprotein determinants of antibody resistance. Compared with the envelope glycoproteins derived from the parental SHIV-89.6, the envelope glycoproteins of the passaged virus were resistant to antibodies directed against the gp120 V3 variable loop and the CD4 binding site. By contrast, both viral envelope glycoproteins were equally sensitive to neutralization by two antibodies, 2G12 and 2F5, that recognize poorly immunogenic structures on gp120 and gp41, respectively. Changes in the V2 and V3 variable loops of gp120 were necessary and sufficient for full resistance to the IgG1b12 antibody, which is directed against the CD4 binding site. Changes in the V3 loop specified complete resistance to a V3 loop-directed antibody, while changes in the V1/V2 loops conferred partial resistance to this antibody. The epitopes of the neutralizing antibodies were not disrupted by the resistance-associated changes. These results indicate that in vivo selection occurs for HIV-1 envelope glycoproteins with variable loop conformations that restrict the access of antibodies to immunogenic neutralization epitopes.
Human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2) cause acquired immunodeficiency syndrome (AIDS) in humans (2, 6, 18). The related simian immunodeficiency virus (SIV) can cause AIDS-like illness in Old World monkeys (10, 33). Infection with these viruses frequently leads to depletion of CD4-positive T cells, which is the central feature of the associated immunodeficiency.
Entry of primate immunodeficiency viruses into target cells is mediated by the envelope glycoproteins, which are organized into a trimeric complex on the virion surface (4, 29, 60). The gp120 exterior envelope glycoprotein binds the viral receptors, CD4 and members of the chemokine receptor family (1, 5, 8, 9, 11, 12, 16, 61). Receptor binding is thought to trigger conformational changes in the envelope glycoproteins that lead to fusion of the viral and target cell membrane by the gp41 transmembrane envelope glycoprotein (49, 55).
During natural infection, both neutralizing and nonneutralizing antibodies are generated against the HIV-1 and SIV envelope glycoproteins. Neutralizing antibodies have been suggested to play a role in preventing infection or in decreasing virus replication and delaying disease progression (3, 7, 13, 14, 19, 44). The development of a safe, effective HIV-1 vaccine would benefit from an understanding of the structural determinants in the envelope glycoproteins that lead to the production of broadly cross-reactive, neutralizing antibodies. The gp120 glycoprotein is the target for most virus-neutralizing antibodies and has evolved variable regions (V1 to V5), some of which are surface-exposed loops, to evade immune responses (35, 42, 64). In addition, the envelope glycoproteins, particularly gp120, are extensively glycosylated (32). Structural studies of HIV-1 gp120 have revealed the spatial relationships among conserved and variable epitopes on this glycoprotein (31, 62).
The humoral immune response to the HIV-1 envelope glycoproteins during natural infection has been studied by characterization of epitopes recognized by monoclonal antibodies from infected humans. Most envelope glycoprotein-directed antibodies are not neutralizing and appear to be elicited by dissociated gp120 and gp41 subunits (20, 30, 59). Neutralizing antibodies that arise relatively early in infection are directed against the gp120 V2 or V3 variable loops (15, 21, 26). The latter antibodies are capable of blocking chemokine receptor binding but are restricted in their antiviral activity to particular viral strains (37, 43). Antibodies that neutralize a broader range of HIV-1 isolates typically arise later in the course of natural infection. Based on the frequency of monoclonal antibodies identified in HIV-1-infected individuals, the majority of broadly neutralizing antibodies are directed against discontinuous gp120 epitopes near the CD4 binding site (CD4BS) (54, 56). Less commonly, broadly neutralizing antibodies are directed against CD4-induced (CD4i) epitopes, which are discontinuous gp120 structures near the chemokine receptor binding site that are better exposed after CD4 binding occurs (47, 55). Two neutralizing antibodies have been isolated only once from separate HIV-1-infected individuals and presumably are directed against poorly immunogenic epitopes. One of these, 2G12, recognizes a carbohydrate-dependent epitope on the surface of gp120 thought to face outward on the assembled envelope glycoprotein trimer (58). The other antibody, 2F5, is directed against a linear gp41 epitope located proximal to the viral membrane (41).
Primary, clinical isolates of HIV-1 are more resistant to neutralization by antibodies than viruses propagated in tissue culture. The capability of the neutralizing antibody to bind the trimeric HIV-1 envelope glycoprotein complex better predicts neutralizing activity than does antibody binding to monomeric gp120 (17, 48, 50, 51). In some cases, it has been shown that features of the gp120 V1/V2 and V3 variable loops specify the relative resistance of primary HIV-1 isolates to neutralizing antibodies (27, 40, 53).
