Abstract
Background
Stuttering priapism is recurrent, self-limited episodes of sustained penile erection and is common in patients with sickle cell disease (SCD). Prevention of stuttering priapism is important to avoid progression to episodes of ischemic priapism which can cause erectile dysfunction. Priapism has been shown to be associated with increased nocturnal hypoxemia in patients with SCD.
Case Description
A 43-year-old male with nocturnal episodes of stuttering priapism that was refractory to treatment with multiple medications was found to have obstructive sleep apnea (OSA). Following treatment of this condition with a continuous positive airway pressure (CPAP), the patient had immediate symptom relief and has had three months without an episode of priapism.
Conclusions
OSA should be considered as an underlying cause of nocturnal stuttering priapism in patients with SCD, particularly in patients who present with stuttering priapism later in life or patients who present strictly with nocturnal episodes. Appropriate management of OSA can significantly decrease the incidence of stuttering priapism in patients with SCD.
Keywords: Priapism, stuttering priapism, obstructive sleep apnea (OSA), sickle cell disease (SCD), case report
Highlight box.
Key findings
• Treatment of obstructive sleep apnea (OSA) can alleviate stuttering priapism in patients with sickle cell disease.
• OSA screening may be helpful in patients with refractory stuttering priapism.
What is known and what is new?
• Patients with sickle cell disease are at increased risk of stuttering priapism.
• This report presents a novel case of a patient with refractory stuttering priapism achieving complete symptom improvement following OSA diagnosis and treatment.
What is the implication, and what should change now?
• OSA should be investigated and managed as an underlying cause of stuttering priapism patients with sickle cell disease.
• Controlled studies comparing continuous positive airway pressure ± medications could provide a clear answer to a possible association.
Introduction
Stuttering priapism is defined as recurrent, self-limited episodes of sustained penile erection typically lasting less than 4 hours (1). In one third of cases these episodes progress to complete ischemic priapism (lasting >4 hours) which damages the erectile bodies and can cause permanent erectile dysfunction (1,2). Although this condition is relatively uncommon in the general population, males with sickle cell disease (SCD) are at an increased risk, affecting between 30% and 45% of all adult males with SCD (3). The pathophysiology of priapism in SCD is thought to be due to sickling, hemolysis, and reduced nitric oxide availability (4). Sickling episodes are often precipitated by hypoxic conditions (5). Obstructive sleep apnea (OSA) is a common cause of nocturnal hypoxic episodes due to partial and complete airway obstructions during sleep (6). OSA has been associated with higher rates of many SCD complications including neurologic and cardiovascular complications (7). Here we discuss treatment of OSA for prevention of refractory stuttering priapism in a patient with SCD, which has not been previously reported in the literature. We present this case in accordance with the CARE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-94/rc).
Case presentation
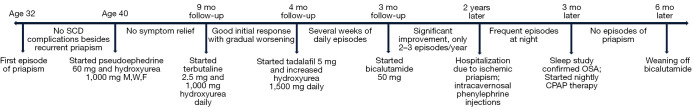
The patient is a 43-year-old African-American male diagnosed with sickle cell anemia (HbSS disease confirmed by genotyping) that was managed with hydroxyurea for sickle crisis prevention. He had not experienced any significant symptoms attributed to his medical condition until the age of 32 years old when he presented with an episode of priapism which was managed by treating the underlying hemoglobinopathy (Figure 1).
Figure 1.
Timeline of symptoms and treatment adjustments. SCD, sickle cell disease; M, Monday; W, Wednesday; F, Friday; mo, months; OSA, obstructive sleep apnea; CPAP, continuous positive airway pressure.
His disease course had been rather uneventful until the age 40 years old when he started experiencing recurrent painful nighttime bouts of priapism lasting up to 2 hours. This resulted in multiple visits to the emergency department. He was diagnosed with stuttering priapism due to his SCD and was commenced on pseudoephedrine 60 mg at bedtime and hydroxyurea 1,000 mg every Monday, Wednesday, and Friday.
Further follow-up 9 months later revealed that oral pseudoephedrine had not resulted in meaningful pain and symptom relief. After discussing the available treatment options, it was decided to start the patient on terbutaline 2.5 mg daily. Around this same time his hydroxyurea dose was changed to 1,000 mg daily. The patient initially responded well to the combination of pseudoephedrine and terbutaline, but the effect appeared to decline within 4 months of treatment.
