Abstract
Importance
Feline calicivirus (FCV)-associated viral systemic disease (VSD) is a severe systemic disease caused by virulent FCV strains and has a very poor prognosis.
Objective
To evaluate the clinical characteristics of a nosocomial FCV-VSD outbreak involving 18 cats in Korea.
Methods
Medical records of cats diagnosed with FCV-VSD from March to September 2018 at a referral veterinary hospital were reviewed. The patient’s signalment, history, clinical features, diagnosis, treatment, and prognosis were evaluated.
Results
Two outbreaks involving 18 cats diagnosed with FCV-VSD occurred over a 6-month period at a referral hospital in Korea. Anorexia, lethargy, fever, and limb edema were the most commonly observed clinical symptoms. Lymphopenia and macrothrombocytopenia were the most common hematological findings, and hyperbilirubinemia and increased levels of aspartate aminotransferase, creatine kinase, and serum amyloid A were the most frequent results of serum biochemistry. FCV was detected by reverse transcription polymerase chain reaction in 11 patients and the remaining 7 were suspected with FCV-VSD. The overall mortality rate was 72.2%. The hospital was closed and disinfected twice, and no additional outbreaks have occurred since the last patient.
Conclusions and Relevance
The clinical and diagnostic characteristics and outcomes of FCV-VSD described in this study can be used to recognize and contain infectious diseases through quick action. To the best of the authors’ knowledge, this is the first report of a nosocomial outbreak of FCV-VSD in Asia.
Keywords: Feline calicivirus, viral systemic disease, nosocomial infection, edema, ulcerative dermatitis, cats
INTRODUCTION
Feline calicivirus (FCV) is a highly contagious virus belonging to the Caliciviridae family and is one of the major causes of upper respiratory tract disease (URTD) in cats [1]. FCV infection typically causes mild, acute, and self-limiting URTD characterized by sneezing, nasal discharge, dyspnea, and conjunctivitis. However, severe pneumonia, limping syndrome, paw and mouth disease, and enteritis due to FCV infection have also been reported [2,3,4,5]. Although asymptomatic carriers are often identified, FCV is isolated more frequently in individuals with URTD than in those without it [6]. FCV is a small RNA virus with numerous strains and shows strong genetic and antigenic variability [1,7]. Because of this diversity, differences in clinical symptoms or severity are possible; however, to date, no FCV strain associated with specific clinical symptoms has been reported [7].
FCV-associated viral systemic disease (FCV-VSD) is a severe systemic disease caused by virulent FCV strains with a high mortality rate [8]. Although the exact pathogenesis of FCV-VSD is unknown, it differs clinically from the typical FCV infection. The severity of the clinical symptoms of FCV-VSD varies and may initially begin with acute URTD [9]. However, unlike the typical FCV infection, it involves characteristic clinical symptoms, such as pyrexia, facial and/or limb edema, oral ulceration, ulcerative dermatitis, and jaundice [10,11]. Vaccination does not completely protect against FCV-VSD, and adult vaccinated cats tend to have more severe disease than kittens [12]. The exposed individuals shed the virus through ocular and nasal discharge, saliva, and feces, and the virus can also be rapidly transmitted through people or fomites [8]. FCV generally survives in the environment for several days [13] but has been reported to survive for up to a month under low-temperature conditions [14]. The incubation period for URTD caused by FCV infection is usually 2–10 days, whereas the incubation period for FCV-VSD is usually 1–5 days [12]. Epizootic spread has been reported both in multi-cat households and veterinary facilities [8,11]. Previous reports of FCV-VSD outbreaks have been mostly limited to the United States or European countries [8,10,11,15]. To date, single cases of FCV-VSD in China and Korea have been reported [16,17,18], but none of simultaneous outbreaks involving multiple cats in any Asian country.
This paper describes the clinical characteristics, risk factors, diagnostic techniques, and prognosis of a nosocomial outbreak of FCV-VSD that occurred over a 6-month period at a referral hospital in Korea.
METHODS
Data collection
Medical records of cats from March to September 2018 at a referral veterinary hospital were reviewed. Data collected from the records included signalment, clinical features, diagnostic approach, treatment, and prognosis. To determine the characteristics of nosocomial infection, we evaluated the initial chief complaint at the time of admission to the hospital, the period in the hospital of each affected cat, and the time of onset of clinical signs related to FCV-VSD.
We assessed the presence of animals living with the patients and their vaccination status against FCV, feline herpesvirus type 1, and feline panleukopenia virus. Regarding vaccination status, those who were vaccinated within the last 3 years were classified as “sufficient,” whereas those who were vaccinated more than 3 years ago were classified as “insufficient.” Furthermore, those who had not been vaccinated or had an unknown vaccination status were classified as “unvaccinated.”
When symptoms related to FCV-VSD developed, samples were collected as oral and laryngeal swabs, scrapings from skin lesions, blood, or liver tissue and submitted to the veterinary diagnostic laboratory (PobaniLab, Korea). Skin samples were obtained using the general deep skin scraping method, not only in cases presenting with skin lesions, such as ulcerations or erosions, but also in cases exhibiting only edema. Liver tissues were acquired during postmortem examination. FCV was detected by reverse transcription quantitative real time polymerase chain reaction (RT-qPCR) as described previously [19]. Briefly, viral RNAs were extracted from the requested specimens using the Boom nucleic acid extraction method, which employs silica-coated magnetic beads. Subsequently, RT-qPCR was conducted using a previously referenced FCV-specific primers and probe panel, utilizing a one-step RT-qPCR master mix in accordance with the manufacturer’s protocol (TAKARA Bio, Japan). The thermocycler used for the RT-qPCR was the RGQ 5plex (QIAGEN, Germany).
Complete blood count (CBC) and serum chemical profile were measured at the time of onset of clinical signs related to FCV-VSD. Patients whose symptoms improved and were discharged following therapeutic intervention were considered survivors, and patients who died during hospitalization despite treatment were considered non-survivors.
