Abstract
Benzodiazepines remain widely used for the treatment of anxiety disorders despite prominent, often limiting side effects including sedation, muscle relaxation, and ataxia. A compound producing a robust anxiolytic action comparable to benzodiazepines, but lacking these limiting side effects at therapeutic doses (an anxioselective agent), would represent an important advance in the treatment of generalized anxiety disorder, and perhaps other anxiety disorders. Here we report that the pyrazolo[1,5-a]-pyrimidine, ocinaplon, exhibits an anxioselective profile in both preclinical procedures and in patients with generalized anxiety disorder, the most common of the anxiety disorders. In rats, ocinaplon produces significant muscle relaxation, ataxia, and sedation only at doses >25-fold higher than the minimum effective dose (3.1 mg/kg) in the Vogel “conflict” test. This anticonflict effect is blocked by flumazenil (Ro 15-1788), indicating that like benzodiazepines, ocinaplon produces an anxiolytic action through allosteric modulation of GABAA receptors. Nonetheless, in eight recombinant GABAA receptor isoforms expressed in Xenopus oocytes, the potency and efficacy of ocinaplon to potentiate GABA responses varied with subunit composition not only in an absolute sense, but also relative to the prototypical benzodiazepine, diazepam. In a double blind, placebo controlled clinical trial, a 2-week regimen of ocinaplon (total daily dose of 180-240 mg) produced statistically significant reductions in the Hamilton rating scale for anxiety scores. In this study, the incidence of benzodiazepine-like side effects (e.g., sedation, dizziness) in ocinaplon-treated patients did not differ from placebo. These findings indicate that ocinaplon represents a unique approach both for the treatment and understanding of anxiety disorders.
Keywords: generalized anxiety disorder, benzodiazepines
Benzodiazepines remain widely used for the treatment of anxiety disorders (1, 2) despite significant limiting side effects including sedation, muscle relaxation, and ataxia. This spectrum of pharmacological actions is produced by augmenting the actions of the inhibitory neurotransmitter GABA through an allosteric modulation of GABAA receptors (3, 4), a family of heteropentameric, ligand-gated ion channels (5). Although there are at least seven subunit classes, the majority of GABAA receptors are composed of α-, β-, and γ-subunits (6), with multiple subtypes of each that can assemble to form GABA-gated ion channels (5, 7). This subunit repertoire allows for remarkable receptor diversity, and >12 distinct GABAA receptor isoforms may be present in the mammalian nervous system (8).
Benzodiazepines bind to and act upon a subset of GABAA receptors containing β- and γ-subunits in combination with α1-, α2-, α3-, or α5-subunits. The identification of novel molecules acting at the benzodiazepine recognition site of the GABAA receptor began >25 years ago (9, 10), and remains an area of intense interest (2, 5, 11, 12). The impetus for identifying such molecules was the discovery that the triazolopyridazine, CL 218,872 (9, 10), exhibited selectivity for “Type I” benzodiazepine receptors (subsequently identified as GABAA receptors containing an α1-subunit; refs. 5 and 13) and a low efficacy relative to benzodiazepines in potentiating GABA-gated chloride currents (14, 15). CL 218,872 produced anxiolytic-like effects in animal models at doses substantially lower than those producing benzodiazepine-like side effects (e.g., sedation, ataxia, muscle relaxation) (16). A compound exhibiting this “anxioselective” profile in humans would represent a significant advance in the treatment and understanding of anxiety disorders.
A number of compounds have been identified that exhibit GABAA receptor subtype selectivity and/or lower efficacy than benzodiazepines to enhance GABA-stimulated chloride currents (reviewed in ref. 2). Some compounds, such as bretazenil and abecarnil, exhibit an anxioselective profile in animals (17, 18), but data from clinical trials do not support the anxioselectivity predicted from preclinical results (19, 20). We now report that ocinaplon (DOV 273,547; 2-pyridinyl[7-(4-pyridinyl)pyrazolo[1,5-a]-pyrimidin-3-yl]methanone) fulfills both preclinical and clinical criteria for an anxioselective agent.
Methods
“Thirsty Rat” Conflict Test. The procedure used in this study was essentially as originally described by Vogel et al. (21). The effects of drugs or vehicle (n ≥ 8 animals per dose) were evaluated 1 h after oral administration. See Supporting Text, which is published as supporting information on the PNAS web site, for further details.
Blockade of Pentylenetetrazole-Induced Convulsions. The anticonvulsant properties of ocinaplon and diazepam were evaluated essentially as described (10, 22) using pentylenetetrazole (19 mg/kg, i.v.) as the convulsant agent. Rats (n ≥ 4 per dose) were challenged with pentylenetetrazole 1 h after oral administration of ocinaplon, diazepam, or vehicle. See Supporting Text for further details.
Evaluation of Motor Function in Rats. Motor activity. One hour after oral administration of ocinaplon, diazepam, or vehicle, individual animals (n = 12-24 per dose) were placed in activity chambers equipped with photoelectric cells, and activity was recorded for 5 min. The ED50 was the dose that reduced activity levels to 50% in comparison with vehicle-treated control animals (10).
Inclined screen. Rats (n ≥ 8 per dose) were placed individually on an inclined screen (60°) 1 h after oral administration of vehicle, ocinaplon, or diazepam. The number of animals remaining on the screen for at least 30 min was recorded. The ED50 is the dose producing screen failures in 50% of the rats (10).
