Abstract
Dietary ω-3 polyunsaturated fatty acid (PUFA) influences the expression of a number of genes in the brain. Zinc transporter (ZnT) 3 has been identified as a putative transporter of zinc into synaptic vesicles of neurons and is found in brain areas such as hippocampus and cortex. Neuronal zinc is involved in the formation of amyloid plaques, a major characteristic of Alzheimer's disease. The present study evaluated the influence of dietary ω-3 PUFA on the expression of the ZnT3 gene in the brains of adult male Sprague-Dawley rats. The rats were raised and/or maintained on a control (CON) diet that contained ω-3 PUFA or a diet deficient (DEF) in ω-3 PUFA. ZnT3 gene expression was analyzed by using real-time PCR, free zinc in brain tissue was determined by zinquin staining, and total zinc concentrations in plasma and cerebrospinal fluid were determined by atomic absorption spectrophotometry. Compared with CON-raised animals, DEF-raised animals had increased expression of ZnT3 in the brain that was associated with an increased level of free zinc in the hippocampus. In addition, compared with CON-raised animals, DEF-raised animals had decreased plasma zinc level. No difference in cerebrospinal fluid zinc level was observed. The results suggest that overexpression of ZnT3 due to a perinatal ω-3 PUFA deficiency caused abnormal zinc metabolism in the brain. Conceivably, the influence of dietary ω-3 PUFA on brain zinc metabolism could explain the observation made in population studies that the consumption of fish is associated with a reduced risk of dementia and Alzheimer's disease.
Keywords: docosahexaenoic acid, Alzheimer's disease, essential fatty acid deficiency, gene expression, zinc transporter
The ω-6 and ω-3 fatty acid families are the two most common polyunsaturated fatty acids (PUFA). The shortest ω-6 PUFA, linoleic acid, and the shortest ω-3 PUFA, α-linolenic acid (ALA, 18:3 ω-3), are essential fatty acids, because they cannot be synthesized by mammals (1) and hence must be ingested or passed from maternal stores. Once ingested, essential fatty acids can be metabolized by a series of desaturation and chain elongation enzymes within the body into longer more unsaturated fatty acids. For example, in the case of the ω-3 PUFA, ALA is metabolized in the liver and other tissues to eicosapentaenoic acid (EPA, 20:5 ω-3) and docosahexaenoic acid (DHA, 22:6 ω-3). The most biologically significant long-chain ω-3 PUFA are EPA and DHA. DHA is the predominant ω-3 PUFA in the mammalian nervous system, comprising ≈3% of the dry weight of the brain (2). The best dietary sources of DHA are fish and fish oils. Because many Western people do not consume fish regularly, deficiency of ω-3 PUFA is frequent in our society. It is believed that the ratio of ω-6 to ω-3 PUFA during the early part of human evolution was close to 1, whereas today the ratio is 10:1 or more (3). The uneven ratio compromises the utilization of ω-3 PUFA in the body, because linoleic acid competes with ALA for the enzymes of desaturation during metabolism. In addition, human studies indicate that consumption of a diet high in linoleic acid (typical of Western diets) reduces the level of DHA in tissues, presumably through competition with DHA in acyltransferase reactions (4).
Alzheimer's disease (AD) is one of the major neurodegenerative conditions commonly found in aged populations. In developed countries, the incidence of AD is 2.8% in the age range 70-74 years and rises to 11.1% in the age range 80-84 years (5). It has also been estimated that, between 1990 and 2010, the number of AD cases will rise by 40% (6). Deficits in specific nutrients [e.g., vitamin B12, vitamin B6, and homocysteine, (7)] have been suggested as possible causative factors in the etiology of AD. DHA is abundant in neuronal membrane phospholipids (8), and there is now a growing body of evidence concerning the beneficial effects of ω-3 PUFA in the amelioration of a number of neuropsychological disorders, such as depression and bipolar affective disorder, as well as in improving learning and memory (9). Furthermore, a number of studies report possible links between dietary fish intake, as well as the ω-3 PUFA status in the body, and protection from the incidence of AD (10-15).
The manipulation of ω-3 PUFA and/or the ratio of ω-3 PUFA to ω-6 PUFA influences the expression of many genes in the brain. Feeding diets rich in ALA or DHA plus eicosapentaenoic acid led to the overexpression of a number of genes in the brain that control synaptic plasticity, signal transduction, cytoskeleton and membrane association, ion channel formation, energy metabolism, and regulatory proteins (16-18). The alteration of dietary PUFA changes the genes in the hippocampus associated with learning and cognitive function (16, 18). Also, short-term administration of DHA to rats caused increased transcription of a gene that has a role in scavenging the amyloid β-protein (19). The deposition of amyloid plaques and neurofibrillary tangles is a major characteristic of AD (20). Thus, dietary ω-3 PUFA, and DHA in particular, can influence the transcription of a number of key genes and exert a protective action against the incidence of AD.