Case classification
Exposure history, clinical signs, and RT-qPCR results were used to classify patients as confirmed, suspected, or possible FCV-VSD according to the classification criteria described previously, with some modifications [8]. In brief, if FCV was positive on RT-qPCR and characteristic clinical signs associated with FCV-VSD (e.g., fever, facial/limb edema, ulcerative dermatitis, or jaundice) were observed, the case was classified as “confirmed.” “Suspected” patients were those who showed characteristic clinical signs and had a history of exposure but were negative for FCV on RT-qPCR, or the test could not be otherwise requested because of sudden death. Exposure history was defined as a hospitalization period of more than 24 h overlapping with that of a confirmed patient. Conversely, “possible FCV-VSD” patients were defined as patients with a history of exposure and showing characteristic clinical signs but did not develop edema or survived.
Statistical analysis
Statistical analyses were performed with the use of IBM SPSS Statistics 27.0 software (IBM Corp., USA). The Fisher’s exact test was used to determine whether there were differences in sex, environment, or vaccination status between the survivors and non-survivors groups. The Mann-Whitney U test was performed to compare differences according to age between the two groups. For all statistical analyses, a p value less than 0.05 was considered statistically significant.
RESULTS
Eighteen cats were included in this study (Table 1). Except for one cat of unknown age (case 13), the age range of the patients was 4 months to 10 years (median 4 years old). Eight cats were domestic shorthair cats, while 10 were purebred cats. Most of the patients were neutered. Except for case 13, who was rescued and had stayed in a shelter where many cats lived together, all were indoor house cats. Nine of them had no companion animals, while the remaining 9 lived in multi-cat households. None of the patients cohabited with dogs. Although most patients had a history of vaccination, only 6 were considered sufficiently vaccinated, while 8 had insufficient vaccination status. The remaining 4 were classified as unvaccinated. The exact vaccine protocols for the vaccinated patients are unknown. There was no significant difference in survival with age, sex, multi-cat environment, or vaccination status (p = 0.33, p = 0.727, p = 1.0, and p = 0.647, respectively).
Table 1. Signalment, history taking results, visit date, hospitalization period, and initial chief complaint of cats with feline calicivirus-associated viral systemic disease in Korea.
| Case No. | Age (yr) | Breed | Sex | Environment | Vaccination statusa | Date of last vaccination | Date in 2018 | Initial chief complaint | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Presentation | Hospitalization period | |||||||||
| Outbreak 1 | ||||||||||
| 1 | 1 yr | DSH | SF | Multi-cat household | Unvaccinated | Unknown | 06/Mar | 6–9/Mar | Limb edema, upper respiratory signs | |
| 2 | 10 yr | DSH | NM | Single-cat household | Insufficient | 9 yr ago | 06/Mar | 19–29/Mar | Chronic kidney disease, pancreatitis | |
| 3 | 10 yr | DSH | SF | Single-cat household | Insufficient | 9 yr ago | 17/Mar | 17–26/Mar | Left ureteral urolith, azotemia | |
| 4 | 4 yr | DSH | NM | Single-cat household | Unvaccinated | Unknown | 10/Mar | 21–30/Mar | Idiopathic cystitis, perineal urethrostomy | |
| 5 | 4 mon | Russian blue | F | Single-cat household | Unvaccinated | None | 22/Mar | 22/Mar–5/Apr | Left femoral fracture | |
| 6 | 3 yr | Scottish fold | SF | Multi-cat household | Sufficient | 2 yr ago | 24/Mar | 29/Mar–1/Apr | Mastectomy due to recurrent mastitis | |
| 7 | 4 yr | Siamese | NM | Single-cat household | Insufficient | 3 yr ago | 26/Mar | 26/Mar–3/Apr | Intestinal foreign body, pancreatitis | |
| 8 | 8 yr | Russian blue | NM | Multi-cat household | Insufficient | 7 yr ago | 27/Mar | None | Medical checkup | |
| 9 | 6 yr | Persian | NM | Multi-cat household | Insufficient | 4 yr ago | 27/Mar | None | Medical checkup | |
| 10 | 2 yr | Siamese | NM | Multi-cat household | Sufficient | 1 yr ago | 12/Apr | 12–19/Apr | Idiopathic cystitis, dysuria | |
| 11 | 6 yr | DSH | NM | Single-cat household | Sufficient | 2 yr ago | 04/May | 9–10/May | Pancreatitis | |
| 12 | 10 yr | DSH | SF | Multi-cat household | Sufficient | 1 yr ago | 06/May | 6–12/May | Uterine metritis, pyometra, fever, tenesmus | |
| Outbreak 2 | ||||||||||
| 13 | 4 yr | DSH | NM | Multi-cat household | Insufficient | 3 yr ago | 12/Aug | 12–21/Aug | Degloving tail Injury after the cat has been outside the house | |
| 14 | 2 yr | Siamese | SF | Single-cat household | Insufficient | 11 yr ago | 13/Aug | 14–21/Aug, 25–26/Aug | Ureterolith, pyelonephritis | |
| 15 | Unknown | Siamese | M | Multi-cat household | Unvaccinated | None | 14/Aug | 14/Aug–7/Sep | Femoral fracture, pancreatitis | |
| 16 | 9 yr | Abyssinian | NM | Single-cat household | Sufficient | 1 yr ago | 19/Aug | 26/Aug–1/Sep | Chronic kidney disease, ureterolith, cystolith | |
| 17 | 8 mon | Munchkin | M | Single-cat household | Sufficient | 4 mon ago | 22/Aug | 1–5/Aug, 12–26/Aug | Portosystemic shunt, hepatic encephalopathy | |
| 18 | 8 yr | DSH | SF | Multi-cat household | Insufficient | 3 yr ago | 06/Sep | 6–7/Sep | Hindlimb limping, lethargy, anorexia, fever, vomiting, pancreatitis | |
DSH, domestic shorthair; SF, spayed female; NM, neutered male; F, female; M, male.
aIndividuals who had previously been vaccinated were further divided into sufficient and insufficient. If there was a history of vaccination within 3 years, it was classified as sufficient.