Next it was decided to add daily dosages of tadalafil 5 mg to his drug regimen and his hydroxyurea dosage was increased to 1,500 mg per day by his hematologist. After 3 months of sustained pain relief, he complained of symptom reemergence and painful stuttering priapism. After experiencing several weeks of painful, nightly priapism he was started on bicalutamide 50 mg per day, in addition to his previous medications. His symptoms were well controlled for approximately 2 years with this drug combination. The patient only experienced 2–3 episodes of priapism per year on this medication regimen until he experienced an episode of acute penile swelling and a refractory erection. Considering this acute exacerbation, he was admitted to the hospital and received intracavernosal phenylephrine injections and oxycodone for pain control. He was advised to continue his previous medications upon discharge from the hospital, but he started experiencing worsening of his symptoms in the absence of any predisposing factor such as infectious diseases.
A detailed review of the patient’s history revealed that he was only experiencing these symptoms during nighttime. On further questioning his partner declared that he was a loud snorer. We hypothesized that hypoxia due to OSA may be a reason for the ineffectiveness of the multiple therapies used in this patient. As a result of this suspicion a sleep study was ordered. His sleep study results indicated that his nighttime O2 saturation had a minimum of 85% and had a stretch of saturation below 90% for 2 minutes consistent with OSA. Complete resolution of symptoms was achieved immediately after starting continuous positive airway pressure (CPAP) and he did not experience even minimal symptoms of painful erections thereafter. Evaluation after 3 months of drug therapy plus CPAP shows adequate control of the previously refractory priapism episodes and retaining normal erections. Follow-up at 6 months pot CPAP revealed normal erectile function during sexual arousal and no episode of priapism; therefore, we decided to wean off the bicalutamide. He does not report any irregularities at 7 months post CPAP (1 month without bicalutamide and only with CPAP).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Stuttering priapism is more common in patients with SCD than the general population (3). It is important that this condition be managed appropriately to avoid permanent damage—erectile dysfunction is 5-fold higher in SCD patients that experience priapism than in the general population (8). Also, priapism is associated with anxiety, depression, and worse quality of life in SCD patients (9). Our patient’s priapism episodes were refractory to multiple lines of preventative medications. Medications that have shown effectiveness for the prevention of priapism in patients with SCD include hydroxyurea at the maximally tolerated dose (10), PDE-5 inhibitors (sildenafil and tadalafil) (11), alpha adrenergic agonists (etilefrine, oral pseudoephedrine) (12,13), anti-androgens (bicalutamide) (14), and alpha-5 reductase inhibitor (finasteride) (15), though there are no guidelines on the subject. Our patient was taking pseudoephedrine 60 mg, terbutaline 2.5 mg, tadalafil 5 mg, hydroxyurea 1,500 mg, and bicalutamide 50 mg yet still experiencing stuttering priapism with an episode of acute ischemic priapism requiring hospitalization and intracavernosal phenylephrine injections. Patients with ischemic priapism that is refractory to medical management require more invasive therapies. Although our patient has never required aspiration of blood from the corpus cavernosum, this would be the next step of management of ischemic priapism that is unresponsive to phenylephrine injections (16). If this intervention is unsuccessful, a surgical shunt may be necessary (17). These invasive procedures come with risks such as infection or urethral injury (17), therefore priapism prevention is key and when SCD patients present with ischemic priapism it should prompt assessment for secondary conditions like OSA which may be contributing.
It is also important to consider contributing comorbidities when a patient presents with an atypical disease course. Our patient’s case was unusual in that his initial presentation of priapism was later in life, at age 32 years old, whereas 90% patients with SCD who develop priapism have their first episode before age 20 years old (18). When patients with SCD present with priapism later in life it is important to assess for comorbidities that may be contributing, particularly OSA, because the prevalence of OSA increases monotonically with age (19). Another sign that a patient with stuttering priapism may have OSA is if their priapism episodes only occur at night, as was the case for our patient. However, this is not a definitive sign of OSA, as priapism is more common at night for all patients with SCD. Mantadakis et al. surveyed 98 males with SCD, of whom 75% reported episodes during sleep with the average time of episodes at 4:00 AM (20). SCD patients who have a history of priapism have been found to have physiologic differences in sleep which may explain this propensity for nocturnal erections.
