Abstract
There are numerous approaches for transcatheter aortic valve replacement (TAVR); however, access-related complications remain a point of concern. We analyzed consecutive patients who underwent TAVR for severe aortic stenosis via the brachiocephalic artery (BCA) without sternotomy (TBc group, n = 10) and via the trans-ascending aortic (TAo group, n = 8). The median BCA diameter and distance between the access point and suprasternal notch or superior margin of the clavicle were 11.3 mm and 8.3 mm, respectively. No patients in the TBc group underwent a partial sternotomy. Compared with the TAo group, the TBc group exhibited a shorter mean procedure time and lower blood loss volume as well as shorter duration of hospitalization. TAVR through the BCA may be a safe and feasible alternative for ascending aorta access. Studies with longer follow-up analysis and more patients are warranted to confirm our findings.
Key Words: alternative access, aortic valve stenosis, brachiocephalic artery, innominate artery, transcatheter aortic valve replacement
Graphical Abstract
Transcatheter aortic valve replacement (TAVR) is a viable treatment procedure for patients with symptomatic severe aortic valve stenosis and involves a significant mortality risk.1 The minimally invasive transfemoral (TF) approach for retrograde implantation of the transcatheter heart valve is the most accepted procedure. However, some patients lack healthy iliac or femoral arteries.2 Moreover, vascular complications, especially arterial injury at the access point, influence the clinical outcomes of TF-TAVR.2,3 Accordingly, alternative approaches, including transapical, trans-ascending aortic (TAo), subclavian, axillary, caval, and carotid access, have been widely adopted.
Given that a few case series have shown the feasibility of the procedure with partial sternotomy,4 we compared a case series of 10 consecutive patients who underwent TAVR procedures via brachiocephalic artery (BCA) access using an Medtronic transcatheter valve (Evolut R and Evolut PRO+, Medtronic, Inc) and the Edwards transcatheter valve (Sapien 3, Edwards Lifesciences) without sternotomy as well as through alternative access sites, such as the TAo approach for TAVR. We describe the current technical and decisional considerations as well as the limitations of the trans-brachiocephalic (TBc) approach.
Methods
Patient Characteristics