Zinc is also regarded as an important nutrient, because it plays an essential role in biological systems as a catalytic and/or structural cofactor in numerous zinc-dependent enzymes, in signal transduction, and as a component of transcription factors (21). The uptake of zinc by eukaryotic cells from the extracellular environment to the cytoplasm is mediated by proteins called zinc transporters (ZnT) (22). Intracellular zinc is bound to metalloenzymes, metalloproteins, nucleoproteins, and nucleic acids, thus little zinc is available as free or loosely bound [referred as mobile or reactive zinc (23)]. Interest in and understanding of eukaryotic ZnT have increased in recent times. Two families of ZnT, namely SLC39 Zrt-Irt-like Protein (ZIP) and SLC30 Cation Diffusion Family (CDF), have so far been identified (24). ZIP family ZnT are important for cellular uptake of zinc, whereas CDF members assist in zinc efflux out of the cell. The current standard nomenclature for the ZIP and ZnT families is SLC39 (ZIP) and SLC30 (ZnT). SLC nomenclature has superseded ZIP and CDF classification (see ref. 25).
A member of the SLC30 family of ZnT, ZnT3, has been identified in synaptic vesicles of neurons and is found in high abundance in brain areas such as hippocampus and cortex (26). ZnT3 is present in areas of the hippocampus including the dentate gyrus and pyramidal cells in the CA3 and CAI regions. Because of its specific intracellular localization, ZnT3 may function to transport zinc into synaptic vesicles (26).
Abnormalities in brain zinc metabolism can induce β-amyloid formation (27). The major component of the plaque is amyloid β-peptide (Aβ) derived from proteolytic cleavage of the β-amyloid precursor protein (APP) (28, 29). There is compelling evidence that zinc can trigger the aggregation of Aβ and the accumulation of plaques (27). Zinc is one of the metals found in high levels in brains from human AD patients and transgenic Tg2576 mice (APP mice), which are prone to develop amyloid plaque pathology (30, 31). The chelation of zinc has caused a precipitation of Aβ from AD brains (31). Furthermore, mice lacking the ZnT3 gene (APP mice) had a reduced amyloid plaque load (32). Metallothionein III, another zinc-binding protein in the brain (33), antagonizes the neurotoxic and neurotrophic effects of Aβ (34). Thus, at present, it appears that the expression of ZnT3 in the brain may play a major role in zinc homeostasis in the brain, etiology in β-amyloid formation, and the development of AD.
In this study, we demonstrate that a dietary supply of ω-3 PUFA early in life influences adult brain ZnT3 gene expression, plasma levels, and free zinc in the hippocampus.
Methods
Animals. All studies used Sprague-Dawley rats from the breeding colony at the Howard Florey Institute. They were maintained on a 12-h light:12-h dark cycle and were housed in suspended wire-mesh cages in a temperature-controlled room (21 ± 1°C). Unless otherwise specified, animals were given free access to pelleted food and water. All experimental procedures were approved by the Animal Experimentation Ethics Committee of the Howard Florey Institute, which operates under the guidelines of the National Health and Medical Research Council.
Diet Composition. The diets contained identical amounts of protein, carbohydrate, vitamins, minerals, and fat (7 g of fat/100 g of diet, dry weight). The composition of the diets complied with guidelines of the Nutrition Research Council (35) and is shown in Table 1. The lipid component comprised either 7% safflower oil [deficient diet (DEF) in ω or 5.84% safflower oil combined with 1.16% flaxseed oil [control diet (CON) in ω-3]. The DEF diet contained 6.7% palmitic acid, 2.6% stearic acid, 16.7% oleic acid, 71.9% linoleic acid, and 0.2% ALA of diet fat, with no long-chain ω-3 PUFA. The CON diet contained 6.5% palmitic acid, 2.3% stearic acid, 15.0% oleic acid, 63.0% linoleic acid, and 11.4% of diet fatty acids as ALA, with no long-chain ω-3 PUFA such as eicosapentaenoic acid and DHA. Both diets contained 21 mg of zinc per kg of diet. The diet content of other minerals was 0.45% calcium, 0.3% phosphorus, 0.09% magnesium, 0.13% sodium, 0.16% chloride, 0.4% potassium, 0.23% sulfur, iron 110 mg/kg, copper 5.7 mg/kg, iodine 0.23 mg/kg, manganese 7.1 mg/kg, selenium 0.15 mg/kg, chromium 2.0 mg/kg, lithium 0.1 mg/kg, boron 0.7 mg/kg, nickel 0.55 mg/kg, and vanadium 0.1 mg/kg. The vitamin content for vitamins A (retinol) and D (cholecalciferol) was 4,000 and 1,000 units/kg, respectively; the contents for other vitamins were vitamin E (α-tocopherol acetate) 75 mg/kg, vitamin K (menadione) 1 mg/kg, thiamine 6 mg/kg, riboflavin 6 mg/kg, niacin 30 mg/kg, pyridoxine 7 mg/kg, pantothenic acid 16 mg/kg, biotin 0.2 mg/kg, folic acid 2 mg/kg, vitamin B12 100 mg/kg, and choline 1,600 mg/kg. Both diets were custom-made by Glen Forrest Stockfeeders, Glen Forrest, WA, Australia; vacuum-sealed under nitrogen; and stored at 4°C until presentation to the animals at room temperature.