Animal models of HIV-1 infection are useful to study the immune responses to the primate immunodeficiency viruses and the role of these responses in protection from infection or disease. Particularly useful in studying humoral immune responses is the infection of macaques with chimeric simian-human immunodeficiency viruses (SHIV), which contain the HIV-1 envelope glycoproteins (15, 34, 45). Macaques are efficiently infected by SHIV-89.6, which contains the envelope glycoproteins derived from a primary HIV-1 isolate, but do not develop clinical sequelae as a result of the infection (46). In vivo passage of SHIV-89.6 resulted in the generation of a pathogenic virus, SHIV-89.6P, that rapidly depleted CD4-positive lymphocytes in infected macaques (45). A molecular proviral clone of SHIV-89.6P was used to generate an infectious virus, SHIV-KB9 (24); SHIV-KB9 causes rapid and severe depletion of CD4+ T lymphocytes in rhesus macaques within 2 weeks after inoculation (24, 25). Sequence analysis of SHIV-KB9 showed that the proviral genome contained nucleotide changes in the long terminal repeat and coding changes in the tat and env genes (24). Most of the observed changes occurred in the env gene, resulting in 12 single amino acid substitutions in the gp120 and gp41 ectodomains. Moreover, a 140-bp env deletion resulted in the substitution of the carboxyl terminus of the SIVmac239 gp41 glycoprotein for that of the HIV-1 gp41 glycoprotein. Characterization of the molecularly cloned virus, KB9, from the SHIV-89.6P virus stock revealed that the passaged viruses were more resistant to some neutralizing antibodies than the parental SHIV-89.6 (25). Here we examine the range of HIV-1-neutralizing antibodies to which the KB9 virus is more resistant than SHIV-89.6. We also examine the changes in the envelope glycoproteins of SHIV-KB9 that determine the relative resistance to specific neutralizing antibodies.
To examine the sensitivity of SHIV-89.6 and SHIV-KB9 to neutralization by a panel of HIV-1-specific monoclonal antibodies, recombinant viruses expressing chloramphenicol acetyltransferase (CAT) and pseudotyped with either the 89.6 or KB9 envelope glycoproteins were produced (22). These recombinant viruses were incubated with monoclonal antibodies prior to infection of CEMx174 lymphocytes. Like most primary HIV-1 isolates, SHIV-89.6 was neutralized efficiently by only a subset of HIV-1-neutralizing antibodies. Even at concentrations as high as 20 μg/ml, the 17b antibody, which recognizes a CD4i epitope, failed to neutralize the 89.6 virus (Table 1). By contrast, viruses with the 89.6 envelope glycoproteins were neutralized by the IgG1b12, AG1121, 2G12, and 2F5 antibodies. IgG1b12 represents the most potent member of the CD4BS-directed antibodies (48), and the murine AG1121 antibody (Immunodiagnostics Inc.) is directed against the gp120 V3 loop. Viruses with the KB9 envelope glycoproteins were more resistant than viruses with the 89.6 envelope glycoproteins to neutralization by IgG1b12 and by AG1121 (Table 1) (25). The viruses were neutralized equivalently by the 2G12 and 2F5 antibodies. The results indicate that the passage-associated changes in the KB9 envelope glycoproteins specify resistance to antibodies directed against the gp120 CD4BS and V3 epitopes.
TABLE 1.
Neutralization of the 89.6 and KB9 envelope glycoproteins
| MAb | Epitope | Neutralizationa (IC50 [μg/ml])
|
|
|---|---|---|---|
| 89.6 | KB9 | ||
| 2G12 | Carbohydrate dependent | 0.03 | 0.03 |
| 2F5 | gp41 | 0.7 | 0.7 |
| IgG1b12 | CD4BS | 0.2 | >5 |
| AG1121 | V3 | 3 | >12 |
| 17b | CD4i | >20 | ND |
IC50, 50% inhibitory concentration based on single-round entry assays performed with various amounts of antibody. ND, not determined.