The clinical signs, diagnosis, and prognosis of each patient with FCV-VSD are described in Table 2. The outbreak is divided into 2 periods. The first outbreak began with the admission of a patient (case 1) who visited the hospital presenting with fever and upper respiratory signs. Of the 12 patients in the first outbreak, 6, 4, and 2 cats were classified as confirmed, suspected, and possible FCV-VSD, respectively. Due to the first outbreak, the hospital was closed for a week from May 12 to 19 and disinfected several times with commercial sodium hypochlorite. No new patients were encountered for the next 3 months. The second outbreak began with the admission of case 13 in August. Outbreak 2 included 6 patients, 5 confirmed and 1 suspected FCV-VSD. From September 7 to September 21, the hospital was closed for the second time, and thorough disinfection was performed. Cases 1 and 18 visited the hospital with clinical symptoms suspected to be FCV-VSD at the time of presentation, but the other patients visited the hospital with initial chief complaints unrelated to FCV infection.
Table 2. Clinical characteristics, diagnosis, treatment and prognosis of cats with feline calicivirus-associated viral systemic disease in Korea.
| Case No. | Onset of clinical symptoms | Clinical symptoms | Areas affected by limb edema | Calicivirus statusa | Sampling site (result) for RT-qPCR | Diagnosis of FCV-VSDb | rFeIFN-ω treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Outbreak 1 | |||||||||
| 1 | 04/Mar | Anorexia, lethargy, fever, limb edema, nasal discharge, sneezing, ocular discharge, ulcerative dermatitis | All four limbs | Unavailable | Suspected | X | Died | ||
| 2 | 23/Mar | Anorexia, lethargy, fever, limb edema, ulcerative dermatitis, nasal discharge, sneezing | All four limbs | Positive | Oral/laryngeal (+), blood (-) | Confirmed | X | Died | |
| 3 | 26/Mar | Anorexia, lethargy, fever, hypersalivation, jaundice | Unavailable | Suspected | X | Died | |||
| 4 | 26/Mar | Anorexia, lethargy, fever, limb edema, ulcerative dermatitis, hypersalivation, vomiting | Lt forelimb | Negative | Oral/laryngeal (-), blood (-) | Suspected | O | Survived | |
| 5 | 02/Apr | Anorexia, lethargy, fever, facial/limb edema, cough | Rt forelimb and Rt hindlimb | Positive | Oral/laryngeal (-), blood (-), liver tissue (+) | Confirmed | X | Died | |
| 6 | 29/Mar | Anorexia, lethargy, fever, limb edema, crusting skin lesions, delayed wound healing | All four limbs | Unavailable | Suspected | X | Died | ||
| 7 | 27/Mar | Anorexia, lethargy, fever, facial/limb edema | Lt forelimb and Lt hindlimb | Negative | Oral/laryngeal (-), blood (-) | Possible | X | Died | |
| 8 | 29/Mar | Lethargy, fever, oral ulceration, ulcerative keratitis, limb edema | All four limbs | Positive | Oral/laryngeal (+) | Confirmed | X | Died | |
| 9 | 04/Apr | Lethargy, oral ulceration, ulcerative keratitis, facial edema | Negative | Oral/laryngeal (-) | Possible | O | Survived | ||
| 10 | 17/Apr | Anorexia, lethargy, fever, facial/limb edema, ulcerative dermatitis | Forelimbs | Positive | Oral/laryngeal (+), skin (+) | Confirmed | X | Survived | |
| 11 | 09/May | Anorexia, lethargy, dull mental status, limb edema, jaundice | All four limbs | Positive | Oral/laryngeal (+), blood (-) | Confirmed | X | Died | |
| 12 | 11/May | Anorexia, lethargy, fever, limb edema | Rt forelimb and Rt hindlimb | Positive | Skin (+), liver tissue (+) | Confirmed | X | Died | |
| Outbreak 2 | |||||||||
| 13 | 15/Aug | Anorexia, nausea, fever, nasal discharge | Positive | Oral/laryngeal (-), skin (+) | Confirmed | X | Died | ||
| 14 | 25/Aug | Anorexia, fever, limb edema, diarrhea | Forelimbs | Positive | Oral/laryngeal (-), skin (+) | Confirmed | O | Survived | |
| 15 | 29/Aug | Lethargy, facial/limb edema, delayed wound healing, nasal discharge, dyspnea, cough, pneumonia | All four limbs | Positive | Oral/laryngeal (+), blood (-) | Confirmed | X | Survived | |
| 16 | 28/Aug | Anorexia, fever, facial edema | Positive | Oral/laryngeal (+), skin (-), blood (-) | Confirmed | O | Died | ||
| 17 | 26/Aug | Anorexia, lethargy, fever, limb edema, delayed wound healing | Hindlimbs | Unavailable | Suspected | X | Died | ||
| 18 | 06/Sep | Anorexia, lethargy, jaundice, hindlimb limping without edema | Positive | Oral/laryngeal (+), blood (-) | Confirmed | O | Died | ||
FCV-VSD, feline calicivirus-associated viral systemic disease; RT-qPCR, reverse transcription quantitative real time polymerase chain reaction; rFeIFN-ω, recombinant-feline interferon-omega; Lt, left; Rt, right.
aDetection of callicivirus by RT-qPCR.
bAccording to exposure history, clinical signs, and RT-qPCR results, patients were classified as confirmed, suspected, or possible FCV-VSD.