Between August 2019 and April 2021, we performed 175 consecutive TAVRs; among them, 157 and 18 were performed through the femoral artery and alternative vessels, respectively. Among the 18 procedures, 10 were performed using the TBc approach and 8 using the TAo approach via partial sternotomy/mini-thoracotomy. In the TBc group, 9 patients were treated with the self-expanding bioprosthesis and 1 patient with the balloon-expandable bioprosthesis. In the TAo group, 5 patients were treated with the self-expanding bioprosthesis and 3 with the balloon-expandable bioprosthesis. Table 1 presents the patient characteristics.
Table 1.
Baseline Patient Characteristics and Procedural and Clinical Outcomes According to Approach
| TBc (n = 10) | TAo (n = 8) | P Value | |
|---|---|---|---|
| Baseline characteristics | |||
| Female | 10 (100) | 4 (50) | 0.011 |
| Age, y | 87.9 ± 4.6 | 85.7 ± 3.5 | 0.298 |
| EuroSCORE II, % | 8.4 ± 5.5 | 9.1 ± 2.9 | 0.781 |
| Aortic valve area, cm2 | 0.58 ± 0.20 | 0.69 ± 0.12 | 0.206 |
| Mean aortic valve gradient, mm Hg | 48.8 ± 12.5 | 57.1 ± 19.4 | 0.287 |
| LVEF, % | 54.8 ± 9.9 | 61.1 ± 13.0 | 0.265 |
| Previous CABG or PCI | 3 (30) | 2 (25) | 0.813 |
| Previous stroke | 1 (10) | 0 | 0.357 |
| CKD stage 4 or 5 | 3 (30) | 3 (37.5) | 0.737 |
| Procedural outcomes | |||
| Mean procedure time, min | 83.7 ± 13.8 | 119.2 ± 27.0 | 0.0023 |
| Mean blood loss, mL | 44.5 ± 42.1 | 289.8 ± 145.8 | <0.001 |
| Mean blood transfusion, units | 0.6 ± 1.3 | 1.7 ± 2.2 | 0.197 |
| Mean fluoroscopy time, min | 26.7 ± 5.5 | 22.1 ± 8.8 | 0.190 |
| Mean volume of contrast medium, mL | 43.9 ± 15.8 | 62.1 ± 45.9 | 0.256 |
| Clinical outcomes | |||
| All-cause mortality | 0 | 1 (12.5) | 0.250 |
| All stroke (disabling/nondisabling) | 0 | 0 | |
| Life-threatening bleeding | 0 | 0 | |
| Acute kidney injury | 0 | 1 (12.5) | 0.250 |
| Coronary artery obstruction | 0 | 0 | |
| Valve-related dysfunction | 0 | 0 | |
| Aortic regurgitation more than moderate | 0 | 0 | |
| New permanent PM implantation | 0 | 1 (12.5) | 0.250 |
| Mean hospital stay, d | 7.1 ± 3.0 | 13.1 ± 7.7 | 0.0385 |
Values are n (%) or mean ± SD.
CABG = coronary artery bypass grafting; CKD = chronic kidney disease; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; PM = pacemaker; TAo = trans-ascending aortic; TBc = trans-brachiocephalic.
All patients provided informed consent for undergoing the procedures; this study was approved by the Institutional Review Board of Kurashiki Central Hospital (reference number 3678). These patients were considered to be at high risk of surgical aortic valve replacement and consequently indicated for TAVR.
Preoperative Investigations
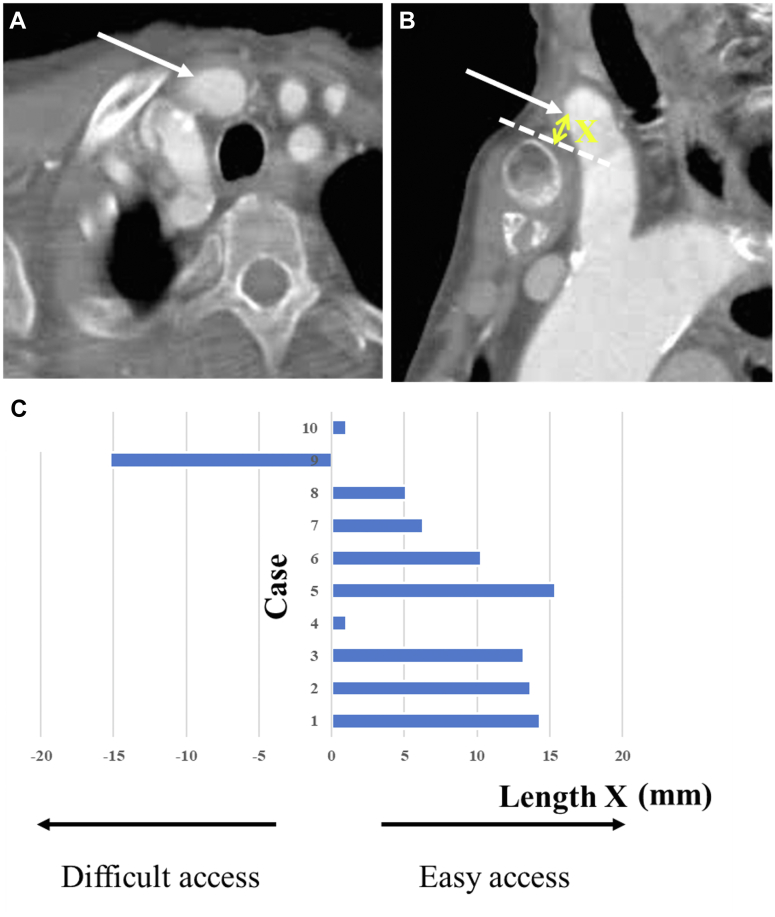
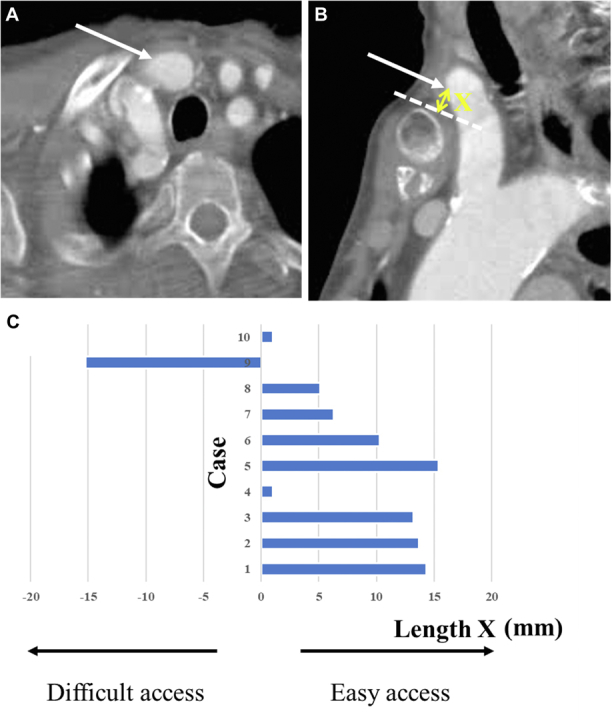