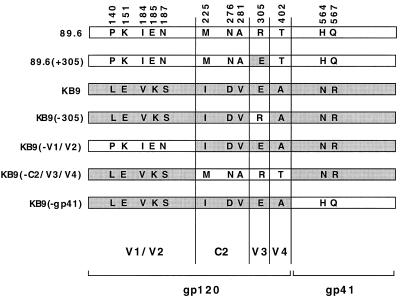
To identify envelope glycoprotein determinants of the differential sensitivity of SHIV-89.6 and SHIV-KB9 to antibody neutralization, chimeric envelope glycoproteins containing elements of the 89.6 and KB9 glycoproteins were created and tested (Fig. 1). The recombinant envelope glycoproteins are named according to the residues or regions that differ from those of the designated parental envelope glycoprotein. For example, KB9(−n) refers to an envelope glycoprotein that is identical to KB9 except for the amino acid residue at the indicated position, which is that of 89.6. Likewise, 89.6(+n) refers to an envelope glycoprotein that is identical to 89.6 except for the residue at the indicated position, which is that of KB9. Other recombinant proteins tested were KB9(−gp41), KB9(−V1/V2), and KB9(−C2/V3/V4), which were identical to KB9 except in the indicated regions, where the sequences were those of 89.6.
FIG. 1.
Primary amino acid sequence of the 89.6, KB9, and recombinant envelope glycoproteins. The envelope glycoprotein ectodomains of the parental SHIV-89.6, the passage-derived SHIV-KB9, and some of the recombinant glycoproteins used in this study are shown in the regions where the sequences differ. Regions derived from KB9 are shaded grey. The residue numbers correspond to those of the prototypic HXBc2 envelope glycoproteins, according to current convention (28), and are therefore different than those previously reported (15). The gp120 regions in which the amino acids are located are listed at the bottom. The gp41 cytoplasmic tail sequences of the envelope glycoproteins, derived from either 89.6 or KB9, correspond to those of the recombinant’s name. The derivation of the cytoplasmic tail from the 89.6 or KB9 envelope glycoproteins does not influence the neutralization sensitivity of the virus (25).
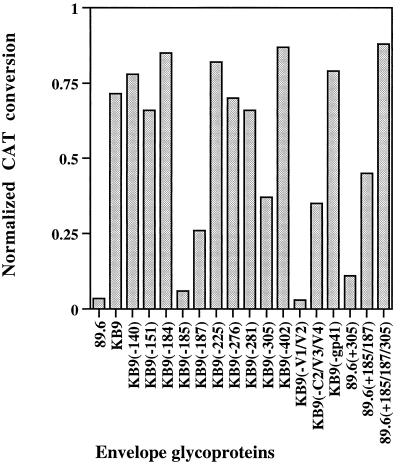
The 89.6, KB9, and chimeric envelope glycoproteins were tested for the ability to support infection of CEMx174 cells and for sensitivity to neutralization by IgG1b12, using the env complementation assay (22). All of the envelope glycoproteins efficiently supported virus entry into CEMx174 cells in the absence of antibody (data not shown). The sensitivity of viruses with the chimeric envelope glycoproteins to IgG1b12 neutralization is shown in Fig. 2. The results with the KB9(−V1/V2), KB9(−185), and KB9(−187) envelope glycoproteins indicate that the KB9 V1/V2 variable loops, in particular residues 185 and 187, are necessary for a high level of resistance to IgG1b12. The level of KB9 neutralization resistance was also influenced by the V3 loop change at residue 305. Although an intermediate level of neutralization resistance could be conferred on the 89.6 envelope glycoproteins by the V2 loop changes at residues 185 and 187, the 89.6(+185/187/305) envelope glycoprotein with an additional V3 loop change specified a level of neutralization resistance equivalent to that seen for the KB9 envelope glycoproteins. Thus, the passage-associated changes in the V2 loop are necessary but not sufficient for the full level of IgG1b12 resistance achieved by the KB9 envelope glycoproteins. The V3 loop change at residue 305 is not sufficient on its own for IgG1b12 resistance but contributes to the degree of neutralization resistance. The passage-associated changes in the gp120 V1, C2, and V4 regions and in the gp41 ectodomain were not necessary for the resistance of the KB9 envelope glycoproteins to neutralization by the IgG1b12 antibody.
FIG. 2.
Sensitivity of recombinant viruses to neutralization by the IgG1b12 antibody. Recombinant viruses bearing the envelope glycoproteins listed on the horizontal axis were incubated for 1 h at 37°C in either the absence or presence of 5 μg of the IgG1b12 antibody per ml. The viruses were then incubated with CEMx174 target cells, and CAT activity was measured 48 h later. For each virus, the CAT activity observed in the target cells following incubation of the virus with antibody was divided by the observed CAT activity when no antibody was included. Thus, a value of 1 indicates no neutralization, whereas a value of 0 indicates complete neutralization. The values shown represent means of duplicate experiments, with experimental variation less than 20% of the values shown.