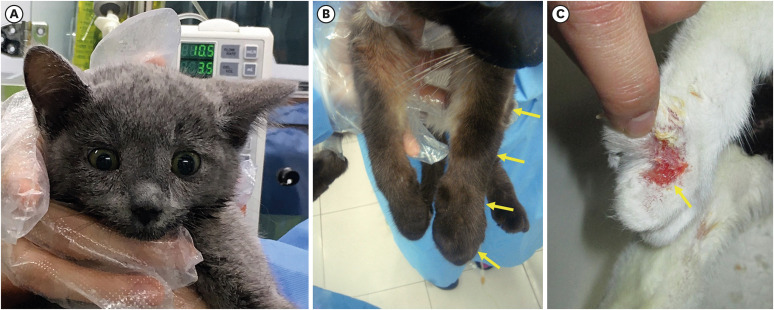
Anorexia (n = 15), lethargy (n = 15), and fever (n = 14) were common nonspecific clinical signs observed in most of the patients. Facial (Fig. 1A) and limb (Fig. 1B) edema were observed in 6 and 13 animals, respectively. Case 18 presented with a hindlimb limp without edema. Limb edema was variously identified as affecting all limbs (n = 6), the anterior or posterior limbs (n = 3), unilateral limbs (n = 3), or only one leg (n = 1). Skin lesions, such as ulcerative dermatitis (Fig. 1C), crusting skin lesions, and delayed wound healing after venipuncture or surgery, were observed in 6 cats. All patients with skin ulcerations or crusts were preceded by leg edema. No improvement in skin lesions was observed in the deceased cats but was observed in the surviving cats. Oral ulceration, ulcerative keratitis, jaundice, and URTD-related symptoms (e.g., nasal discharge, sneezing, and coughing) were observed in 2, 2, 3, and 5 cats, respectively. Alterations in mental status and diarrhea were observed in 1 cat each.
Fig. 1. Clinical features of cats with feline calicivirus-associated viral systemic disease. (A) Facial edema in case 3, (B) edema of the left forelimb in case 5 (yellow arrows), and (C) crusted ulcerative skin lesion in case 2 (yellow arrow).
CBC and serum biochemistry were performed on 17 cats, and the findings are shown in Table 3. Lymphopenia, elevated mean platelet volume, and thrombocytopenia were the predominant abnormalities, observed in descending order. Mild to moderate anemia, neutrophilia, and basophilia were also observed in some patients. Increased aspartate aminotransferase (AST) levels were observed in 12 of 14 assessed cats, and hyperbilirubinemia in 12 of 16 cats assessed. Creatine kinase (CK) and serum amyloid A (SAA) levels were measured in 7 and 10 animals, respectively, and were significantly elevated in all individuals evaluated.
Table 3. Hematologic and serum chemistry findings in 18 cats with feline calicivirus-associated viral systemic disease.
| Parameter | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | Case 14 | Case 15 | Case 16 | Case 17 | Case 18 | Mean ± SD | Reference interval | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete blood count | |||||||||||||||||||||
| RBC (×106/L) | 9.08 | 3.34 | 8.25 | 4.41 | 6.69 | 7.11 | 7.28 | 9.47 | 7.83 | 8.48 | 7.18 | 5.40 | 5.77 | 4.12 | 4.74 | 3.34 | NA | 7.16 | 6.5 ± 1.9 | 6.0–10.1 | |
| Hb (g/dL) | 14.1 | 6.0 | 11.9 | 7.5 | 30.1 | 12.6 | 11.5 | 14.1 | 12.6 | 13.3 | 10.1 | 8.1 | 8.8 | 6.1 | 7.2 | 5.2 | NA | 11.7 | 11.2 ± 5.5 | 8.1–14.2 | |
| PCV (%) | 42.4 | 18.0 | 37.0 | 22.6 | 30.1 | 36.6 | 33.5 | 45.9 | 41.6 | 37.0 | 32.6 | 25.1 | 26.3 | 18.7 | 21.3 | 14.7 | NA | 35.2 | 30.5 ± 9.1 | 27.7–46.8 | |
| Reticulocytes (%) | 0.15 | 0.28 | 0.09 | 0.69 | 0.31 | 0.63 | 0.49 | 0.2 | 0.10 | 0.27 | 0.24 | 0.47 | 0.97 | 0.69 | 0.91 | 0.64 | NA | 0.33 | 0.4 ± 0.3 | 0–1.3 | |
| Reticulocytes (×103/L) | 13.7 | 9.5 | 7.1 | 30.4 | 20.7 | 44.6 | 35.4 | 18.9 | 7.8 | 22.7 | 16.9 | 25.4 | 55.7 | 28.4 | 43.2 | 21.5 | NA | 13.7 | 24.5 ± 13.4 | 15.0–81.0 | |
| WBC (×103/L) | 4.38 | 44.21 | 23.51 | 15.91 | 4.89 | 17.74 | 7.65 | 5.73 | 12.60 | 14.75 | 6.94 | 2.93 | 12.11 | 22.77 | 34.21 | 0.06 | NA | 11.39 | 14.2 ± 11.3 | 6.3–19.6 | |
| Neutrophil (×103/L) | 3.32 | 40.72 | 22.65 | 14.55 | 3.42 | 15.02 | 6.23 | 4.66 | 11.79 | 12.66 | 6.19 | 1.97 | 10.55 | 18.77 | 30.16 | 0 | NA | 10.26 | 12.5 ± 10.4 | 3.0–13.4 | |
| Lymphocyte (×103/L) | 0.4 | 1.79 | 0.31 | 0.61 | 1.36 | 1.03 | 1.22 | 0.76 | 0.32 | 0.49 | 0.64 | 0.89 | 1.29 | 3.25 | 2.70 | 0.06 | NA | 0.93 | 1.1 ± 0.8 | 2.0–7.2 | |
| Monocyte (×103/L) | 0.41 | 1.4 | 0.32 | 0.72 | 0.05 | 0.33 | 0.03 | 0.1 | 0.34 | 0.63 | 0.05 | 0.03 | 0.09 | 0.36 | 0.86 | 0 | NA | 0.08 | 0.3 ± 0.4 | 0–1.0 | |
| Eosinophil (×103/L) | 0.06 | 0.11 | 0.14 | 0 | 0.03 | 1.25 | 0.06 | 0.03 | 0.03 | 0.93 | 0.00 | 0.00 | 0.03 | 0.12 | 0.20 | 0 | NA | 0.06 | 0.2 ± 0.3 | 0–1.7 | |
| Basophil (×103/L) | 0.01 | 0.06 | 0.04 | 0.01 | 0.49 | 0.07 | 0.80 | 0.01 | 0.00 | 0.30 | 0.01 | 0.00 | 0.10 | 0.22 | 0.07 | 0 | NA | 0.02 | 0.1 ± 0.2 | 0–0.1 | |
| Platelets (×103/L) | 74 | 141 | 79 | 141 | 287 | 118 | 193 | 141 | 172 | 177 | 245 | 148 | 412 | 104 | 108 | 171 | NA | 169 | 169.4 ± 80.4 | 156.4–626.4 | |
| MPV (fL) | 21.5 | 30.7 | 22.2 | 30.7 | 22.0 | 12.9 | 23.1 | 20.6 | 20.6 | 14.8 | 16.6 | 25.8 | 20.1 | 27.6 | 23.0 | 23.2 | NA | 23.7 | 22.3 ± 4.7 | 8.6–18.9 | |
| Serum chemistry | |||||||||||||||||||||
| AST (U/L) | 194 | 217 | 70 | 72 | 50 | 83 | 366 | 441 | 34 | 91 | 120 | 58 | 242 | 36 | NA | 142.9 ± 120.6 | 12–46 | ||||