Table 1. Dietary composition.
| Composition | CON | DEF |
|---|---|---|
| Ingredient | ||
| Sucrose | 10.69 | 10.69 |
| Casein* | 20.0 | 20.0 |
| Cellulose | 5.0 | 5.0 |
| Starch | 39.75 | 39.75 |
| Dextrinized starch | 13.2 | 13.2 |
| Trace minerals | 0.14 | 0.14 |
| Calcium carbonate | 1.3 | 1.3 |
| Sodium chloride | 0.26 | 0.26 |
| Potassium dihydrogen phosphate | 0.69 | 0.69 |
| Potassium sulfate | 0.16 | 0.16 |
| Potassium citrate | 0.25 | 0.25 |
| Vitamins | 1.0 | 1.0 |
| Choline chloride, 50% | 0.25 | 0.25 |
| Lipid source† | ||
| Safflower oil | 5.84 | 7.00 |
| Flax oil | 1.16 | 0.0 |
| ω-3 fatty acids (ALA) | 0.80 | 0.01 |
Composition (g/100 g) of the semisynthetic diets that were sufficient (CON) and deficient (DEF) in ω-3 polyunsaturated fatty acids.
Commercial casein, not vitamin free.
Neither diet contained EPA or DHA.
Experimental Groups. Experiment 1. One week before mating, adult female Sprague-Dawley rats (n = 16) were placed on one of two diets: DEF (n = 8) or CON (n = 8). Once the pups were born, they were maintained on their mothers' diets (either CON or DEF) during lactation. At weaning (3 weeks), the pups were separated from their mothers. Only male pups born in litter sizes of 8-12 rats were selected for the experimental groups. Pups from eligible litter sizes were allocated evenly to reduce the number of littermates in the same dietary group.
Pups born of CON mothers were maintained on CON diets after weaning (CON, n = 7), whereas pups born of a DEF mother were split into two groups. One group was maintained on the DEF diet (DEF, n = 7), and the other was switched to the CON diet at weaning [born to deficient mothers and raised from weaning on a CON diet (DEF-CON), n = 7]. The DEF-CON group investigated the importance of lactation as a critical period for obtaining ω-3 PUFA. Studies on brain lipids and gene expression were conducted when the animals were >18 weeks old. In animals that were initially ω-3 PUFA-deficient, a period of 10 weeks is adequate for repletion of neural DHA levels to occur (36).
Experiment 2. For the evaluation of plasma and cerebrospinal fluid (CSF) zinc levels and free brain zinc, the above experimental paradigm was repeated with n = 8 animals in each group [born to CON mothers and raised until adulthood on a CON diet (CON-CON), born to DEF mothers and raised until adulthood on a CON diet (DEF-CON), and born to DEF mothers and raised until adulthood on a DEF diet (DEF-DEF)]. The animals were at least 18 weeks of age when the samples were collected.
Fatty Acid Profiling of Experimental Diets. Total lipids of diet samples were extracted as described by Folch et al. (37), and further procedures were followed, as described (38). The fatty acid methyl esters were then separated and measured on a Shimadzu gas chromatograph with flame ionization detection. A 60-m × 0.25-mm BPX-70 fused silica capillary column (SGE Scientific, Melbourne, Australia) with a film thickness of 0.25 μm was used in conjunction with a Shimadzu autoinjector. High-purity helium was used as a carrier gas. A temperature-gradient program was used with an initial temperature of 120°C, increasing at 3°C/min to 220°C. Identification of the fatty acid methyl esters was made by comparison with the retention times of gas liquid chromatography reference standard mixtures (Nu Chek Prep, Elysian, MN).
Brain Membrane Phospholipid Profile. To confirm the effectiveness of the dietary manipulations in altering DHA composition in brain membrane phospholipids, an analysis of the brain phospholipid fatty acids was performed by using brain tissue pooled from three animals belonging to the three dietary groups in Experiment 1. At 18 weeks of age, the rats were killed and their brains collected and stored at -80°C. The pooled brains were each homogenized and lipids extracted from each group into 5 ml of chloroform-methanol 1:1 (containing 10 mg/liter of butylated hydroxy-toluene antioxidant). Phospholipid classes from the pooled samples were separated in duplicate by TLC (silica gel 60, Merck) by using the solvent system composed of chloroform/methanol/acetic acid, 28% ammonium hydroxide/distilled water (50:35:4:1:1, vol/vol), which separated the four main glycerophospholipid classes, phosphatidyl ethanolamine, phosphatidyl inositol, phosphatidyl choline, and phosphatidyl serine, into distinct narrow bands capable of being visualized under UV light after spraying the plate with dichlorofluorescein. The methyl esters of the fatty acids of the phospholipid classes were prepared by saponification by using KOH (0.68 mol/liter in methanol) followed by esterification with 14% boron trifluoride in methanol. The fatty acid methyl esters were separated by capillary gas liquid chromatography, as described above.
Collection of CSF. A sample of CSF was collected under light anesthesia (pentobarbital sodium/Nembutal, 50 mg/kg body weight). For that purpose, the following procedure was used: the rat's head was placed in a stereotaxic frame, and the back of head was shaved between and below the ears. A 1.5-cm midline incision was made in the skin below the occipital notch. Then, the surrounding muscle and connective tissue were cleared away. A cutoff 23-gauge needle attached to P47 polyethylene tubing of ≈50 cm in length was prepared. Then the needle was inserted to a depth of 2 mm into the back of the head ≈1 mm below the base of the skull, piercing the cisterna magna. CSF was siphoned out by gravity or gentle suction by using a syringe, and only clear samples were retained. The samples were centrifuged to remove any cellular debris and the supernatant stored frozen (-80°C) until further analysis was done.
Collection of Plasma. A blood sample (10 ml) was drawn from the left ventricle of the heart into a syringe containing 50 units of sodium heparin as an anticoagulant. The sample was immediately chilled on ice until centrifugation. Plasma was collected and stored at -80°C until analysis of zinc.