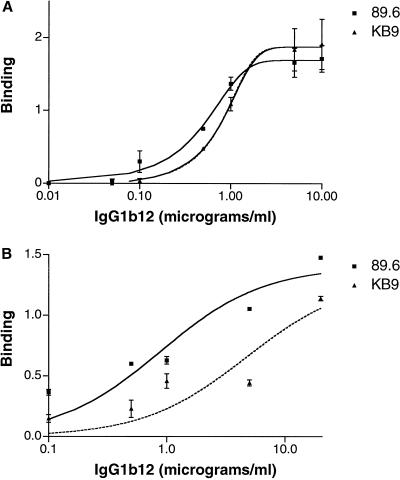
The observed resistance of the KB9 envelope glycoproteins to neutralization by IgG1b12 could be due to passage-associated sequence changes that result in a loss or reduction of antibody binding. Indeed, V2 loop changes have previously been shown to affect the ability of IgG1b12 to bind the monomeric gp120 glycoprotein (48). To address this possibility, we examined the binding of IgG1b12 to 89.6 and KB9 soluble gp120 monomers and oligomeric envelope glycoprotein complexes expressed on the surface of transfected 293T cells. Figure 3A shows that the IgG1b12 antibody bound soluble 89.6 and KB9 gp120 monomers comparably. Differences in the efficiency of IgG1b12 binding to the 89.6 and KB9 envelope glycoproteins were more apparent when antibody binding to envelope glycoprotein complexes on the cell surface were examined (Fig. 3B). These results indicate that the IgG1b12 epitope is preserved on the KB9 envelope glycoproteins but may be less available for antibody binding in the context of the oligomeric envelope glycoprotein complex.
FIG. 3.
Binding of the IgG1b12 antibody to monomeric, soluble gp120 and oligomeric, cell surface-expressed 89.6 and KB9 envelope glycoproteins. (A) The 89.6 and KB9 soluble gp120 glycoproteins were produced from a plasmid in which a stop codon had been introduced into the region encoding the gp120-gp41 junction. Twenty-four hours after transfection of 293T cells with the gp120-expressing plasmid and a plasmid expressing HIV-1 Tat, the cells were labeled with [35S]cysteine and [35S]methionine; the supernatant was harvested 16 h later. The amounts of soluble 89.6 and KB9 gp120 glycoproteins in the supernatants were estimated by precipitation with an excess of serum from an HIV-1-infected individual. The estimates were used to adjust the amounts of the radiolabeled 89.6 and KB9 gp120 glycoproteins in the solutions, which were then adjusted to identical volumes and used for subsequent immunoprecipitation. Normalized solutions of the radiolabeled 89.6 and KB9 gp120 glycoproteins were then incubated with different amounts of the IgG1b12 antibody and precipitated with protein A-Sepharose beads. The final concentration of antibody is reported on the horizontal axis. In parallel, the gp120 samples were again precipitated with serum from an HIV-1-infected person to control for amounts of the 89.6 and KB9 gp120 glycoproteins. The amount of precipitated gp120 was determined by densitometry of sodium dodecyl sulfate-polyacrylamide gels. Values of gp120 precipitated by IgG1b12 were normalized to the amounts precipitated by the serum from the HIV-1-infected person and are reported on the vertical axis in arbitrary densitometric units. (B) The full-length 89.6 and KB9 envelope glycoproteins were expressed in transfected 293T cells along with the HIV-1 Tat protein. Sixteen hours after radiolabeling, the cells were incubated with different amounts of the IgG1b12 antibody in 2% fetal calf serum for 2 h at 37°C. The cells were then washed in phosphate-buffered saline and 2% fetal calf serum and lysed in NP-40 buffer prior to precipitation with protein A-Sepharose beads. The binding of the IgG1b12 antibody was normalized to that seen when the serum of an HIV-1-infected individual was used, as described above.