Electrocardiogram-triggered multidetector computed tomography images were routinely studied for accurate measurements of the aortic valve annulus, sinotubular junction, sinus of Valsalva, and other structures (Siemens AG). The bifurcation of the BCA into the right subclavian and right common carotid arteries is typically either behind the sternum/right clavicle or cranially higher. The need for sternotomy was preoperatively determined. Specifically, if the BCA bifurcation reached the upper rim level of the manubrium or passed across it slightly, the BCA was accessed suprasternally without sternotomy (Figures 1A and 1B); alternatively, TAo access with partial sternotomy or right mini thoracotomy was applied as the second-best alternative approach.
Figure 1.
Preoperative CT Images and Data
The distance between the access point and suprasternal notch or superior margin of the clavicle: axial view (A) and sagittal view (B). (C) Preoperative computed tomography (CT) data. Dotted line indicates the upper border of the manubrium; the arrows indicate access site; and X indicates the distance between the access point and suprasternal notch or superior margin of the clavicle.
Operative Technique
A 2- to 3-cm oblique cervical incision was made, the sheath was introduced from the access point, and a stiff guidewire was passed through the aortic valve. Subsequently, slow and gradual deployment was performed during rapid ventricular pacing. Transesophageal echocardiography was used to ensure correct placement and to identify perivalvular leaks.
Discharge and Follow-Up
According to the requirements of our outpatient clinic, patients were closely monitored postoperatively through patient interviews, clinical examination, follow-up echocardiography, and enhanced computed tomography scans.
Statistical Analysis
The Shapiro-Wilk test was performed to test for normality. Continuous variables are presented as mean ± SD (normally distributed) or median and range (non-normally distributed). The Student’s t-test was used to compare normally distributed variables between groups, and the Mann-Whitney U test was used for non-normally distributed variables. The chi-square or Fisher exact test was used to compare categorical variables between groups, as appropriate. Statistical analyses were performed by using SPSS version 22 (IBM SPSS Statistics, IBM Corporation).
Results
Preoperative
The median BCA diameter was 11.3 mm (range, 10.1 to 16.2 mm); the median distance between the access point and the aortic valve annulus was 12.7 mm (range, 11.1 to 14.1 mm). The median distance between the access point and suprasternal notch or superior margin of the clavicle (Length X in Figure 1B) was 8.3 mm (range, –15.2 to 15.4 mm). If the access point was beneath the sternal manubrium or the right clavicle, X was a negative in value. Figure 1C describes the computed tomography data.