Rod walking. Rats were trained to traverse a wooden rod inclined at 17°. One hour after orally administered diazepam and ocinaplon, the number of animals (n = 10 per dose) unable to traverse the rod was evaluated (22). The ED50 was that dose causing 50% of the rats to fall. ED50 values were calculated by the probit analysis method of Finney (23).
Squirrel Monkey Conflict Procedure. The effects of ocinaplon, diazepam, and vehicle were evaluated in a conflict procedure (22, 24) using adult, male squirrel monkeys (Saimiri sciureus). Drugs or vehicle were administered by gavage 60 min before placing the animals in the operant conditioning chamber. The mean reinforced responses, mean conflict responses, and mean conflict response failures were calculated for each dosage level. Significant differences between drug responding and control level (obtained on the day before treatment) were determined by using a paired t test. See Supporting Text for further details.
Clinical Study in Generalized Anxiety Disorder. Twenty centers in Germany received Ethics Committee approval to participate in this study, with 15 centers enrolling patients. The study was performed in accordance with the declaration of Helsinki, Good Clinical Practice on Medicinal Products in the European Community, Food and Drug Administration Good Clinical Practice Regulations, and International Harmonization Good Clinical Practice Guidelines. Study-related procedures were not initiated until a subject had given written informed consent on an Institutional Review Board-approved consent form. Eligible outpatients ≥18 years of age who met the DSM-IV criteria for general anxiety disorder (GAD), had sufficient initial symptom severity to require treatment [total score on the Hamilton Rating Scale for Anxiety (HAM-A) ≥ 22], and did not have coexisting depressive symptoms [total score on the Hamilton Rating Scale for Depression (HAM-D) ≤ 15] were included in a placebo run-in period (single-blind, 1-week dosing). Those individuals whose HAM-A scores did not decrease by >10% during the run-in period were enrolled in a 14-day, double-blind treatment period and randomized to one of three treatment groups. In addition to the principal efficacy measure, change in the HAM-A (25), secondary measures of efficacy included the clinical global impression scale, and the patient's self-rating scale for generalized anxiety (26). Repeated weekly assessments of symptom severity over time served as the dependent variable in the analyses for efficacy. Adverse experiences were evaluated at baseline (i.e., end of screening/placebo run-in period) and at the beginning of every week thereafter. Each nonlaboratory adverse experience reported by the patients was rated for its severity and for its relationship with the study drug. Vital signs were evaluated by monitoring sitting blood pressure, respiratory rate, temperature, and pulse rate. Screening and safety analysis consisted of blood chemistry and liver function tests in conjunction with urinalysis and hematologic testing (complete blood count).
Inhibition of Radioligand Binding to Native GABAA Receptors. The ability of ocinaplon to inhibit [3H]flunitrazepam binding to native GABAA receptors prepared from rat cortical and cerebellar membranes was performed essentially as described (ref. 27; see Supporting Text for further details).
Evaluation of GABA-Gated Currents in Recombinant Human GABAA Receptors Expressed in Xenopus Oocytes. cRNAs encoding GABAA receptor α1, 2-, 3-, or 5-, β2, and γ2- or 3-subunits were microinjected into Xenopus laevis oocytes. Forty-eight hours later, the effects of ocinaplon and diazepam were measured on GABA-gated inward currents using a Warner two-electrode voltage clamp amplifier in voltage clamp mode as described (see ref. 28 and Supporting Text for further details).
Animals. Studies in both rats and monkeys were conducted at Lederle Laboratories (Pearl River, NY). Protocols were approved by the Animal Care and Use Committee consistent with National Institutes of Health guidelines. Studies in X. laevis frogs were approved by the Animal Care and Use Committee, Boston University School of Medicine. Male CD rats (Charles River Breeding Laboratories) weighing 120-220 g were used in these experiments. Adult, male squirrel monkeys (S. sciureus) were obtained from a local colony. Female, oocyte-positive X. laevis frogs were purchased from Xenopus One (Ann Arbor, MI) or Nasco (Fort Atkinson, WI).
Drugs. Ocinaplon was manufactured by ACRAF SpA (Aprilia, Italy). Diazepam, flumazenil, and other reagents were obtained from Sigma/Research Biochemicals. Drugs were suspended in 0.5% methylcellulose containing 0.1% Tween 80.
Results
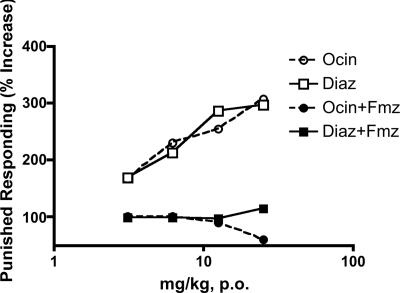
Preclinical Studies in Rodents and Primates. The ability of a drug to increase punished responding in “conflict” procedures is highly predictive of an antianxiety effect in humans (21, 22, 29). Ocinaplon produced a dose-related increase in punished responding in a “thirsty rat” conflict procedure (21) with a potency and efficacy comparable to the prototypic benzodiazepine, diazepam (Fig. 1). The minimum effective dose for each drug was 3.1 mg/kg when administered orally 1 h before testing. The ability of flumazenil (Ro 15-1788) to antagonize the anticonflict action of ocinaplon (Fig. 1) indicates that, like benzodiazepines (30), this effect is mediated by GABAA receptors. Furthermore, ocinaplon was as potent and effective as diazepam in reducing pentylenetetrazole-induced convulsions [ED50 values of 9.6 (95% CI; range, 7.9-12.1) mg/kg and 7.5 (95% CI; range, 5.3-10.6) mg/kg orally, respectively], another preclinical test that is highly predictive of an antianxiety effect in humans (22).