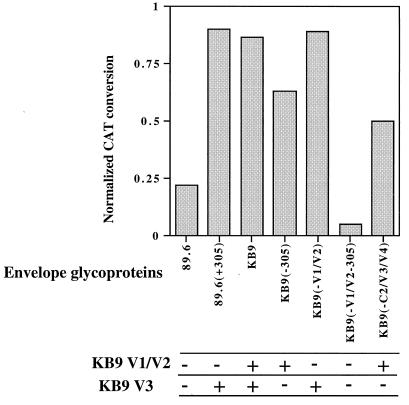
The envelope glycoprotein determinants of SHIV-KB9 resistance to neutralization by a V3 loop-directed antibody, AG1121, were investigated, using the panel of recombinant envelope glycoproteins. All of the viruses pseudotyped with envelope glycoproteins containing the passage-associated glutamate residue at position 305, including the 89.6(+305) mutant, exhibited resistance to neutralization by AG1121 (Fig. 4). The envelope glycoprotein variants with the wild-type arginine at position 305 specified either an intermediate level of neutralization or neutralization comparable to that of the 89.6 glycoproteins. The envelope glycoproteins, KB9(−305) and KB9(−C2/V3/V4), which specified intermediate neutralization phenotypes, contained the KB9 V1/V2 variable loops. Thus, the KB9-associated residue at position 305 within the V3 loop is sufficient to confer complete resistance to neutralization by the AG1121 antibody. In the absence of this change, the presence of the KB9 V1/V2 loops can specify an intermediate level of neutralization resistance. Only when both V1/V2 and V3 sequences of the KB9 envelope glycoprotein were reverted to the 89.6 sequences in the KB9(−V1/V2-305) mutant was a fully neutralization-sensitive phenotype observed.
FIG. 4.
Sensitivity of recombinant viruses to neutralization by the AG1121 anti-V3 antibody. Recombinant CAT-expressing viruses bearing the envelope glycoproteins indicated on the horizontal axis were tested for sensitivity to 12 μg of the AG1121 antibody per ml as described in the legend to Fig. 2. The origin of the V1/V2 and V3 loops in each envelope glycoprotein is indicated at the bottom as − (wild-type 89.6 sequences) or + (KB9 sequences).
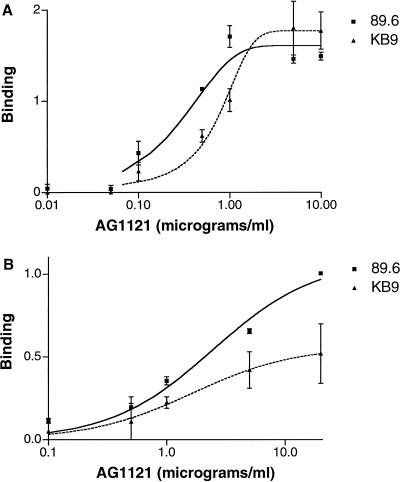
The binding of the AG1121 anti-V3 loop antibody to the 89.6 and KB9 envelope glycoproteins was examined. Figure 5A shows that the AG1121 antibody recognized both monomeric gp120 envelope glycoproteins, indicating that the epitope was intact on both envelope glycoproteins. The affinity of the AG1121 antibody for the 89.6 gp120 monomer was approximately 2.7-fold that for the KB9 gp120 glycoprotein. The AG1121 antibody also bound the KB9 envelope glycoprotein complex on the cell surface less efficiently than the 89.6 envelope glycoproteins (Fig. 5B).
FIG. 5.
Binding of the AG1121 anti-V3 antibody to monomeric, soluble gp120 and oligomeric, cell surface-expressed 89.6 and KB9 envelope glycoproteins. (A) Binding of the AG1121 antibody to 89.6 and KB9 soluble gp120 monomers was determined as described in the legend to Fig. 3A. (B) Binding of the AG1121 antibody to full-length 89.6 and KB9 envelope glycoproteins expressed on the surface of 293T cells was determined as described in the legend to Fig. 3B. Reported values are normalized by reference to the serum from an HIV-1-infected individual to control for cell surface expression levels.
In this study, we used a panel of neutralizing antibodies to characterize the antigenic differences between two primary HIV-1 envelope glycoproteins that differ by only 12 amino acids in the exterior domains. These 12 changes arose during in vivo passage of SHIV-89.6 and, in part, may have resulted from selective pressure exerted by the host immune response. A previous study indicated that for the first few months after infection with SHIV-89.6 or SHIV-KB9, the neutralizing antibody responses are restricted to the infecting virus and are directed against a very limited number of epitopes (15). The determinants of these strain-specific epitopes were localized to the V2 and/or V3 loops of the HIV-1 gp120 envelope glycoprotein. Our observation that the KB9 envelope glycoproteins conferred on viruses greater resistance to a V3 loop-directed monoclonal antibody than did the 89.6 envelope glycoproteins is consistent with the presence of selective V3-directed antibodies during the in vivo evolution of SHIV-KB9. The increased resistance to a CD4BS antibody conferred by the KB9 envelope glycoproteins raises the possibility that antibodies directed against the CD4-binding region also shaped the evolution of SHIV-KB9. More broadly neutralizing antibodies like CD4BS antibodies tend to arise later in the course of HIV-1 infection in humans or SHIV infection in monkeys. However, it is possible that lower-affinity members of this antibody group exerted selective pressure in vivo without being detected in the neutralization assay. Alternatively, resistance to the CD4BS antibody could have arisen as a consequence of selective pressure exerted by V2- and V3-directed antibodies. Whatever the nature of the humoral immune response that drove the evolution of SHIV-KB9, it is noteworthy that the protection against CD4BS antibodies is precise and does not extend to CD4 itself, which binds equivalently to the 89.6 and KB9 envelope glycoproteins (25).