| ALT (U/L) | 95 | 11 | 23 | 21 | 134 | 51 | 19 | 85 | 110 | 103 | 19 | 61 | 9 | 11 | 43 | NA | 39 | 49.6 ± 39.7 | 28–106 | ||
| ALP (U/L) | 19 | 34 | 24 | 13 | 10 | 21 | 45 | 10 | 10 | 9 | 26 | 9 | 10 | 8 | 7 | NA | 19 | 17.1 ± 10.1 | 14–71 | ||
| GGT (U/L) | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 1 | NA | 9 | 1.2 ± 2.2 | 0–4 | ||||
| TBIL (mg/dL) | 2.8 | 2.9 | 0.6 | 0.4 | 0.5 | 1.0 | 0.9 | 0.2 | 0.5 | 4.1 | 0.7 | 1.1 | 0.2 | 0.1 | 0.1 | NA | 1.2 | 1.1 ± 1.1 | 0–0.2 | ||
| TP (g/dL) | 6.4 | 6.5 | 7.3 | 5.9 | 6.5 | 6.5 | 5.6 | 8.7 | 6.5 | 5.8 | 7.0 | 5.8 | 5.4 | NA | 7.5 | 6.5 ± 0.8 | 5.5–7.5 | ||||
| ALB (g/dL) | 3 | 2.6 | 3.3 | 3.8 | 2.2 | 3.1 | 2.9 | 2.0 | 3.1 | 3.2 | 2.9 | 3.1 | 2.9 | 2.3 | NA | 2.8 | 2.9 ± 0.4 | 2.7–3.9 | |||
| GLOB (g/dL) | 3.4 | 3.2 | 3.5 | 3.7 | 3.4 | 3.6 | 3.6 | 5.6 | 3.3 | 2.9 | 3.9 | 2.9 | 3.1 | NA | 4.7 | 3.6 ± 0.7 | 2.8–5.1 | ||||
| CK (U/L) | 10,298 | 2,538 | 1,415 | 473 | 673 | 673 | NA | 3,221 | 2,750.6 ± 3,229.1 | 0–314 | |||||||||||
| fSAA (μg/mL) | 116.0 | 65.9 | 70.7 | 118.0 | 165.0 | > 200 | 103.0 | > 200 | 8.4 | NA | 131.0 | 108.7 ± 30.0 | 0–5 | ||||||||
RBC, red blood cell; Hb, hemoglobin; PCV, packed cell volume; WBC, total white blood cell count; MPV, mean platelet volume; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; TBIL, total bilirubin; TP, total protein; ALB, albumin; GLOB, globulin; CK, creatine kinase; fSAA, feline serum amyloid A; NA, not applicable.
Positive RT-qPCR for FCV was found in 7 of 13 oral and laryngeal swab samples from separate patients. Furthermore, 4 of 5 skin samples and all 2 liver tissue samples tested positive, but all 8 blood samples were negative. The quantification results of viral loads for positive samples are presented in Supplementary Table 1. Additionally, the reverse transcription polymerase chain reaction test results for other viruses, including feline herpesvirus, reovirus, and influenza A virus, were negative for all cats included in this study (data not shown).
Thirteen patients eventually died, and the mortality rate was 72.2%. The average age of deceased and surviving patients was 5.7 and 3.5 years, respectively. The mortality rate (83.3%) of sufficiently vaccinated individuals was significantly higher than that of the other patients (66.7%; unvaccinated or insufficiently vaccinated). Autopsies were conducted on the two deceased patients (cases 5 and 12). In both cats, severe edema in the dermis and subcutis, along with infiltration of mild macrophages and plasmacytes in dermal vessels and surrounding skin tissue, were confirmed (data not shown). However, no findings suggestive of systemic infectious, inflammatory, toxic, or neoplastic disease were observed in the remaining organs. Three (60%) of 5 patients who received feline recombinant interferon omega (Virbagen Omega; Virbac SA, France) survived. Of 13 patients who did not receive interferon omega, only 2 survived. In addition, broad-spectrum antibiotics, fluid therapy, and feeding via a nasoesophageal tube or force feeding were administered depending on the patient’s condition.
DISCUSSION
This study describes two separate outbreaks of FCV-VSD involving 18 cats over a 6-month period in Korea. Considering the survival period of FCV and the excellent antiviral effect of sodium hypochlorite [20], each outbreak in this study may have been caused by the external influx of patients (cases 1 and 13). However, the origin of the infection in cases 1 and 13, the first patients of outbreaks 1 and 2, respectively, was unknown. The routes of movement and people exposed in the hospital commonly overlapped with those of the confirmed cases; however, in cases 10, 11, and 12, the routes of contact with the confirmed cats were difficult to infer. Additionally, in case 18, the patient may have had symptoms related to FCV-VSD from the time of admission. In previous reports, the onset of FCV-VSD occurred among shelter cats, but the origin was unknown in other cases [11,12]. Furthermore, considering that FCV-VSD is highly likely to be transmitted through fomites and humans [8,11,15], the viability of FCV in the environment and its resistance to disinfectants cannot be ruled out. In addition, a virulent strain that induced FCV-VSD was being shed by some patients up to 16 weeks after recovery [8]. Despite the contagious nature of FCV, FCV-VSD outbreaks have been limited to veterinary clinics and have not spread within the community [12]. After the outbreak in this study, no patients suspected of spreading were observed in nearby hospitals or the surrounding community. However, considering the possibility of transmission by external influx, spread to the wider community cannot be completely ruled out.