Fixation, Isolation of Brains, and Preparation of Brain Sections. First, the rats were deeply anesthetized with sodium thiopentone (Nembutal, 50 mg i.p.). Then rats were perfused transcardially with 50-100 ml of normal saline followed by 250-300 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2). Then the brains were removed and placed in the fixative for 2-3 h. Subsequently, brains were transferred to a 20% sucrose solution in phosphate buffer, in which they were left overnight at 4°C. Finally, serial or alternate 40-μm coronal sections were cut from hippocampal regions by using a freezing microtome.
Zinquin Staining to Visualize the Free Zinc Pools in Specific Brain Areas. The brain sections that represent the neuronal cells in hippocampal and cortical regions were stained as explained (39).
Acquisition and Storage of Brain Samples, RNA Preparation, and Amplification. The brain samples were obtained from adult animals and then frozen in liquid nitrogen and stored at -80°C until the RNA extraction was carried out. For RNA extraction, n = 4 animals from each experimental group were used. RNA preparations and amplification were performed as described (16).
RT-PCR Procedure. Quantitative RT-PCR was performed as described (24). The PCR primers used were as follows: actin, forward primer, 5′-TTCAACACCCCAGCCATGT-3′; reverse primer, 5′-GCATACAGGGACAACACAGCC-3′ and ZnT3, forward primer, 5′-CTTCCTGGAGATCCTGGCC-3′; reverse primer, 5′-CACCAGGAACAGCACCTTCA-3′. Reactions were performed in triplicate on two different pools (n = 5) representing biological replicates. The relative expression ratio was normalized to β-actin. Nontemplate control sample was used for each PCR run to check the genomic DNA contaminations of cDNA template and primer-dimer formation. Analysis of results was performed by using the Pfaffl method (40). Results are expressed as the arithmetical mean with their standard error (mean ± SE), and those values were considered regulated where the average-fold change (increase or decrease) of the six data points was at least 2.0-fold.
Plasma and CSF Zinc Assay by Flame and Furnace Atomic Absorption Spectrophotometry. Plasma and CSF samples (25-μl volumes) are digested in nitric acid (final concentration 0.5%), vortexed, and then centrifuged. The analysis was carried out as described (41). The supernatants were analyzed by air/acetylene flame or furnace atomic absorption spectrophotometry by using a Varian SpectrAA-800 atomic absorption spectrophotometer, with graphite tube atomizer and programmable sample dispenser. For each batch of samples, a quality control was analyzed to ensure that samples fell within the expected range.
Statistical Analysis Data reported in Fig. 1 were analyzed by using Student's t test (statistica 5.0, Statsoft, Tulsa, OK).
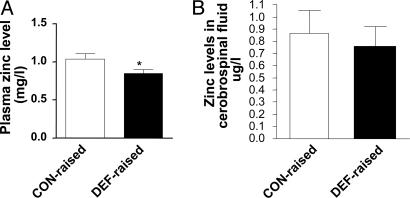
Fig. 1.
Concentrations of zinc in plasma (A) and CSF (B) in CON-raised (empty bars, CON-CON) and DEF-raised (filled bars, DEF-DEF + DEF-CON) groups. The values are expressed as mean ± SEM for n = 8 for CON- and n = 12 for DEF-raised animals for plasma zinc level and n = 7 for CON- and n = 12 for DEF-raised animals for CSF zinc levels. *, significant difference at P < 0.05.
Results
Brain Membrane Phospholipid Profile. Analysis of the fatty acid profiles of the major DHA-containing membrane phospholipids (phosphatidyl-ethanolamine and phosphatidyl-serine) in brain tissue confirmed that the DEF group had a reduced proportion of DHA (22:6n-3) and total ω-3 PUFA proportion compared with the CON and DEF-CON groups (Table 2). The results also confirmed that dietary replenishment of ω-3 PUFA after weaning in the DEF-CON group restored the membrane phospholipid proportion of 22:6n-3 and total ω-3 PUFA to that of the CON group. In the DEF group, the loss of 22:6n-3 was largely replaced by a 22-carbon ω-6 PUFA, which is a marker of ω-3 PUFA deficiency, 22:5n-6, as shown in Table 2.
Table 2. Membrane phospholipid profile of brain tissue (% of total fatty acids).