The KB9 and 89.6 envelope glycoproteins were equally sensitive to neutralization by the 2G12 and 2F5 antibodies, which appear to be infrequently elicited in HIV-1-infected humans. Apparently, there is little selective pressure in SHIV-infected monkeys to vary or protect these two conserved epitopes. One explanation for this situation is that SHIV-infected monkeys, like HIV-1-infected humans, rarely generate neutralizing antibodies that resemble 2G12 or 2F5. The high degree of glycosylation of the HIV-1 gp120 surface that contacts the 2G12 antibody has been suggested to contribute to the poor immunogenicity of the epitope (58). The linear 2F5 epitope in gp41 has also proven to be very poorly immunogenic, for reasons that remain unclear (41).
The changes responsible for resistance to both the AG1121 V3-directed antibody and the IgG1b12 CD4BS antibody mapped to the V2 and V3 loops of the HIV-1 gp120 glycoprotein. This is consistent with previous observations that the major gp120 variable loops not only contain neutralization epitopes but can modulate the interaction of antibodies with conserved gp120 epitopes related to the receptor binding regions (53, 62). V3 loop changes in residue 305 were primarily responsible for the resistance of SHIV-KB9 to neutralization by the AG1121 antibody. Although the AG1121 antibody can still bind the monomeric KB9 gp120 envelope glycoprotein, it does so with affinity lower than that seen for the AG1121 interaction with the 89.6 gp120 glycoprotein. Our data do not allow us to distinguish whether the residue 305 phenotypes are mediated by partial perturbation of the AG1121 epitope or by secondary effects on V3 conformation and exposure of the epitope. The V2 loop changes in residues 185 and 187, which allowed partial resistance to AG1121 neutralization, probably operate by masking the V3 epitope. The proximity of the HIV-1 gp120 V2 and V3 loops has been suggested by the generation of neutralizing antibodies in SHIV-infected monkeys that apparently recognize structures dependent on both V2 and V3 sequences (15). Although the V2 and V3 variable loops were deleted from the gp120 core that has been structurally characterized by X-ray crystallography (31, 62), proximity of these loops is consistent with this structure. Finally, the V2 loop has been shown to mask CD4i epitopes (63), which are thought to reside immediately adjacent to the V3 loop.
Resistance to neutralization by the CD4BS antibody IgG1b12 was conferred primarily by passage-associated changes in the V2 loop, with the residue at position 185 playing a dominant role. The KB9 gp120 monomer is still efficiently recognized by the IgG1b12 antibody, suggesting that the epitope is intact in the neutralization-resistant virus. Previous studies have suggested a proximity of the V2 loop and the IgG1b12 epitope (48), a possibility consistent with the relationship of the V2 loop and the CD4BS proposed on the basis of antibody competition and X-ray crystallographic analyses (31, 62). Mo and colleagues showed that after in vitro passage in the presence of IgG1b12, a single amino acid substitution at position 185 was sufficient to create a virus resistant to IgG1b12 neutralization (38). In contrast to most CD4BS antibodies, IgG1b12 recognizes a V1/V2 loop-deleted gp120 glycoprotein less efficiently than the wild-type glycoprotein (48). Thus, residues in the V2 loop can play a major role in the maintenance or exposure of the IgG1b12 epitope.
V3 loop changes augmented the degree of IgG1b12 resistance specified by the V2 loop changes. It is noteworthy that in vivo passage resulted in reciprocal charge substitutions in the two residues at positions 185 and 305 implicated in the resistance phenotype. This observation, the shift in the glycosylation site at the adjacent position 187 in the KB9 V2 loop (24), and the identification of apparently discontinuous neutralization epitopes spanning V2 and V3 (15) have led to the model that the V2 and V3 variable loops are more proximal in the KB9 than in the 89.6 envelope glycoproteins. Such proximity would explain the cooperativity in resistance to IgG1b12 neutralization observed in our study.