Fig. 1.
Anticonflict actions of ocinaplon and diazepam: blockade by flumazenil. Adult, male CD rats (Charles River Breeding Laboratories) were orally administered vehicle (0.5% methylcellulose containing 0.1% Tween 80), ocinaplon, or diazepam suspended in vehicle. Sixty minutes later, the effect of these treatments was evaluated in the “thirsty rat conflict” test, essentially as described (21). Flumazenil (12.5 mg/kg, i.p.) was administered 30 min before testing. This dose of flumazenil does not affect performance in the thirsty rat conflict test (data not shown). The minimum effective dose (the first dose producing a statistically significant difference from vehicle treated rats) of both ocinaplon and diazepam was 3.1 mg/kg. Values represent the mean (n ≥ 8 animals per dose) increase in punished responding compared to vehicle treated rats. Open circles, ocinaplon; open squares, diazepam; filled circles, ocinaplon plus flumazenil; filled squares, diazepam plus flumazenil.
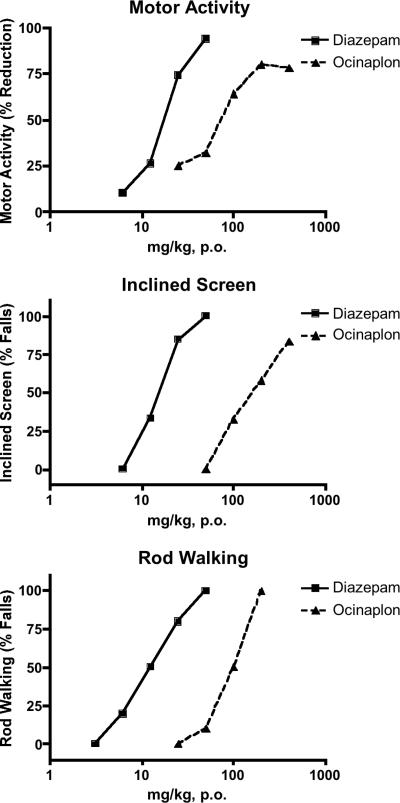
Ocinaplon produced performance deficits comparable to diazepam in the motor activity, rod-walking, and inclined screen tests (Fig. 2) that are considered predictive of side effects (sedation, ataxia, and muscle relaxation, respectively) typically associated with benzodiazepines (22). However, ocinaplon was 5- to 10-fold less potent than diazepam in disrupting performance in these tests, in contrast to its potency in the thirsty rat conflict and antipentylenetetrazole tests. This differential potency of ocinaplon in rodents is consistent with the profile of an “anxioselective” agent.
Fig. 2.
Effects of ocinaplon and diazepam on motor function in rats. Compounds were evaluated 60 min after oral administration. (Top) Motor activity. Values represent the mean % decrease in motor activity of 12-24 rats per dose compared to vehicle treated animals. The ED50 of diazepam and ocinaplon was 17.5 and 81.7 mg/kg, respectively. (Middle) Inclined screen. The effect of diazepam and ocinaplon was evaluated on the ability of rats to remain on an inclined (60°) screen for 30 min. The ED50 of diazepam and ocinaplon was 15.5 (3.5-24.9, 95% CI) and 172.2 (123.3-244.5, 95% CI) mg/kg, respectively. (Bottom) Rod walking. Animals were trained to traverse a rod inclined at 17°. Values represent the mean of 10 rats per dose. The ED50 of diazepam and ocinaplon was 13.8 (2.7-20.4, 95% CI) and 92 (68-124, 95% CI) mg/kg, respectively.
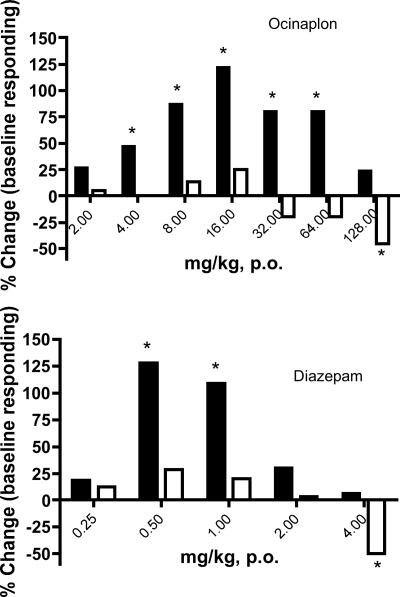
Ocinaplon produced a similar anxioselective profile in nonhuman primates. Squirrel monkeys were trained to bar press under conditions of both reward and punishment in a conflict procedure that is sensitive to clinically effective anxiolytics (22, 24). The minimum effective dose (MED) for ocinaplon to increase punished responding was 4 mg/kg orally; a significant increase in punished responding was maintained until a dose of 64 mg/kg. At a dose of 128 mg/kg, both punished and food reinforced responding were decreased (Fig. 3). The MED for diazepam to increase punished responding in this procedure was 0.5 mg/kg. However, this anticonflict effect was no longer apparent at doses ≥2 mg/kg, presumably because of disruption of motor activity that also significantly reduced responding under a reinforcing (nonpunishment) schedule at a dose of 4 mg/kg (Fig. 3).
Fig. 3.