| PE
|
PS
|
|||||
|---|---|---|---|---|---|---|
| Fatty acid | CON | DEF | DEF-CON | CON | DEF | DEF-CON |
| 16:0 | 5.91 | 6.00 | 5.87 | 1.90 | 1.12 | 1.34 |
| 16:1 | 0.28 | 0.20 | 0.35 | 0.10 | 0.04 | 0.07 |
| 18:0 | 16.36 | 16.37 | 15.98 | 40.02 | 39.00 | 39.62 |
| 18:1n-9+n-7 | 23.26 | 22.36 | 24.10 | 25.78 | 23.34 | 25.25 |
| 18:2n-6 | 0.28 | 0.19 | 0.27 | 0.29 | 0.30 | 0.28 |
| 18:3n-3 | 0.12 | 0.11 | 0.12 | ND* | ND | ND |
| 20:0 | 0.23 | 0.27 | 0.24 | 0.56 | 0.54 | 0.52 |
| 20:1 | 5.24 | 5.34 | 5.52 | 3.71 | 4.24 | 3.84 |
| 20:2n-6 | 0.11 | 0.10 | 0.11 | 0.20 | 0.19 | 0.19 |
| 20:3n-6 | 0.34 | 0.24 | 0.32 | 0.28 | 0.19 | 0.27 |
| 20:4n-6 | 10.72 | 11.66 | 10.57 | 1.09 | 3.54 | 3.12 |
| 20:5n-3 | 0.11 | 0.13 | 0.11 | 0.20 | 0.21 | 0.23 |
| 22:4n-6 | 5.45 | 6.45 | 5.13 | 2.82 | 3.49 | 2.72 |
| 22:5n-6 | 0.47 | 6.71 | 0.43 | 1.26 | 10.09 | 1.03 |
| 22:5n-3 | 0.30 | 0.04 | 0.27 | 0.19 | 0.03 | 0.18 |
| 22:6n-3 (DHA) | 17.78 | 10.58 | 17.39 | 19.36 | 11.52 | 19.24 |
| Total ω-6 | 17.38 | 25.35 | 16.83 | 5.94 | 17.80 | 7.60 |
| Total ω-3 | 18.31 | 10.86 | 17.89 | 19.75 | 11.76 | 19.65 |
| Total PUFA | 35.69 | 36.21 | 34.72 | 25.69 | 29.56 | 27.26 |
| Other† | 13.24 | 13.24 | 13.23 | 2.25 | 2.16 | 2.12 |
Male rats were raised on control (CON) or deficient (DEF) diets or were provided from deficient dams and then received the control diet after weaning (DEF-CON). After the end of experiments at 18 weeks of age, rats were killed, three brains from each group were pooled, and the fatty acid composition (%) of membrane phospholipids was analyzed in duplicate. PE, phosphatidylethanolamine; PS, phosphatidyl serine. The results confirmed that DHA content was decreased in the DEF group and restored in the DEF-CON group.
ND, below the limit of detection, 0.02%.
Includes 16- and 18-carbon aldehydes, 17:0, 22:0, and 24:1.
Gene Expression Analysis by Real-Time RT-PCR. In Experiment 1, the ZnT3 gene was significantly overexpressed in DEF-DEF (ratio, 4.37 ± 0.87) and DEF-CON (ratio, 2.22 ± 0.45) animals compared with CON-CON when analyzed by quantitative real-time RT-PCR. The up-regulation of ZnT3 was more pronounced in brains obtained from DEF-DEF animals, whereas a normalization effect could be detected in the DEF-CON group; however, a significant increase in mRNA level was recorded in the DEF-CON group compared with the animals maintained on the control diet.
Plasma and CSF Zinc Levels. In Experiment 2, the plasma zinc levels were significantly decreased in animals subjected to ω-3 PUFA deficiency during early life (DEF-raised, DEF-DEF, and DEF-CON) compared with those raised on an ω-3 PUFA-sufficient diet (CON-CON) (Fig. 1 A). CSF zinc levels were not altered (Fig. 1B).
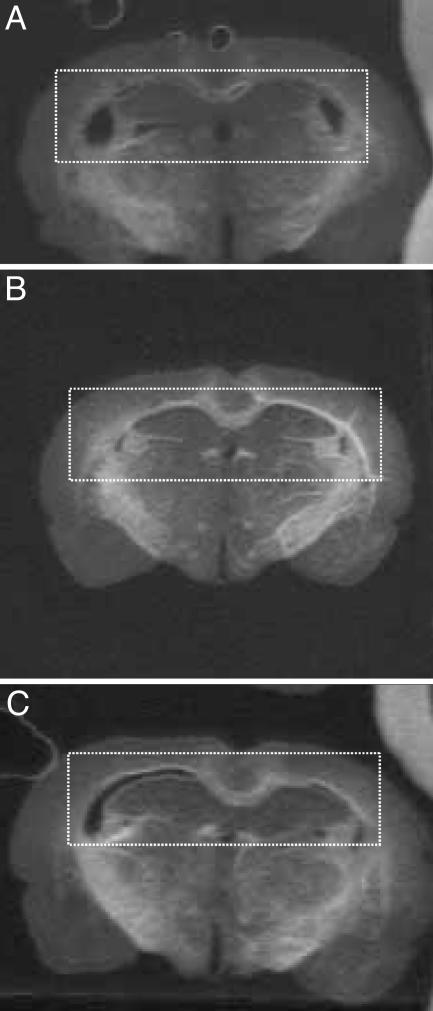
Zinquin Stain in Brain Sections. Zinquin was used to detect free zinc pools and loosely bound zinc. The intensity of the zinquin label was increased in brain sections from DEF-raised (DEF-CON and DEF-DEF) compared with CON-raised (CON-CON) animals. The intensity was mostly concentrated in hippocampal regions that have zinc-containing mossy fibers, such as the CA1 region and the dentate gyrus [highlighted by dashed boxes (Fig. 2)].
Fig. 2.
Zinc labeling in the brain. Coronal brain sections represent hippocampal regions from rats belonging to CON-CON (A), DEF-CON (B), and DEF-DEF (C) groups. The bright areas indicate zinquin labeling for free zinc pools. The dashed boxes highlight the hippocampal region that has zinc-containing mossy fibers. The results indicate that sections representing the DEF-CON and DEF-DEF groups show intense staining for zinc compared with the CON-CON group.