These studies illustrate examples of the in vivo evolution of primary HIV-1 envelope glycoproteins in response to selective forces that include neutralizing antibodies. Further analysis may provide insights into the structural relationships of variable and conserved neutralization epitopes on the HIV-1 envelope glycoproteins and might suggest avenues for intervention.
Acknowledgments
We thank Dennis Burton, Alexandra Trkola, and Herman Katinger for reagents and Yvette McLaughlin and Sheri Farnum for manuscript preparation.
This work was supported by National Institutes of Health grants AI33832 and AI31783. Dana-Farber Cancer Institute is the recipient of a Center for AIDS Research award from the National Institutes of Health. This work was also supported by the Mathers Charitable Foundation, the Friends 10, Douglas and Judy Krupp, and the late William F. McCarty-Cooper.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 3.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 4.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 5.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, MacKay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 6.Clavel F. HIV-2, the West African AIDS virus. AIDS. 1987;1:135–140. [PubMed] [Google Scholar]
- 7.Conley A J, Kessler II J A, Boots L J, McKenna P M, Schleif W A, Emini E A, Mark III G E, Katinger H, Cobb E K, Lunceford S M, Rouse S R, Murthy K K. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 9.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers R C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 11.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 12.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 13.Emini E A, Nara P L, Schleif W A, Lewis J A, Davide J P, Lee D R, Kessler J, Conley S, Matsushita S, Putney S D, et al. Antibody-mediated in vitro neutralization of human immunodeficiency virus type 1 abolishes infectivity for chimpanzees. J Virol. 1990;64:3674–3678. doi: 10.1128/jvi.64.8.3674-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, et al. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 15.Etemad-Moghadam B, Karlsson G B, Halloran M, Sun Y, Schenten D, Fernandes M, Letvin N L, Sodroski J. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys. J Virol. 1998;72:8437–8445. doi: 10.1128/jvi.72.10.8437-8445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 17.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, White G, Foster P, Markham P D. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 19.Girard M, Kieny M P, Pinter A, Barre-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore E, et al. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gnann J W, Jr, Nelson J A, Oldstone M B. Fine mapping of an immunodominant domain in the transmembrane glycoprotein of human immunodeficiency virus. J Virol. 1987;61:2639–2641. doi: 10.1128/jvi.61.8.2639-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorny M K, Moore J P, Conley A J, Karwowska S, Sodroski J, Williams C, Burda S, Boots L J, Zolla-Pazner S. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J Virol. 1994;68:8312–8320. doi: 10.1128/jvi.68.12.8312-8320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho D D, Fung M S, Cao Y Z, Li X L, Sun C, Chang T W, Sun N C. Another discontinuous epitope on glycoprotein gp120 that is important in human immunodeficiency virus type 1 neutralization is identified by a monoclonal antibody. Proc Natl Acad Sci USA. 1991;88:8949–8952. doi: 10.1073/pnas.88.20.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson G B, Halloran M, Li J, Park I W, Gomila R, Reimann K A, Axthelm M K, Lliff S A, Letvin N L, Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenealy W R, Matthews T J, Ganfield M C, Langlois A J, Waselefsky D M, Petteway S R., Jr Antibodies from human immunodeficiency virus-infected individuals bind to a short amino acid sequence that elicits neutralizing antibodies in animals. AIDS Res Hum Retroviruses. 1989;5:173–182. doi: 10.1089/aid.1989.5.173. [DOI] [PubMed] [Google Scholar]
- 27.Koito A, Harrowe G, Levy J A, Cheng-Mayer C. Functional role of the V1/V2 region of human immunodeficiency virus type 1 envelope glycoprotein gp120 in infection of primary macrophages and soluble CD4 neutralization. J Virol. 1994;68:2253–2259. doi: 10.1128/jvi.68.4.2253-2259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korber B T, Foley B, Kuiken C, Pillai S, Sodroski J G. Numbering position in HIV relative to HXB2. In: Korber B, Brander C, Haynes B, Koup R, Moore J, Walker B, editors. Human retroviruses and AIDS 1999. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos National Laboratories, Los Alamos, N.Mex, p. IV-27–IV-35. 1998. [Google Scholar]
- 29.Kowalski M, Potz J, Basiripour L, Dorfman T, Goh W C, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987;237:1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- 30.Krowka J F, Singh B, Stites D P, Maino V C, Narindray D, Hollander H, Jain S, Chen H, Blackwood L, Steimer K S. Epitopes of human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins recognized by antibodies in the sera of HIV-1-infected individuals. Clin Immunol Immunopathol. 1991;59:53–64. doi: 10.1016/0090-1229(91)90081-k. [DOI] [PubMed] [Google Scholar]