Intraoperative
BCA access was obtained suprasternally in all patients. No patient underwent partial sternotomy for BCA access; however, in the TAo group, 6 patients underwent partial sternotomy and 2 underwent right mini-thoracotomy. The mean procedure time was shorter in the TBc group than in the TAo group (83.7 ± 13.8 minutes vs 119.2 ± 27.0 minutes; P = 0.002) (Table 1). After valve implantation, the mean gradient decreased from 48.8 ± 12.5 mm Hg to 7.4 ± 3.3 mm Hg in the TBc group and from 57.1 ± 19.4 mm Hg to 9.5 ± 1.8 mm Hg in the TAo group. No perioperative cerebral or cardiac ischemia was observed in either group; furthermore, no surgical conversion to full sternotomy was required.
Postoperative Outcomes
During the early postoperative course, no significant complications occurred in the TBc group. However, one hospital death due to nonocclusive mesenteric ischemia occurred in the TAo group. The mean hospitalization length was shorter in the TBc group than in the TAo group (7.1 ± 3.0 days vs 13.1 ± 7.7 days; P = 0.038).
The 30-day survival rate was 100% and 87.5% in the TBc and TAo groups, respectively. Table 1 summarizes the early safety clinical endpoints as proposed by the Valve Academic Research Consortium 3 criteria.5 There were no TAVR-related complications in the TBc group. Contrastingly, in the TAo group, one patient required temporary hemodialysis for acute kidney injury and another required implantation of a permanent pacemaker.
Early Follow-Up Data
The mean follow-up period was 181.8 days, with no deaths occurring during this period. There were no early valve reinterventions or open conversions; moreover, no valve-related dysfunction was observed in either group. None of the patients has required aortic valve reintervention.
Discussion
This study is the first to report the outcomes of TBc-TAVR without sternotomy wherein primary alternative access was achieved via the TBc approach in case of poor TF access. No vascular or access-related complications were experienced at the BCA or ascending aorta access sites. Our findings indicate that BCA access could be feasible and safe, with low rates of major vascular complications, bleeding, and stroke compared with ascending aorta access if the BCA is positioned suprasternally or accessible without sternotomy. The reported outcomes are consistent with previously reported TF-TAVR outcomes.6
Since August 2019, in cases in which femoral access is inappropriate, we have favored the BCA as an access point; if unsuitable, the next alternative is direct ascending aorta access through a second or third right mini-thoracotomy or through a partial sternotomy.
The following conditions should be satisfied for BCA access in TAVR7: 1) BCA diameter (>8 mm on preoperative computed tomography imaging) large enough to insert the 18-F delivery sheath, which allows blood flow through the gap between the sheath and vessel; 2) absence of BCA occlusion increasing the risk of intraoperative cerebral ischemia; and 3) no calcifications in the artery causing vascular stenosis. Contralateral or left atherosclerosis in the carotid artery with significant stenosis or occlusion are contraindications for TBc-TAVR.
There is a low risk of periprocedural distal ischemia due to insertion of the TAVR introducer sheath because the diameter of the BCA is larger than that of the subclavian and carotid arteries. In this case series, no clinically assessed stroke or temporary ischemic attack occurred in either group. The alternative BCA access could have a low risk of mortality and morbidity that is similar, or even superior, to that with the TF approach.