Discussion
The most exciting observation made in this study was the up-regulation in the expression of the ZnT3 gene in the brains of rats raised on diets deficient in ω-3 PUFA compared with the ω-3 PUFA sufficient group. When coupled with our other data showing reduced plasma levels of zinc and increased brain zinc, the results are consistent with a scheme whereby increased expression of ZnT3 leads to sequestration of zinc from extracellular fluid into selective areas of the brain involved in memory formation.
Permissive Role of Brain Zinc in AD. ZnT3 has been identified as a putative transporter of zinc into synaptic vesicles of neurons and is found abundantly in brain areas such as hippocampus and cortex (26). In the hippocampus, ZnT3 is abundantly found in areas such as the dentate gyrus and the pyramidal cells in the CA3 and CAI regions. Neuronal zinc has been reported to be involved in the formation of amyloid plaques (AP), which is a major characteristic of AD (27). Zinc increases the aggregation of Aβ and can also stabilize β-amyloid deposits The absence of ZnT3 activity reduced AP formation in transgenic APP mice that express the amyloid pathology (32). Thus, the up-regulation of ZnT3 due to ω-3 PUFA deficiency during the perinatal period could lead to a predisposition to AD during adulthood.
This speculation is further supported by the observed changes in plasma zinc levels. Low plasma levels of zinc have been associated with the incidence of AD in humans (11, 42). Similarly, AD patients treated with the selective metal chelator clioquinol showed an increase in plasma zinc and a superior cognitive performance relative to patients receiving placebo (43). The significant reduction of plasma zinc that we observed in animals raised on an ω-3 PUFA-deficient diet could be a result of an increased transportation of zinc into tissues from the circulating zinc pools by overexpressed ZnT3 or an effect of ω-3 PUFA deficiency on zinc absorption, although this does not appear to be consistent with studies showing that ω-6 PUFA promote zinc absorption (44-46).
The increased intensity of zinquin labeling of cellular-free zinc that we observed in the hippocampus and associated mossy fibers is evidence for increased zinc accumulation in the brains of rats raised on ω-3 PUFA-deficient diets.
The importance of zinc in AD has been further confirmed by a study showing that, after treatment with metal chelators, the aggregated Aβ from postmortem AD brains could be dissolved (31). Further, treatment of AD patients with clioquinol reduced the level of circulating Aβ, in addition to the effects on plasma zinc and cognitive performance, mentioned above. In addition to its promotion of the aggregation of Aβ, zinc also markedly increased G protein activation mediated by soluble Aβ in cultured cortical membranes (47). Therefore, the abnormalities of zinc metabolism in brain arising from dysregulation of gene expression may create a predisposition to pathological conditions such as AD.
ω-3 PUFA and AD. There is a growing body of evidence from epidemiological data and animal studies suggesting that ω-3 PUFA supply has a link to AD (10-15). Most importantly, dietary intake of ω-3 PUFA and eating fish once per week reduced the risk of developing AD by 60% compared with never or rarely eating fish (48). Furthermore, intake of fish at least once per week was associated with a one-third reduction of dementia risk (15). Postmortem analysis of the brain membrane fatty acid profile of AD patients has revealed a deficiency in DHA levels of some phospholipid classes compared with non-AD brains (13). Our dietary strategy was similarly effective in reducing DHA levels in brain phospholipids, but the upregulation of the ZnT3 gene was prolonged into adult life irrespective of the repletion of dietary ω-3 PUFA. This result was consistent with a perinatal effect of the dietary ω-3 PUFA supply previously observed for blood pressure (49). Recent data have suggested a mechanism through which dietary ω-3 PUFA act to prevent or ameliorate neuropathological damage associated with AD. Calon et al. (50) showed that dendritic damage in the mouse APP transgene could be inhibited by dietary ω-3 PUFA supplementation and provided evidence that DHA prevented oxidative stress associated with increased Aβ from damaging dendritic actin, a crucial component of the neuronal morphology underlying memory formation. Further, spatial memory performance in maze testing was improved by high levels of dietary DHA (18).
Conclusion
There are now compelling data for the role of zinc in the promotion of Aβ aggregation in the brain and the ameliorative effects of reducing free zinc on AD pathology. Similarly, the deficiency of ω-3 PUFA, in particular brain membrane DHA, is steadily gaining recognition as a factor in the pathogenesis of diseases such as AD. Our data on the perinatal influence of dietary ω-3 PUFA depletion and the expression of the ZnT3 in the brain (and subsequent sequestration of zinc into brain tissue) provide an important link between studies that have shown the positive effects of dietary DHA and reduced brain zinc on the pathology of AD.
Acknowledgments
This work was supported by grants from the Hungarian Scientific Research Fund (OTKA, F 042850 and TS 044836) and from the National Office for Research and Technology (NKTH, RET-08/2004). K.K. is supported by the János Bolyai Fellowship of the Hungarian Ministry of Education. We acknowledge financial support from the Association pour la Recherche sur le Cancer (DP0212079, to H.S.W.), National Health and Medical Research Council (232306, to M.L.M.), Association pour la Recherche sur le Cancer (DP0346830, to R.S.W., H.S.W., and A.J.S.), and National Health and Medical Research Council (217011, to R.S.W.).
Author contributions: R.S.W., A.J.S., and A.P.J. designed research; A.P.J., M.L.A., M.L.M., H.S.W., R.S.W., K.K., A.J.S., and L.G.P. performed research; M.L.A. contributed new reagents/analytic tools; R.S.W., A.P.J., A.J.S., M.L.M., M.L.A., L.G.P., and J.E.H. analyzed data; and A.P.J., R.S.W., A.J.S., M.L.A., M.L.M., J.E.H., and L.G.P. wrote the paper.