Risk factors for URTD due to FCV infection include poor environmental hygiene, living with more than one dog, multi-cat households, intact reproductive status, and co-infection with other pathogens causing URTD [21,22]. Although no known risk factors for FCV-VSD have been identified, adult cats tend to show more severe disease than kittens [8]. In this study, no significant risk factors linked to FCV-VSD were identified. Although deceased individuals have an average age slightly higher than that of survivors, all two patients under one year of age were included in the deceased group. The majority of patients included in this study presented with pre-existing conditions unrelated to FCV-VSD upon their initial hospital visit. However, there was no discernible pattern or uniformity among these conditions. Further research is warranted to elucidate risk factors, encompassing host health status and environmental variables, crucial for the diagnosis, treatment, and prevention of FCV-VSD.
The protective efficacy of the FCV vaccine against FCV-VSD is controversial. Because the occurrence of FCV-VSD was reported in regularly vaccinated individuals in some studies, the number of strains included in vaccines may be insufficient to prevent the occurrence of FCV-VSD [8,11,15]. However, one study reported that oral vaccination led to mild self-limiting clinical symptoms during FCV infection [10]. Another study demonstrated that FCV vaccination had a partial protective effect against FCV-VSD and that virus neutralization assays may underestimate the protective effect of the vaccine [23]. Although developing a vaccine for FCV infection is difficult because of its antigenically diverse nature [24], regular vaccination is still essential.
Forty-six percent of patients with limb edema had edema in all four legs, but no trend toward a specific leg was found. In some patients with limb edema, progression to skin lesions, such as erosions, ulcers, or crusts, was observed, which was similar to the progression observed in a previous study [4]. However, unlike in the previous study, lesions on footpads were not clearly observed in the patients in the present study. In case 18, the patient visited the hospital with hindlimb limping without edema and fever as the main symptoms and was diagnosed with FCV on the day of presentation. These symptoms were similar to limping syndrome caused by FCV [25]. However, limping syndrome usually showed mild URTD symptoms, and lameness usually only persisted temporarily [3,16,25]. In case 18 in the present study, lameness persisted, was accompanied by pancreatitis, and eventually led to death. Respiratory signs such as sneezing, nasal discharge, and coughing were observed in some patients with FCV-VSD [15]. Conversely, in the present study, respiratory signs were not a prominent initial symptom, and the incidence of those signs was low. In addition, the incidence of oral ulceration was only 12.5%, which was significantly lower than those reported in previous studies [11,15]. One study reported that clinical symptoms became more severe and outcomes worsened during the outbreak; however, no such increase in virulence was noted in this study [11]. The phenotypic diversity of FCV infections, including not only typical URTD but also limping syndrome and VSD, may be due to the genetic and antigenic diversity of FCV [9,25].
Lymphopenia is observed in several viral infections, including FCV-VSD, and is associated with disease severity, although the exact mechanism is unknown [4,26]. In previous studies, lymphopenia, neutrophilia, and thrombocytopenia were observed on CBC in some cats with FCV-VSD [8,27]. No previous studies have been conducted on the mechanism or frequency of macrothrombocytopenia caused by FCV infection. However, thrombocytopenia frequently occurs in feline viral infections, particularly feline leukemia virus (FeLV) infections, and pathological changes, such as the decreased life span of FeLV-infected platelets, have been reported [28,29]. However, whether these changes in platelets actually cause changes in platelet function or hemostasis in patients with FeLV is unclear [29]. Considering that thrombocytopenia is often identified in patients with FCV-VSD, unlike typical calicivirus infections, clinical awareness of the possibility of pathological changes in platelets due to infections by virulent strains should be considered.
Liver enzyme elevation and hyperbilirubinemia in FCV-VSD are caused by liver necrosis, congestion, or pancreatitis [9,15]. Hyperbilirubinemia was commonly observed in this study, but only 3 patients developed jaundice. This finding indicates the possibility that progression to jaundice may have been overlooked because of the high mortality rate. Elevated CK along with high AST activity may indicate possible muscle damage, which would be supported by the absence of significant elevations in other liver enzyme values. However, no pathological findings were identified in the internal organs, including the liver or muscles, in both patients who underwent autopsy. An increase in SAA, which is usually observed in inflammatory diseases [30], was also confirmed in all patients with FCV-VSD in this study. However, hypoproteinemia and hypoalbuminemia, which have been reported previously [8,15], could not be confirmed in the patients in the present study. Further investigation is warranted to ascertain the potential utility of these hematologic and serum biochemical parameters as prognostic indicators or therapeutic response markers in FCV-VSD.
FCV infection cannot be ruled out with a negative RT-qPCR result, and factors such as sampling method, storage, and transport may affect the test results [9]. RT-qPCR is as sensitive and specific as viral isolation in detecting FCV, and has been demonstrated to detect a high level of FCV from an oronasal swab in a typical FCV infection [31,32]. However, in the present study, the virus detection rate in skin samples and liver tissue was higher than that in oral or laryngeal samples. In addition, only a few RNA copies were confirmed in some oropharyngeal swabs, whereas numerous RNA copies were confirmed in skin scraps or liver tissue samples (Supplementary Table 1). In FCV-VSD, not all patients show respiratory signs; therefore, sampling through multiple routes is recommended to reduce the possibility of false negatives. Further investigation is necessary to explore optimal sampling sites and techniques, as well as to develop new analysis methods for FCV detection.