A study assessing the pattern of penile rigidity during sleep in adults with SCD and a history of priapism found that 77.8% of cases had an apnea hypopnea index (AHI) of greater than 5 events per hour compared to 31.2% of SCD patients without priapism. The patients with priapism history in this study also had an increase in oxyhemoglobin desaturation, arousals, and periodic leg movements compared to the control group. The oxyhemoglobin saturation is particularly important as oxyhemoglobin saturation less than 90% was associated with priapism after adjusting for lung involvement and AHI (21). The association between priapism and oxyhemoglobin desaturation in patients with SCD is further supported by a study conducted by Stauffer et al. which found a higher proportion of hemolytic complications, including priapism, in SCD patients with nocturnal hypoxemia, measured as oxyhemoglobin saturation less than 90%. However, this study also found that the proportion of patients suffering from nocturnal hypoxemia did not differ between those with and without OSA and therefore this study did not find an increased risk of priapism associated with OSA (defined by AHI) (22). AHI is a common measurement in the evaluation of OSA but does not take into account the duration and depth of abnormal respiratory events (23). Overall, the findings from these two studies indicate that oxyhemoglobin desaturation is the most important risk factor for priapism in patients with SCD.
Treatment with a CPAP has been shown to improve oxyhemoglobin saturation and reduce AHI in patients with OSA (24). As SCD patients with history of priapism have been found to have an increased AHI and oxyhemoglobin desaturation (21), CPAP therapy would be expected to provide great benefit for these patients. The impact of CPAP treatment of OSA on the frequency of priapism episodes in patients with SCD has not previously been reported. Our patient’s case demonstrates the significant impact of CPAP therapy on priapism symptoms in patients with SCD. He had immediate improvement upon the initiation of CPAP therapy with three priapism free months since beginning treatment for his underlying OSA. This remarkable improvement for a patient who had not found symptomatic relief despite the usage multiple medications underscores the importance of assessing for and treating OSA in SCD patients with nighttime priapism.
Considering the rarity of our findings, we believe the current existing guidelines should be used first for treating stuttering priapism in all patients regardless of sleep abnormalities. However, we do not see any harm in including a focus on OSA during the initial workup such as screening the patients with the STOP-Bang questionnaire that provides an insight regarding sleep abnormalities. From our experience, we believe pharmacotherapy should be used per the current guidelines and OSA could be treated after determining the lack of function by drug therapy. At this time, we are unable to say whether or not CPAP therapy without initial pharmacotherapy could lead to favorable results.
Conclusions
This patient’s case demonstrates the value of identification and management of OSA for the treatment of refractory stuttering priapism in patients with SCD. Treating OSA led to rapid improvement of this patient’s stuttering priapism symptoms and so far, he has enjoyed three months free from any stuttering priapism episodes. OSA should particularly be considered in patients with nocturnal episodes of stuttering priapism, patients whose priapism has been refractory to multiple medications, and SCD patients with initial onset of stuttering priapism symptoms in adulthood.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-94/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-94/coif). The authors have no conflicts of interest to declare.
References
- 1.Abdeen BM, Leslie SW. Stuttering Priapism. [Updated 2023 Oct 31]. In: StatPearls [Internet]. StatPearls Publishing; 2023 Jan. Accessed December 19, 2023. [Google Scholar]
- 2.Liguori G, Rizzo M, Boschian R, et al. The management of stuttering priapism. Minerva Urol Nefrol 2020;72:173-86. 10.23736/S0393-2249.19.03323-X [DOI] [PubMed] [Google Scholar]
- 3.Ekong A, Berg L, Amos RJ, et al. Regular automated red cell exchange transfusion in the management of stuttering priapism complicating sickle cell disease. Br J Haematol 2018;180:585-8. 10.1111/bjh.14393 [DOI] [PubMed] [Google Scholar]