Abbreviations: Aβ, amyloid β-peptide; AD, Alzheimer's disease; ALA, α-linolenic acid; CSF, cerebrospinal fluid; DHA, docosahexaenoic acid; PUFA, polyunsaturated fatty acid; ZnT, zinc transporter; APP, amyloid precursor protein; CON, control diet; DEF, deficient diet; DEF-CON, born to deficient mothers and raised from weaning on a CON diet; CON-CON, born to CON mothers and raised until adulthood on a CON diet; DEF-DEF, born to DEF mothers and raised until adulthood on a DEF diet.
References
- 1.Sinclair, A. J., Attar-Bashi, N. M. & Li, D. (2002) Lipids 37, 1113-1123. [DOI] [PubMed] [Google Scholar]
- 2.Svennerholm, L. (1968) J. Lipid Res. 9, 570-579. [PubMed] [Google Scholar]
- 3.Eaton, S. B., Eaton, S. B., 3rd, Sinclair, A. J., Cordain, L. & Mann, N. J. (1998) World Rev. Nutr. Diet. 83, 12-23. [DOI] [PubMed] [Google Scholar]
- 4.Mantzioris, E., James, M. J., Gibson, R. A. & Cleland, L. G. (1994) Am. J. Clin. Nutr. 59, 1304-1309. [DOI] [PubMed] [Google Scholar]
- 5.Jorm, A. F., Korten, A. E. & Henderson, A. S. (1987) Acta. Psychiatr. Scand. 76, 465-479. [DOI] [PubMed] [Google Scholar]
- 6.Tully, A. M., Roche, H. M., Doyle, R., Fallon, C., Bruce, I., Lawlor, B., Coakley, D. & Gibney, M. J. (2003) Br. J. Nutr. 89, 483-489. [DOI] [PubMed] [Google Scholar]
- 7.Nourhashemi, F., Gillette-Guyonnet, S., Andrieu, S., Ghisolfi, A., Ousset, P. J., Grandjean, H., Grand, A., Pous, J., Vellas, B. & Albarede, J. L. (2000) Am. J. Clin. Nutr. 71, 643S-649S. [DOI] [PubMed] [Google Scholar]
- 8.Bazan, N. G. & Scott, B. L. (1990) Ups. J. Med. Sci. Suppl. 48, 97-107. [PubMed] [Google Scholar]
- 9.Freeman, M. P. (2000) Ann. Clin. Psychiatry 12, 159-165. [DOI] [PubMed] [Google Scholar]
- 10.Conquer, J. A., Tierney, M. C., Zecevic, J., Bettger, W. J. & Fisher, R. H. (2000) Lipids 35, 1305-1312. [DOI] [PubMed] [Google Scholar]
- 11.Kyle, D. J., Schaefer, E., Patton, G. & Beiser, A. (1999) Lipids 34, Suppl, S245. [DOI] [PubMed] [Google Scholar]
- 12.Corrigan, F. M., Horrobin, D. F., Skinner, E. R., Besson, J. A. & Cooper, M. B. (1998) Int. J. Biochem. Cell Biol. 30, 197-207. [DOI] [PubMed] [Google Scholar]
- 13.Prasad, M. R., Lovell, M. A., Yatin, M., Dhillon, H. & Markesbery, W. R. (1998) Neurochem. Res. 23, 81-88. [DOI] [PubMed] [Google Scholar]
- 14.Morris, M. C., Evans, D. A., Bienias, J. L., Tangney, C. C., Bennett, D. A., Aggarwal, N., Schneider, J. & Wilson, R. S. (2003) Arch. Neurol. 60, 194-200. [DOI] [PubMed] [Google Scholar]
- 15.Barberger-Gateau, P., Letenneur, L., Deschamps, V., Peres, K., Dartigues, J. F. & Renaud, S. (2002) Br. Med. J. 325, 932-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitajka, K., Puskas, L. G., Zvara, A., Hackler, L., Jr., Barcelo-Coblijn, G., Yeo, Y. K. & Farkas, T. (2002) Proc. Natl. Acad. Sci. USA 99, 2619-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitajka, K., Sinclair, A. J., Weisinger, R. S., Weisinger, H. S., Mathai, M., Jayasooriya, A. P., Halver, J. E. & Puskas, L. G. (2004) Proc. Natl. Acad. Sci. USA 101, 10931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barcelo-Coblijn, G., Hogyes, E., Kitajka, K., Puskas, L. G., Zvara, A., Hackler, L., Jr., Nyakas, C., Penke, Z. & Farkas, T. (2003) Proc. Natl. Acad. Sci. USA 100, 11321-11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puskas, L. G., Kitajka, K., Nyakas, C., Barcelo-Coblijn, G. & Farkas, T. (2003) Proc. Natl. Acad. Sci. USA 100, 1580-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selkoe, D. J. (1999) Nature 399, A23-31. [DOI] [PubMed] [Google Scholar]
- 21.Gaither, L. A. & Eide, D. J. (2001) J. Biol. Chem. 276, 22258-22264. [DOI] [PubMed] [Google Scholar]
- 22.Zhao, H. & Eide, D. (1996) Proc. Natl. Acad. Sci. USA 93, 2454-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes, J. G. (1996) Am. J. Physiol. 270, C401-C410. [DOI] [PubMed] [Google Scholar]