The mortality rate due to FCV-VSD in this study (72.2%) was similar to or slightly higher than the 40%–79% reported in previous studies [8,10,11]. To date, no effective treatment for FCV-VSD has been established; however, the in vitro efficacy of antivirals, such as nitazoxanide, has been analyzed [33]. Feline recombinant omega interferon has shown clinical efficacy in refractory stomatitis caused by FCV infection [34]. Three out of 5 patients who received feline recombinant interferon omega survived in this study, but only 2 out of 13 patients who did not receive it survived. However, because this study had a small sample size and was not controlled, additional randomized and controlled studies are required to evaluate the clinical efficacy of interferon omega against virulent FCV strains. Moreover, the development of antivirals or potential therapeutic interventions for FCV-VSD is warranted.
In this study, the exact route of infection introduction could not be identified, and internal organs were not evaluated by postmortem examination. Additionally, another limitation is the inability to isolate and genetically analyze FCV strains collected during the outbreak. Several highly virulent strains associated with FCV-VSD have been reported; however, to date, no specific genetic markers for detecting these strains have been identified [9,35]. Although various viral strains have been detected, no specific mutations causing FCV-VSD have been reported to date [33,35]. Therefore, the diagnosis of FCV-VSD is based on history, characteristic systemic clinical signs, positive detection, and poor prognosis. To control outbreaks, closing and disinfecting the affected clinic is most effective [12]. In the animal hospital in the present study, no new FCV-VSD outbreaks have occurred for several years after outbreak 2.
In conclusion, we described the clinical and diagnostic characteristics and outcome of FCV-VSD that occurred consecutively in several cats in Korea. To the best of the author’s knowledge, this is the first report of a nosocomial outbreak of FCV-VSD in Asia. Considering the rapid transmission and poor prognosis of FCV-VSD, veterinarians must recognize the disease and take prompt action to treat patients and prevent infection. Disease control is necessary through the closure of hospitals and thorough disinfection.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Doo-Sung Cheon (Postbio) for assisting with genetic testing for the detection of feline calicivirus.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Park J, Lee D, Hong YJ.
- Data curation: Park J, Lee D.
- Formal analysis: Park J.
- Investigation: Park J, Hong YJ, Hyun JE.
- Supervision: Hyun JE.
- Visualization: Park J.
- Writing - original draft: Park J, Hyun JE.
- Writing - review & editing: Hwang CY, Hyun JE.
SUPPLEMENTARY MATERIAL
Sample collection site for feline calicivirus detection in each patient and qualitative and quantitative test results of RT-qPCR by sample
References
- 1.Radford AD, Coyne KP, Dawson S, Porter CJ, Gaskell RM. Feline calicivirus. Vet Res. 2007;38(2):319–335. doi: 10.1051/vetres:2006056. [DOI] [PubMed] [Google Scholar]
- 2.Slaviero M, Ehlers LP, Argenta FF, Savi C, Lopes BC, Pavarini SP, et al. Causes and lesions of fatal pneumonia in domestic cats. J Comp Pathol. 2021;189:59–71. doi: 10.1016/j.jcpa.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Dawson S, Bennett D, Carter SD, Bennett M, Meanger J, Turner PC, et al. Acute arthritis of cats associated with feline calicivirus infection. Res Vet Sci. 1994;56(2):133–143. doi: 10.1016/0034-5288(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 4.Palombieri A, Sarchese V, Giordano MV, Fruci P, Crisi PE, Aste G, et al. Detection and characterization of feline calicivirus associated with paw and mouth disease. Animals (Basel) 2022;13(1):65. doi: 10.3390/ani13010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Martino B, Lanave G, Di Profio F, Melegari I, Marsilio F, Camero M, et al. Identification of feline calicivirus in cats with enteritis. Transbound Emerg Dis. 2020;67(6):2579–2588. doi: 10.1111/tbed.13605. [DOI] [PubMed] [Google Scholar]
- 6.Binns SH, Dawson S, Speakman AJ, Cuevas LE, Hart CA, Gaskell CJ, et al. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus. J Feline Med Surg. 2000;2(3):123–133. doi: 10.1053/jfms.2000.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissler K, Schneider K, Platzer G, Truyen B, Kaaden OR, Truyen U. Genetic and antigenic heterogeneity among feline calicivirus isolates from distinct disease manifestations. Virus Res. 1997;48(2):193–206. doi: 10.1016/s0168-1702(97)01440-8. [DOI] [PubMed] [Google Scholar]
- 8.Hurley KE, Pesavento PA, Pedersen NC, Poland AM, Wilson E, Foley JE. An outbreak of virulent systemic feline calicivirus disease. J Am Vet Med Assoc. 2004;224(2):241–249. doi: 10.2460/javma.2004.224.241. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann-Lehmann R, Hosie MJ, Hartmann K, Egberink H, Truyen U, Tasker S, et al. Calicivirus infection in cats. Viruses. 2022;14(5):937. doi: 10.3390/v14050937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen NC, Elliott JB, Glasgow A, Poland A, Keel K. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet Microbiol. 2000;73(4):281–300. doi: 10.1016/S0378-1135(00)00183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deschamps JY, Topie E, Roux F. Nosocomial feline calicivirus-associated virulent systemic disease in a veterinary emergency and critical care unit in France. J Feline Med Surg Open Rep. 2015;1(2):2055116915621581. doi: 10.1177/2055116915621581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurley KF, Sykes JE. Update on feline calicivirus: new trends. Vet Clin North Am Small Anim Pract. 2003;33(4):759–772. doi: 10.1016/s0195-5616(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 13.Clay S, Maherchandani S, Malik YS, Goyal SM. Survival on uncommon fomites of feline calicivirus, a surrogate of noroviruses. Am J Infect Control. 2006;34(1):41–43. doi: 10.1016/j.ajic.2005.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadoi K, Kadoi BK. Stability of feline caliciviruses in marine water maintained at different temperatures. New Microbiol. 2001;24(1):17–21. [PubMed] [Google Scholar]