- 4.Nolan VG, Wyszynski DF, Farrer LA, et al. Hemolysis-associated priapism in sickle cell disease. Blood 2005;106:3264-7. 10.1182/blood-2005-04-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun K, Xia Y. New insights into sickle cell disease: a disease of hypoxia. Curr Opin Hematol 2013;20:215-21. 10.1097/MOH.0b013e32835f55f9 [DOI] [PubMed] [Google Scholar]
- 6.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 2015;7:1311-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz T, Schatz J, Roberts CW. Comorbid obstructive sleep apnea and increased risk for sickle cell disease morbidity. Sleep Breath 2018;22:797-804. 10.1007/s11325-018-1630-x [DOI] [PubMed] [Google Scholar]
- 8.Idris IM, Burnett AL, DeBaun MR. Epidemiology and treatment of priapism in sickle cell disease. Hematology Am Soc Hematol Educ Program 2022;2022:450-8. 10.1182/hematology.2022000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Idris IM, Bonnet K, Schlundt D, et al. Psychometric Impact of Priapism on Lives of Adolescents and Adults With Sickle Cell Anemia: A Sequential Independent Mixed-Methods Design. J Pediatr Hematol Oncol 2022;44:19-27. 10.1097/MPH.0000000000002056 [DOI] [PubMed] [Google Scholar]
- 10.Saad ST, Lajolo C, Gilli S, et al. Follow-up of sickle cell disease patients with priapism treated by hydroxyurea. Am J Hematol 2004;77:45-9. 10.1002/ajh.20142 [DOI] [PubMed] [Google Scholar]
- 11.Hou LT, Burnett AL. Regimented Phosphodiesterase Type 5 Inhibitor Use Reduces Emergency Department Visits for Recurrent Ischemic Priapism. J Urol 2021;205:545-53. 10.1097/JU.0000000000001365 [DOI] [PubMed] [Google Scholar]
- 12.Okpala I, Westerdale N, Jegede T, et al. Etilefrine for the prevention of priapism in adult sickle cell disease. Br J Haematol 2002;118:918-21. 10.1046/j.1365-2141.2002.03691.x [DOI] [PubMed] [Google Scholar]
- 13.Mocniak M, Durkin CM, Early K. The use of sudafed for priapism in pediatric patients with sickle cell disease. J Pediatr Nurs 2012;27:82-4. 10.1016/j.pedn.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 14.Dahm P, Rao DS, Donatucci CF. Antiandrogens in the treatment of priapism. Urology 2002;59:138. 10.1016/S0090-4295(01)01492-3 [DOI] [PubMed] [Google Scholar]
- 15.Rachid-Filho D, Cavalcanti AG, Favorito LA, et al. Treatment of recurrent priapism in sickle cell anemia with finasteride: a new approach. Urology 2009;74:1054-7. 10.1016/j.urology.2009.04.071 [DOI] [PubMed] [Google Scholar]
- 16.Bivalacqua TJ, Allen BK, Brock GB, et al. The Diagnosis and Management of Recurrent Ischemic Priapism, Priapism in Sickle Cell Patients, and Non-Ischemic Priapism: An AUA/SMSNA Guideline. J Urol 2022;208:43-52. 10.1097/JU.0000000000002767 [DOI] [PubMed] [Google Scholar]
- 17.Segal RL, Readal N, Pierorazio PM, et al. Corporal Burnett "Snake" surgical maneuver for the treatment of ischemic priapism: long-term followup. J Urol 2013;189:1025-9. 10.1016/j.juro.2012.08.245 [DOI] [PubMed] [Google Scholar]
- 18.Rogers ZR. Priapism in sickle cell disease. Hematol Oncol Clin North Am 2005;19:917-28, viii. 10.1016/j.hoc.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 19.Bixler EO, Vgontzas AN, Ten Have T, et al. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 1998;157:144-8. 10.1164/ajrccm.157.1.9706079 [DOI] [PubMed] [Google Scholar]
- 20.Mantadakis E, Cavender JD, Rogers ZR, et al. Prevalence of priapism in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol 1999;21:518-22. 10.1097/00043426-199911000-00013 [DOI] [PubMed] [Google Scholar]
- 21.Roizenblatt M, Figueiredo MS, Cançado RD, et al. Priapism is associated with sleep hypoxemia in sickle cell disease. J Urol 2012;188:1245-51. 10.1016/j.juro.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 22.Stauffer E, Poutrel S, Cannas G, et al. Nocturnal Hypoxemia Rather Than Obstructive Sleep Apnea Is Associated With Decreased Red Blood Cell Deformability and Enhanced Hemolysis in Patients With Sickle Cell Disease. Front Physiol 2021;12:743399. 10.3389/fphys.2021.743399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temirbekov D, Güneş S, Yazıcı ZM, et al. The Ignored Parameter in the Diagnosis of Obstructive Sleep Apnea Syndrome: The Oxygen Desaturation Index. Turk Arch Otorhinolaryngol 2018;56:1-6. 10.5152/tao.2018.3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta V, Vasu TS, Phillips B, et al. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med 2013;9:271-9. 10.5664/jcsm.2500 [DOI] [PMC free article] [PubMed] [Google Scholar]