Anticonflict actions of ocinaplon (Upper) and diazepam (Lower) in primates. Punished and nonpunished responding in adult, male squirrel monkeys was performed as described (24). Drugs were administered orally 60 min before a test session. A profound sedation was noted in animals administered 4mg/kg diazepam. Although a significant reduction in nonpunished responding was observed with ocinaplon at 128 mg/kg, overt signs of sedation were not noted. Filled bars, punished (conflict) responding; open bars, unpunished responding; *, P < 0.05 paired t test.
Ocinaplon in Patients with GAD. Based on these preclinical results, we initiated a multicenter, double-blind, placebo-controlled safety and efficacy study of ocinaplon in GAD. Eligible patients were enrolled in a 14-day double-blind treatment period and randomized to one of three treatment groups: placebo (n = 42), 60 mg of ocinaplon three times a day (TID) (n = 43), or 120 mg of ocinaplon twice daily (BID) (n = 42). The principal efficacy measure was change in the HAM-A score at the end of the 2-week study.
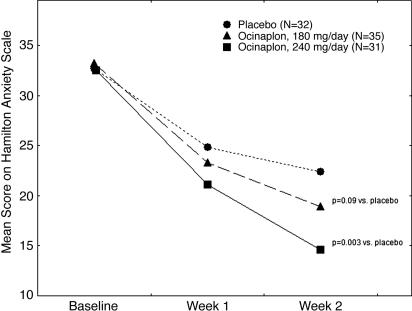
Hierarchical linear modeling analysis of HAM-A scores obtained from the intent-to-treat (ITT) population comprising all randomized subjects (including patients who did not complete the trial) yielded a significant interaction between time and treatment (t = 2.6, P = 0.011) for the global contrast between ocinaplon and placebo. Patients assigned to placebo displayed a mean reduction in HAM-A scores of 9.7 ± 1.4 (standard error) points (P = 0.001) after 2 weeks, whereas the reduction in total HAM-A scores in patients assigned to the 60-mg ocinaplon TID and 120-mg ocinaplon BID arms were 14.1 ± 1.9 and 15.3 ± 1.9 points, respectively. Analyses of the individual treatment group contrasts in the ITT population for the 2-week data demonstrated that 120-mg ocinaplon BID elicited a significantly greater reduction in symptom severity compared to placebo (t = 2.34, P = 0.02). The difference between 60-mg ocinaplon TID and placebo approached statistical significance (t = 1.89, P = 0.06). Investigation of the effect size estimates indicated that the two doses were essentially identical in efficacy (with slight numerical advantage for 120-mg ocinaplon BID compared to 60-mg ocinaplon TID). An analysis of the set of patients completing the study with no protocol deviation (n = 32, 35, and 31 for placebo, 180 mg of ocinaplon, and 240 mg of ocinaplon, respectively) yielded results (Fig. 4) essentially identical to those found in the ITT sample. A statistically significant difference in HAM-A scores for the global contrast between ocinaplon and placebo was observed as early as 1 week (P = 0.022) after the start of dosing. Statistically significant dose × time interactions were also observed for each individual dose of ocinaplon (t = 1.7, df = 66, P = 0.09 for 180 mg/day ocinaplon vs. placebo; t = 3.1, df = 62, P = 0.003 for 240 mg/day ocinaplon) compared to placebo. The reductions in HAM-A scores produced by ocinaplon were paralleled by changes in both the clinical global impression and self-rating scale measures (data not shown).
Fig. 4.
Anxiolytic effects of ocinaplon in GAD. Eligible patients with a diagnosis (HAM-A score of ≥20 at the end of the 1-week placebo run-in period) of GAD were randomized into three groups receiving placebo, 180 mg of ocinaplon (60 mg three times daily), or 240 mg of ocinaplon (120 mg twice daily) for 14 days. For each group and each time point, the depicted values represent the mean total score on the HAM-A scale of evaluable patients completing the study with no protocol deviation. The overall effect of ocinaplon on HAM-A scores was significantly different (P = 0.007) compared with placebo. Filled circles, placebo; open circles, ocinaplon, 180 mg/day; filled squares, ocinaplon 240 mg/day; a: t = 1.7, df = 66, P = 0.09 for Ocinaplon, 180 mg/day vs. placebo; b, t = 3.1, df = 62, P = 0.003 for Ocinaplon 240 mg/day vs. placebo. These t values represent the dose × time interaction for each individual dose of ocinaplon.
There were no treatment-emergent, serious adverse events in this trial. The number of patients in each group with at least one treatment emergent adverse event in the trial was relatively low (Table 1), and the proportion of patients with treatment emergent adverse events was comparable among treatment groups (placebo, 9.5%; 240 mg of ocinaplon, 9.5%; 180 mg of ocinaplon, 11.6%; P = 1.0, Fisher's exact test). No clinically significant, treatment-emergent laboratory abnormalities were detected in the study population. In general, the overall side-effect profile was unremarkable across the entire study population. Of particular note was an absence of significant findings or trends for the central nervous system and other effects normally associated with benzodiazepines such as sedation and dizziness (Table 1 and refs. 31 and 32).