- 15.Schorr-Evans EM, Poland A, Johnson WE, Pedersen NC. An epizootic of highly virulent feline calicivirus disease in a hospital setting in New England. J Feline Med Surg. 2003;5(4):217–226. doi: 10.1016/S1098-612X(03)00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H, Miao Q, Zhu J, Yang Z, Liu G. Isolation and molecular characterization of a virulent systemic feline calicivirus isolated in China. Infect Genet Evol. 2018;65:425–429. doi: 10.1016/j.meegid.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Xin T, Wei J, Jiang Y, Liu X, Song W, et al. Isolation and phylogenetic analysis of strains of feline calicivirus in Beijing, China. Arch Virol. 2021;166(9):2521–2527. doi: 10.1007/s00705-021-05163-2. [DOI] [PubMed] [Google Scholar]
- 18.Park JI, Suh SI, Hyun C. Virulent systemic feline calicivirus infection in a kitten. J Vet Clin. 2015;32(5):445–448. [Google Scholar]
- 19.Chander Y, Tiwari AK, Sajja S, Ramakrishnan MA, Faaberg KS, Goyal SM. TaqMan" RT-PCR assay for the detection of Feline calicivirus. Int J Virol. 2007;3(3):100–106. [Google Scholar]
- 20.Chiu S, Skura B, Petric M, McIntyre L, Gamage B, Isaac-Renton J. Efficacy of common disinfectant/cleaning agents in inactivating murine norovirus and feline calicivirus as surrogate viruses for human norovirus. Am J Infect Control. 2015;43(11):1208–1212. doi: 10.1016/j.ajic.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Helps CR, Lait P, Damhuis A, 2, Björnehammar U, Bolta D, Brovida C, et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: experience from 218 European catteries. Vet Rec. 2005;156(21):669–673. doi: 10.1136/vr.156.21.669. [DOI] [PubMed] [Google Scholar]
- 22.Berger A, Willi B, Meli ML, Boretti FS, Hartnack S, Dreyfus A, et al. Feline calicivirus and other respiratory pathogens in cats with feline calicivirus-related symptoms and in clinically healthy cats in Switzerland. BMC Vet Res. 2015;11(1):282. doi: 10.1186/s12917-015-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willi B, Spiri AM, Meli ML, Samman A, Hoffmann K, Sydler T, et al. Molecular characterization and virus neutralization patterns of severe, non-epizootic forms of feline calicivirus infections resembling virulent systemic disease in cats in Switzerland and in Liechtenstein. Vet Microbiol. 2016;182:202–212. doi: 10.1016/j.vetmic.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Poulet H, Brunet S, Soulier M, Leroy V, Goutebroze S, Chappuis G. Comparison between acute oral/respiratory and chronic stomatitis/gingivitis isolates of feline calicivirus: pathogenicity, antigenic profile and cross-neutralisation studies. Arch Virol. 2000;145(2):243–261. doi: 10.1007/s007050050021. [DOI] [PubMed] [Google Scholar]
- 25.Lanave G, Buonavoglia A, Pellegrini F, Di Martino B, Di Profio F, Diakoudi G, et al. An outbreak of limping syndrome associated with feline calicivirus. Animals (Basel) 2023;13(11):1778. doi: 10.3390/ani13111778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, Zhang Z, Prajapati M, Li Y. Lymphopenia caused by virus infections and the mechanisms beyond. Viruses. 2021;13(9):1876. doi: 10.3390/v13091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds BS, Poulet H, Pingret JL, Jas D, Brunet S, Lemeter C, et al. A nosocomial outbreak of feline calicivirus associated virulent systemic disease in France. J Feline Med Surg. 2009;11(8):633–644. doi: 10.1016/j.jfms.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs RM, Boyce JT, Kociba GJ. Flow cytometric and radioisotopic determinations of platelet survival time in normal cats and feline leukemia virus-infected cats. Cytometry. 1986;7(1):64–69. doi: 10.1002/cyto.990070109. [DOI] [PubMed] [Google Scholar]
- 29.Jordan HL, Grindem CB, Breitschwerdt EB. Thrombocytopenia in cats: a retrospective study of 41 cases. J Vet Intern Med. 1993;7(5):261–265. doi: 10.1111/j.1939-1676.1993.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 30.Yuki M, Aoyama R, Nakagawa M, Hirano T, Naitoh E, Kainuma D. A clinical investigation on serum amyloid A con-centration in client-owned healthy and diseased cats in a primary care animal hospital. Vet Sci. 2020;7(2):45. doi: 10.3390/vetsci7020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz C, Hartmann K, Mueller RS, Helps C, Schulz BS. Sampling sites for detection of feline herpesvirus-1, feline calicivirus and Chlamydia felis in cats with feline upper respiratory tract disease. J Feline Med Surg. 2015;17(12):1012–1019. doi: 10.1177/1098612X15569615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abd-Eldaim MM, Wilkes RP, Thomas KV, Kennedy MA. Development and validation of a TaqMan real-time reverse transcription-PCR for rapid detection of feline calicivirus. Arch Virol. 2009;154(4):555–560. doi: 10.1007/s00705-009-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bordicchia M, Fumian TM, Van Brussel K, Russo AG, Carrai M, Le SJ, et al. Feline calicivirus virulent systemic disease: clinical epidemiology, analysis of viral isolates and in vitro efficacy of novel antivirals in Australian outbreaks. Viruses. 2021;13(10):2040. doi: 10.3390/v13102040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hennet PR, Camy GA, McGahie DM, Albouy MV. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: a randomised, multi-centre, controlled, double-blind study in 39 cats. J Feline Med Surg. 2011;13(8):577–587. doi: 10.1016/j.jfms.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caringella F, Elia G, Decaro N, Martella V, Lanave G, Varello K, et al. Feline calicivirus infection in cats with virulent systemic disease, Italy. Res Vet Sci. 2019;124:46–51. doi: 10.1016/j.rvsc.2019.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample collection site for feline calicivirus detection in each patient and qualitative and quantitative test results of RT-qPCR by sample