Other alternative TAVR approaches have limitations.8 For example, although the transapical approach has been extensively adopted, it is more invasive because it requires thorax opening and is unfavorable for patients with respiratory dysfunction. Moreover, the trans-ascending aortic approach requires a partial sternotomy or anterior thoracotomy. In fact, we observed a shorter mean procedure time and a lower blood loss volume in the TBc group than in the TAo group. In case of contraindications for trans-ascending aortic or transfemoral access, subclavian artery access becomes another candidate. The trans-subclavian approach can be either left- or right-sided. From our experience, the subclavian arteries are often calcified or small, and the TAVR system transverses the aortic arch in patients with diffuse, brittle atheroma (shaggy), which presents a risk of vascular complications. In our practice, we mostly use transaxillary access. The choice of artery depends on the transcatheter heart valve used and the root angle. Transaxillary access with an evolute valve typically requires a lower root angle (<30° to 40°). Another alternative approach for TAVR is the carotid artery, which can be easily accessed, and the access position can be controlled. Because carotid arteries often have a small diameter, the delivery sheath could lead to occlusion and a decrease in cerebral perfusion during the procedure. Finally, transcaval access may be excluded from the list of alternative approaches for large-bore devices as it has the highest risk of major vascular complications.9
Cervical artery tortuosity is more common on the right side in older patients. Honig et al10 reported that it is caused by arterial dilation in response to arterial atherosclerotic changes in the arteries. Furthermore, when left ventricular hypertrophy occurs, the ascending aorta is pushed upward, which causes the BCA to be pushed upward and sag, resulting in flexion and tortuosity. Therefore, in East Asia, which has a large older population, the BCA might be the preferred access site, as observed in our case series.
Study Limitations
This was a small single-center case series with a short follow-up period. Although we described the feasibility and good short-term outcomes with 2 different approaches, large-scale studies with a long-term follow-up period are warranted to show and verify the efficacy of brachiocephalic access.
Conclusions
We found that the brachiocephalic approach could be safe and feasible for TAVR in cases in which TF access is contraindicated. The most important factor when considering the TAVR approach is personalized tailoring of the access point to facilitate a successful outcome.
Funding Support and Author Disclosures
Dr Fuku is a proctor for Medtronic and Edwards Lifesciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 2.Toggweiler S., Leipsic J., Binder R.K., et al. Management of vascular access in transcatheter aortic valve replacement: part 1: basic anatomy, imaging, sheaths, wires, and access routes. J Am Coll Cardiol Intv. 2013;6:643–653. doi: 10.1016/j.jcin.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Toggweiler S., Leipsic J., Binder R.K., et al. Management of vascular access in transcatheter aortic valve replacement: part 2: vascular complications. J Am Coll Cardiol Intv. 2013;6:767–776. doi: 10.1016/j.jcin.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Philipsen T.E., Collas V.M., Rodrigus I.E., et al. Brachiocephalic artery access in transcatheter aortic valve implantation: a valuable alternative: 3-year institutional experience. Interact Cardiovasc Thorac Surg. 2015;21:734–740. doi: 10.1093/icvts/ivv262. [DOI] [PubMed] [Google Scholar]
- 5.Généreux P., Piazza N., Alu M.C., et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77:2717–2746. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Beohar N., Kirtane A.J., Blackstone E., et al. Trends in complications and outcomes of patients undergoing transfemoral transcatheter aortic valve replacement: experience from the PARTNER continued access registry. J Am Coll Cardiol Intv. 2016;9:355–363. doi: 10.1016/j.jcin.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 7.Philipsen T.E., Rodrigus I.E., Claeys M.J., Bosmans J.M. Alternative access in transcatheter aortic valve implantation: brachiocephalic artery access. Innovations (Phila) 2012;7:372–375. doi: 10.1097/IMI.0b013e31827e5934. [DOI] [PubMed] [Google Scholar]
- 8.Bax J.J., Delgado V., Bapat V., et al. Open issues in transcatheter aortic valve implantation. Part 2: procedural issues and outcomes after transcatheter aortic valve implantation. Eur Heart J. 2014;35:2639–2654. doi: 10.1093/eurheartj/ehu257. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama Y., Sakata T., Mikami T., et al. Vascular access for transcatheter aortic valve replacement: a network meta-analysis. J Cardiol. 2023;82:227–233. doi: 10.1016/j.jjcc.2023.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Honig E.I., Dubilier W., Jr., Steinberg I. Significance of the buckled innominate artery. Ann Intern Med. 1953;39:74–80. doi: 10.7326/0003-4819-39-1-74. [DOI] [PubMed] [Google Scholar]