- 24.Gaither, L. A. & Eide, D. J. (2001) Biometals 14, 251-270. [DOI] [PubMed] [Google Scholar]
- 25.Eide, D. J. (2004) Pflügers Arch. 447, 796-800. [DOI] [PubMed] [Google Scholar]
- 26.Palmiter, R. D., Cole, T. B., Quaife, C. J. & Findley, S. D. (1996) Proc. Natl. Acad. Sci. USA 93, 14934-14939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bush, A. I., Pettingell, W. H., Multhaup, G., d Paradis, M., Vonsattel, J. P., Gusella, J. F., Beyreuther, K., Masters, C. L. & Tanzi, R. E. (1994) Science 265, 1464-1467. [DOI] [PubMed] [Google Scholar]
- 28.Masters, C. L., Multhaup, G., Simms, G., Pottgiesser, J., Martins, R. N. & Beyreuther, K. (1985) EMBO J. 4, 2757-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., McDonald, B. L. & Beyreuther, K. (1985) Proc. Natl. Acad. Sci. USA 82, 4245-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh, S. W., Chen, J. W., Motamedi, M., Bell, B., Listiak, K., Pons, N. F., Danscher, G. & Frederickson, C. J. (2000) Brain Res. 852, 268-273. [DOI] [PubMed] [Google Scholar]
- 31.Cherny, R. A., Legg, J. T., McLean, C. A., Fairlie, D. P., Huang, X., Atwood, C. S., Beyreuther, K., Tanzi, R. E., Masters, C. L. & Bush, A. I. (1999) J. Biol. Chem. 274, 23223-23228. [DOI] [PubMed] [Google Scholar]
- 32.Lee, J. Y., Cole, T. B., Palmiter, R. D., Suh, S. W. & Koh, J. Y. (2002) Proc. Natl. Acad. Sci. USA 99, 7705-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, J. Y., Kim, J. H., Palmiter, R. D. & Koh, J. Y. (2003) Exp. Neurol. 184, 337-347. [DOI] [PubMed] [Google Scholar]
- 34.Irie, Y. & Keung, W. M. (2001) Biochem. Biophys. Res. Commun. 282, 416-420. [DOI] [PubMed] [Google Scholar]
- 35.Reeves, P. G., Rossow, K. L. & Lindlauf, J. (1993) J. Nutr. 123, 1923-1931. [DOI] [PubMed] [Google Scholar]
- 36.Weisinger, H. S., Vingrys, A. J., Bui, B. V. & Sinclair, A. J. (1999) Invest. Ophthalmol. Visual Sci. 40, 327-338. [PubMed] [Google Scholar]
- 37.Folch, J., Lees, M. & Sloane Stanley, G. H. (1957) J. Biol. Chem. 226, 497-509. [PubMed] [Google Scholar]
- 38.Weisinger, H. S., Vingrys, A. J. & Sinclair, A. J. (1995) Lipids 30, 471-473. [DOI] [PubMed] [Google Scholar]
- 39.Michalczyk, A. A., Allen, J., Blomeley, R. C. & Ackland, M. L. (2002) Biochem. J. 364, 105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaffl, M. W. (2001) Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ball, M. J. & Ackland, M. L. (2000) Br. J. Nutr. 83, 27-33. [DOI] [PubMed] [Google Scholar]
- 42.Licastro, F., Davis, L. J., Mocchegiani, E. & Fabris, N. (1996) Biol. Trace Elem. Res. 51, 55-62. [DOI] [PubMed] [Google Scholar]
- 43.Ritchie, C. W., Bush, A. I., Mackinnon, A., Macfarlane, S., Mastwyk, M., MacGregor, L., Kiers, L., Cherny, R., Li, Q. X., Tammer, A., et al. (2003) Arch. Neurol. 60, 1685-1691. [DOI] [PubMed] [Google Scholar]
- 44.Cunnane, S. C. (1982) Pediatr. Res. 16, 599-603. [DOI] [PubMed] [Google Scholar]
- 45.Rosenthal, M. J., Hwang, I. K. & Song, M. K. (2001) Life Sci. 70, 337-348. [DOI] [PubMed] [Google Scholar]
- 46.Song, M. K. & Adham, N. F. (1985) Am. J. Clin. Nutr. 41, 1201-1209. [DOI] [PubMed] [Google Scholar]
- 47.Molnar, Z., Kovacs, P., Laczko, I., Soos, K., Fulop, L., Penke, B. & Lengyel, I. (2004) J. Neurochem. 89, 1215-1223. [DOI] [PubMed] [Google Scholar]
- 48.Morris, M. C., Evans, D. A., Bienias, J. L., Tangney, C. C., Bennett, D. A., Wilson, R. S., Aggarwal, N. & Schneider, J. (2003) Arch. Neurol. 60, 940-946. [DOI] [PubMed] [Google Scholar]
- 49.Weisinger, H. S., Armitage, J. A., Sinclair, A. J., Vingrys, A. J., Burns, P. L. & Weisinger, R. S. (2001) Nat. Med. 7, 258-259. [DOI] [PubMed] [Google Scholar]
- 50.Calon, F., Lim, G. P., Yang, F., Morihara, T., Teter, B., Ubeda, O., Rostaing, P., Triller, A., Salem, N., Jr., Ashe, K. H., Frautschy, S. A. & Cole, G. M. (2004) Neuron 43, 633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]