Table 1. Summary of treatment emergent adverse events (AEs).
| Number of patients (%)
|
||||
|---|---|---|---|---|
| MedDRA system organ classification (SOC) | MedDRA preferred term | Placebo (n = 42) | TID60 (n = 43) | BID120 (n = 42) |
| One or more AEs* | All patients with AEs | 4 (9.5) | 5 (11.6) | 4 (9.5) |
| Gastrointestinal disorders | Patients with AEs | 1 (2.4) | 1 (2.3) | 1 (2.4) |
| Abdominal pain upper | 1 (2.4) | 0 | 0 | |
| Nausea | 0 | 1 (2.3) | 1 (2.4) | |
| General disorders and administration site conditions | Patients with AEs | 1 (2.4) | 1 (2.3) | 1 (2.4) |
| Fatigue | 1 (2.4) | 0 | 1 (2.4) | |
| Mucous membrane disorder NOS | 0 | 1 (2.3) | 0 | |
| Immune system disorders | Patients with AEs | 0 | 0 | 1 (2.4) |
| Conjunctivitis allergic | 0 | 0 | 1 (2.4) | |
| Investigations | Patients with AEs | 0 | 1 (2.3) | 0 |
| Heart rate increased | 0 | 1 (2.3) | 0 | |
| Musculoskeletal and connective tissue disorders | Patients with AEs | 2 (4.8) | 0 | 2 (4.8) |
| Back pain | 1 (2.4) | 0 | 0 | |
| Musculoskeletal chest pain | 0 | 0 | 1 (2.4) | |
| Neck pain | 0 | 0 | 1 (2.4) | |
| Torticollis | 1 (2.4) | 0 | 0 | |
| Nervous system disorders | Patients with AEs | 0 | 2 (4.7) | 1 (2.4) |
| Agitation | 0 | 1 (2.3) | 0 | |
| Sedation | 0 | 1 (2.3) | 0 | |
| Somnolence | 0 | 1 (2.3) | 0 | |
| Vertigo | 0 | 0 | 1 (2.4) | |
| Renal and urinary disorders | Patients with AEs | 0 | 1 (2.3) | 0 |
| Cystitis NOS | 0 | 1 (2.3) | 0 | |
| Respiratory, thoracic, and mediastinal disorders | Patients with AEs | 0 | 1 (2.3) | 0 |
| Sinusitis NOS | 0 | 1 (2.3) | 0 | |
Patients reporting individual AEs may not add up to the number of patients within a SOC because a subject may have reported more than one AE with in a SOC term. MeDRA, medical dictionary regulatory applications; NOS, not otherwise specified.
No significant difference among treatments in proportion of patients with AEs.
In Vitro Studies: Native and Recombinant GABAA Receptors. Ocinaplon selectively inhibited [3H]flunitrazepam binding in a broad receptor-binding screen using 30 different ligands (data not shown), consistent with the observation that Ro 15-1788 antagonizes the anticonflict actions of ocinaplon (Fig. 1). Ocinaplon was ≈3-fold more potent in inhibiting [3H]flunitrazepam binding to rat cerebellum than cortex (IC50 values of 1.2 μM and 3.8 μM, respectively, with Hill coefficients 0.9 and 0.7 in cerebellum and cortex, respectively). These radioligand-binding data indicate that compared to diazepam, the prototypic benzodiazepine, ocinaplon is a low-affinity ligand at GABAA receptors that exhibits a modest selectivity for GABAA1 receptors (receptors containing α1-subunits; ref. 5), because this subunit is enriched in cerebellum compared to cortex (6, 33, 34).
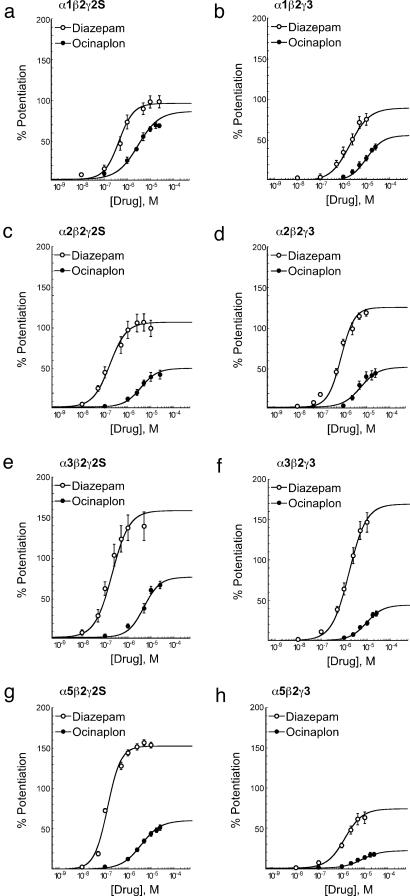
To determine how subunit composition influences the molecular actions of ocinaplon at GABAA receptors, cRNAs corresponding to specific combinations of α1, 2-, 3-, or 5-, β2, and γ2- or 3-subunits were subcloned and microinjected into X. laevis oocytes. Forty-eight hours later, the effects of ocinaplon and diazepam were measured on GABA-gated inward currents in the voltage clamp mode (28). Both the potency and efficacy (that is, the maximal effect) of diazepam to potentiate GABA-stimulated chloride currents varied with subunit composition (Fig. 5 and Table 2). Likewise, the potency and efficacy of ocinaplon varied with subunit composition (see Fig. 5 and Table 2), not only in an absolute sense but also relative to diazepam. Thus, ocinaplon increased GABA-stimulated chloride currents with efficacies ranging from ≈25% to 85% relative to diazepam, and potencies 5- to 35-fold lower than diazepam across these eight recombinant receptor isoforms. The greatest relative potentiation of GABA-stimulated currents was produced in receptors containing the α1-subunit. In those cells containing the α1β2γ2 isoform where ocinaplon displayed the greatest relative efficacy, diazepam was approximately eight times more potent than ocinaplon in its ability to potentiate GABA-stimulated currents (see Table 2), a relative potency comparable to that seen in the squirrel monkey conflict procedure (Fig. 3). The substitution of a γ3-subunit in this α1-containing complex reduced the relative efficacy of ocincaplon with only a small increase in relative potency. Similar substitution of a γ3-subunit in the α2-, α3-, and α5-containing complexes produced larger increases in the relative potency of ocinaplon while decreasing its relative efficacy (Table 2). Ocinaplon may thus be viewed as a partial positive modulator at α2-, α3-, and α5-containing receptors because its efficacy was substantially lower than diazepam at these receptor isoforms (Table 2).