- 31.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibodies. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard C K, Spellman M W, Riddle L, Harris R J, Thomas J N, Gregory T J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 33.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Waldron L M, MacKey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 34.Li J T, Halloran M, Lord C I, Watson A, Ranchalis J, Fung M, Letvin N L, Sodroski J G. Persistent infection of macaques with simian-human immunodeficiency viruses. J Virol. 1995;69:7061–7067. doi: 10.1128/jvi.69.11.7061-7067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda T, Matsushita S, Kuroda M J, Kannagi M, Takatsuki K, Harada S. Generation of neutralization-resistant HIV-1 in vitro due to amino acid interchanges of third hypervariable env region. J Immunol. 1990;145:3240–3246. [PubMed] [Google Scholar]
- 36.Matsushita S, Robert-Guroff M, Rusche J, Koito A, Hattori T, Hoshino H, Javaherian K, Takatsuki K, Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988;62:2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews T J, Langlois A J, Robey W G, Chang N T, Gallo R C, Fischinger P J, Bolognesi D P. Restricted neutralization of divergent human T-lymphotropic virus type III isolates by antibodies to the major envelope glycoprotein. Proc Natl Acad Sci USA. 1986;83:9709–9713. doi: 10.1073/pnas.83.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo H, Stamatatos L, Ip J E, Barbas C F, Parren P W, Burton D R, Moore J P, Ho D D. Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J Virol. 1997;71:6869–6874. doi: 10.1128/jvi.71.9.6869-6874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore J P, Sattentau Q J, Yoshiyama H, Thali M, Charles M, Sullivan N, Poon S W, Fung M S, Traincard F, Pinkus M, Robey G, Robinson J E, Ho D D, Sodroski J. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: human immune response to the V1 and V2 domains. J Virol. 1993;67:6136–6151. doi: 10.1128/jvi.67.10.6136-6151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morikita T, Maeda Y, Fujii S, Matsushita S, Obaru K, Takatsuki K. The V1/V2 region of human immunodeficiency virus type 1 modulates the sensitivity to neutralization by soluble CD4 and cellular tropism. AIDS Res Hum Retroviruses. 1997;13:1291–1299. doi: 10.1089/aid.1997.13.1291. [DOI] [PubMed] [Google Scholar]
- 41.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nara P L, Smit L, Dunlop N, Hatch W, Merges M, Waters D, Kelliher J, Gallo R C, Fischinger P J, Goudsmit J. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990;64:3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palker T J, Clark M E, Langlois A J, Matthews T J, Weinhold K J, Randall R R, Bolognesi D P, Haynes B F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci USA. 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 45.Reimann K A, Li J T, Veazey R, Halloran M, Park I W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 48.Roben P, Moore J P, Thali M, Sodroski J, Barbas III C F, Burton D R. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stamatatos L, Zolla-Pazner S, Gorny M K, Cheng-Mayer C. Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells. Virology. 1997;229:360–369. doi: 10.1006/viro.1997.8443. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passed human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan N, Sun Y, Binley J, Lee J, Barbas III C F, Parren P W, Burton D R, Sodroski J. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 1998;72:6332–6338. doi: 10.1128/jvi.72.8.6332-6338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thali M, Furman C, Ho D D, Robinson J, Tilley S, Pinter A, Sodroski J. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 1992;66:5635–5641. doi: 10.1128/jvi.66.9.5635-5641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thali M, Olshevsky U, Furman C, Gabuzda D, Posner M, Sodroski J. Characterization of a discontinuous human immunodeficiency virus type 1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J Virol. 1991;65:6188–6193. doi: 10.1128/jvi.65.11.6188-6193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 58.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J J, Steel S, Wisniewolski R, Wang C Y. Detection of antibodies to human T-lymphotropic virus type III by using a synthetic peptide of 21 amino acid residues corresponding to a highly antigenic segment of gp41 envelope protein. Proc Natl Acad Sci USA. 1986;83:6159–6163. doi: 10.1073/pnas.83.16.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 61.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardosa A A, Desjardins E, Newman W, Gerard C, Sodroski J. CD4-induced interactions of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;184:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 62.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 63.Wyatt R, Moore J, Accola M, Desjardins E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshiyama H, Mo H, Moore J P, Ho D D. Characterization of mutants of human immunodeficiency virus type 1 that have escaped neutralization by a monoclonal antibody to the gp120 V2 loop. J Virol. 1994;68:974–978. doi: 10.1128/jvi.68.2.974-978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]