Fig. 5.
Potentiation of GABA-gated currents by ocinaplon and diazepam in recombinant rat GABAA receptors expressed in Xenopus oocytes. Potentiation was determined by using an EC10 concentration of GABA. Curves were fitted by using nonlinear regression analysis and represent the mean values obtained from at least five oocytes harvested from at least two batches. Open circles, diazepam; filled circles, ocinaplon.
Table 2. Potencies and efficacies of diazepam and ocinaplon in recombinant GABAA receptors.
| Diazepam (Dz)
|
Ocinaplon (Ocin)
|
Ocin/Dz
|
||||||
|---|---|---|---|---|---|---|---|---|
| Receptor composition | Emax, % potentiation | EC50, μM | pEC50 | Emax, % potentiation | EC50, μM | pEC50 | EC50 | Emax |
| α1β2γ2S | 103 ± 8.4 | 0.36 | 6.45 ± 0.06 | 88 ± 8.6 | 3.07 | 5.51 ± 0.14 | 8.6 | 0.85 |
| α1β2γ3 | 90 ± 8.0 | 1.95 | 5.71 ± 0.06 | 51 ± 3.5 | 10.03 | 5.00 ± 0.05 | 5.1 | 0.57 |
| α2β2γ2S | 108 ± 13.5 | 0.16 | 6.81 ± 0.06 | 52 ± 8.8 | 3.39 | 5.47 ± 0.07 | 21.9 | 0.48 |
| α2β2γ3 | 127 ± 4.2 | 0.78 | 6.11 ± 0.02 | 46 ± 5.2 | 3.83 | 5.42 ± 0.04 | 4.9 | 0.36 |
| α3β2γ2S | 159 ± 16.9 | 0.20 | 6.71 ± 0.14 | 78 ± 7.1 | 4.59 | 5.34 ± 0.06 | 23.5 | 0.49 |
| α3β2γ3 | 166 ± 14.8 | 1.58 | 5.80 ± 0.04 | 41 ± 4.5 | 9.02 | 5.05 ± 0.05 | 5.7 | 0.25 |
| α5β2γ2S | 154 ± 8.0 | 0.11 | 6.96 ± 0.07 | 61 ± 4.2 | 3.79 | 5.42 ± 0.07 | 34.8 | 0.40 |
| α5β2γ3 | 74 ± 9.9 | 1.36 | 5.87 ± 0.03 | 22 ± 0.2 | 7.40 | 5.13 ± 0.05 | 5.4 | 0.30 |
Discussion
Ocinaplon is anxiolytic in rodents and primates, including humans, with a magnitude of effect comparable to benzodiazepines. Thus, in “conflict” procedures that are predictive of anxiolytic activity (22), ocinaplon produced a maximum response comparable to the prototypic benzodiazepine diazepam (Figs. 1 and 3). In GAD patients, the difference from placebo in the HAM-A score (4.4-5.6 points) in both ocinaplon dose groups (180- and 240-mg total daily dose) after 2 weeks of treatment (Fig. 4) was comparable to the difference (5.1 points) recently reported after 4 weeks of treatment with the benzodiazepine, lorazepam (6-mg total daily dose) (32). However, in contrast to the side effect profile typically produced by anxiolytic doses of benzodiazepines (e.g., a high incidence of patients experiencing somnolence, dizziness, and incoordination) (32), the incidence of adverse events with ocinaplon was not different from placebo (Table 1). Additional multicenter studies of longer duration are in progress to confirm both the efficacy of ocinaplon in reducing the symptoms of GAD and its apparent anxioselective profile.
Previously published results using genetically modified “knock-in” mice have emphasized α2-containing receptor isoforms as critical for mediating the anxiolytic effects of benzodiazepines, and α1-containing isoforms as important for mediating the sedative effects (35, 36). Thus, at face value, the present results are at variance with these previously reported data because ocinaplon was most potent and efficacious, both in an absolute sense and relative to diazepam, in receptors containing α1-subunits. Several different interpretations might explain this apparent inconsistency.
First, it is possible that ocinaplon and benzodiazepines act on different GABAA receptor subtypes such that ocinaplon produces its anxiolytic effects through actions on α1-containing complexes, whereas benzodiazepines and perhaps other structural classes of compounds (11, 12) produce anxiolytic effects through actions on α2-containing (GABAA2) receptors. This hypothesis is presently under investigation in selectively bred “knock-in” mice expressing either GABAA1 or GABAA2 receptors that are insensitive to benzodiazepines (35-37). Second, it is possible that only partial activation of α2-containing GABAA receptors by ocinaplon is sufficient to produce a full anxiolytic effect. The low efficacy of L-838,417 (which exhibits an anxioselective profile in animals) relative to benzodiazepines at α2-containing receptors is consistent with this hypothesis (12, 36). However, this hypothesis does not adequately explain the anxioselective property of ocinaplon, given its potency and efficacy at α1-containing receptors, which are presumed to mediate the sedative effects of benzodiazepines. A variation of this hypothesis posits that anxiolysis requires that a compound produce only partial activation, whereas “side effects” require full activation of GABAA receptors relative to a benzodiazepine. However, this hypothesis may also be viewed as flawed because ocinaplon, despite its partial agonist profile at α2-, α3-, and α5-containing receptors (Fig. 5 and Table 2), produces sedation, muscle relaxation, and ataxia, albeit at doses more than one order of magnitude higher than those producing an anxiolytic effect. Rather, a combination of high relative potency and high relative efficacy at certain receptor subtypes would seem to more parsimoniously explain its anxioselective properties. Finally, although studies using knock-in mice indicate that GABAA1 receptors contribute to the sedative and anticonvulsant properties of benzodiazepines (36, 37), pharmacological studies using a selective GABAA1 antagonist (38, 39) indicate that the anticonflict (but not the motor impairing) actions of diazepam (11, 40) are mediated via GABAA1 receptors. There are other significant inconsistencies between data obtained in knock-in mice and pharmacological studies using subtype selective pharmacological agents in wild type animals (41). It is not known whether such differences relate to the behavioral assays used, species differences, or a combination of these factors. Furthermore, given both the array of symptoms associated with GAD and the potential for GABAA receptor heterogeneity, ocinaplon may activate multiple GABAA receptor isoforms, including those not examined in the present study, to produce an anxioselective action in the clinic. The present results demonstrate ocinaplon represents both a significant advance in the treatment of generalized anxiety disorder and a unique tool for studying the molecular substrates of anxiety.
Supplementary Material
Author contributions: B.B., J.S., S.J.R., D.H.F., and P.S. designed research; J.S., B.B., E.K., M.C.G., S.B., and T.T.G. performed research; P.C., E.K., M.C.G., S.B., S.J.R., T.T.G., and P.S. analyzed data; and A.L., P.C., S.J.R., T.T.G., D.H.F., and P.S. wrote the paper.
Abbreviations: GAD, generalized anxiety disorder; HAM-A, Hamilton Rating Scale for Anxiety; HAM-D, Hamilton Rating Scale for Depression.
References
- 1.Stahl, S. M. (2002) J. Clin. Psychiatry 63, 756-757. [DOI] [PubMed] [Google Scholar]
- 2.Atack, J. R. (2003) Curr. Drug Target CNS Neurol. Disord. 2, 213-232. [DOI] [PubMed] [Google Scholar]
- 3.Choi, D. W., Farb, D. H. & Fischbach, G. D. (1977) Nature 269, 342-344. [DOI] [PubMed] [Google Scholar]
- 4.Haefely, W. & Polc, P. (1986) in Benzodiazepine/GABA Receptors and Chloride Channels, eds. Olsen, R. W. & Venter, C. (Liss, New York), pp. 97-133.
- 5.Barnard, E. A., Skolnick, P., Olsen, R. W., Mohler, H., Sieghart, W., Biggio, G., Braestrup, C., Bateson, A. N. & Langer, S. Z. (1998) Pharmacol. Rev. 50, 291-313. [PubMed] [Google Scholar]
- 6.De Blas, A. L. (1996) Mol. Neurobiol. 12, 55-71. [DOI] [PubMed] [Google Scholar]
- 7.Korpi, E. R., Grunder, G. & Luddens, H. (2002) Prog. Neurobiol. 67, 113-159. [DOI] [PubMed] [Google Scholar]
- 8.McKernan, R. M. & Whiting, P. J. (1996) Trends Neurosci. 19, 139-143. [DOI] [PubMed] [Google Scholar]
- 9.Lippa, A. S., Critchett, D., Sano, M. C., Klepner, C. A., Greenblatt, E. N., Coupet, J. & Beer, B. (1979) Pharmacol. Biochem. Behav. 10, 831-843. [DOI] [PubMed] [Google Scholar]
- 10.Lippa, A. S., Coupet, J., Greenblatt, E. N., Klepner, C. A. & Beer, B. (1979) Pharmacol. Biochem. Behav. 11, 99-106. [DOI] [PubMed] [Google Scholar]
- 11.Griebel, G., Perrault, G., Simiand, J., Cohen, C., Granger, P., Decobert, M., Francon, D., Avenet, P., Depoortere, H., Tan, S., et al. (2001) J. Pharmacol. Exp. Ther. 298, 753-768. [PubMed] [Google Scholar]
- 12.Johnstone, T. B., Hogenkamp, D. J., Coyne, L., Su, J., Halliwell, R. F., Tran, M. B., Yoshimura, R. F., Li, W. Y., Wang, J. & Gee, K. W. (2004) Nat. Med. 10, 31-32. [DOI] [PubMed] [Google Scholar]
- 13.Pritchett, D. B., Luddens, H. & Seeburg, P. H. (1989) Science 245, 1389-1392. [DOI] [PubMed] [Google Scholar]
- 14.Chan, C. Y. & Farb, D. H. (1985) J. Neurosci. 5, 2365-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wafford, K. A., Whiting, P. J. & Kemp, J. A. (1993) Mol. Pharmacol. 43, 240-244. [PubMed] [Google Scholar]
- 16.Lippa, A. S., Meyerson, L. R. & Beer, B. (1982) Life Sci. 31, 1409-1417. [DOI] [PubMed] [Google Scholar]
- 17.Haefely, W., Martin, J. R. & Schoch, P. (1990) Trends Pharmacol. Sci. 11, 452-456. [DOI] [PubMed] [Google Scholar]
- 18.Stephens, D. N., Turski, L., Hillman, M., Turner, J. D., Schneider, H. H. & Yamaguchi, M. (1992) Adv. Biochem. Psychopharmacol. 47, 395-405. [PubMed] [Google Scholar]
- 19.Ballenger, J. C., McDonald, S., Noyes, R., Rickels, K., Sussman, N., Woods, S., Patin, J. & Singer, J. (1991) Psychopharmacol. Bull. 27, 171-179. [PubMed] [Google Scholar]
- 20.van Steveninck, A. L., Gieschke, R., Schoemaker, R. C., Roncari, G., Tuk, B., Pieters, M. S., Breimer, D. D. & Cohen, A. F. (1996) Br. J. Clin. Pharmacol. 41, 565-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel, J. R., Beer, B. & Clody, D. E. (1971) Psychopharmacologia 21, 1-7. [DOI] [PubMed] [Google Scholar]
- 22.Lippa, A., Nash, P. A. & Greenblatt, D. J. (1979) in Anxiolytics: Industrial Pharmacology, eds. Fielding, S. & Lal, H. (Futura, Mount Kisco, NY), Vol. 3, pp. 41-81. [Google Scholar]
- 23.Finney, D. J. (1978) Statistical Methods in Biological Assay (Hafner, New York).
- 24.Greenblatt, E. N., Lippa, A. S. & Osterberg, A. C. (1978) Arch Int. Pharmacodyn. Ther. 233, 107-135. [PubMed] [Google Scholar]
- 25.Hamilton, M. (1959) Br. J. Med. Psychol. 23, 50-55. [DOI] [PubMed] [Google Scholar]
- 26.Guy, W. (1976) ECDEU Assessment Manual for Psychopharmacology (National Institute of Mental Health, Rockville, MD), revised edition.
- 27.Liu, R., Hu, R. J., Zhang, P., Skolnick, P. & Cook, J. M. (1996) J. Med. Chem. 39, 1928-1934. [DOI] [PubMed] [Google Scholar]
- 28.Park-Chung, M., Malayev, A., Purdy, R. H., Gibbs, T. T. & Farb, D. H. (1999) Brain Res. 830, 72-87. [DOI] [PubMed] [Google Scholar]
- 29.Millan, M. J. & Brocco, M. (2003) Eur. J. Pharmacol. 463, 67-96. [DOI] [PubMed] [Google Scholar]
- 30.Hunkeler, W., Mohler, H., Pieri, L., Polc, P., Bonetti, E. P., Cumin, R., Schaffner, R. & Haefely, W. (1981) Nature 290, 514-516. [DOI] [PubMed] [Google Scholar]
- 31.Feltner, D. E., Crockatt, J. G., Dubovsky, S. J., Cohn, C. K., Shrivastava, R. K., Targum, S. D., Liu-Dumaw, M., Carter, C. M. & Pande, A. C. (2003) J. Clin. Psychopharmacol. 23, 240-249. [DOI] [PubMed] [Google Scholar]
- 32.Pande, A. C., Crockatt, J. G., Feltner, D. E., Janney, C. A., Smith, W. T., Weisler, R., Londborg, P. D., Bielski, R. J., Zimbroff, D. L., Davidson, J. R. & Liu-Dumaw, M. (2003) Am. J. Psychiatry 160, 533-540. [DOI] [PubMed] [Google Scholar]
- 33.Luddens, H. & Wisden, W. (1991) Trends Pharmacol. Sci. 12, 49-51. [DOI] [PubMed] [Google Scholar]
- 34.Wisden, W., Laurie, D. J., Monyer, H. & Seeburg, P. H. (1992) J. Neurosci. 12, 1040-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low, K., Crestani, F., Keist, R., Benke, D., Brunig, I., Benson, J. A., Fritschy, J. M., Rulicke, T., Bluethmann, H., Mohler, H. & Rudolph, U. (2000) Science 290, 131-134. [DOI] [PubMed] [Google Scholar]
- 36.McKernan, R. M., Rosahl, T. W., Reynolds, D. S., Sur, C., Wafford, K. A., Atack, J. R., Farrar, S., Myers, J., Cook, G., Ferris, P., et al. (2000) Nat. Neurosci. 3, 587-592. [DOI] [PubMed] [Google Scholar]
- 37.Rudolph, U., Crestani, F., Benke, D., Brunig, I., Benson, J. A., Fritschy, J. M., Martin, J. R., Bluethmann, H. & Mohler, H. (1999) Nature 401, 796-800. [DOI] [PubMed] [Google Scholar]
- 38.Cox, E. D., Hagen, T. J., McKernan, R. M. & Cook, J. M. (1995) Med. Chem. Res. 5, 710-718. [Google Scholar]
- 39.Harvey, S. C., Foster, K. L., McKay, P. F., Carroll, M. R., Seyoum, R., Woods, J. E., Jr., Grey, C., Jones, C. M., McCane, S., Cummings, R., et al. (2002) J. Neurosci. 22, 3765-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shannon, H. E., Guzman, F. & Cook, J. M. (1984) Life Sci. 35, 2227-2236. [DOI] [PubMed] [Google Scholar]
- 41.Rosahl, T. W. (2003) Curr. Drug Target CNS Neurol. Disord. 2, 207-212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.