Abstract
In 2002, a Neandertal partial femoral diaphysis was discovered at Les Rochers-de-Villeneuve (Vienne, France). Radiocarbon dated to ≈40,700 14C years before present, this specimen is one of the most recent Middle Paleolithic Neandertals. The diaphysis derives from an archeological level indicating alternating human and carnivore (mostly hyena) occupation of the cave, reinforcing the close proximity and probable competition of Middle Paleolithic humans with large carnivores for resources and space. Morphological aspects of the diaphysis and ancient DNA extracted from it indicate that it is aligned with the Neandertals and is distinct from early modern humans. However, its midshaft cortical bone distribution places it between other Middle Paleolithic Neandertals and the Châtelperronian Neandertal from La Roche-à-Pierrot, supporting a pattern of changing mobility patterns among late Middle Paleolithic Neandertals on the eve of modern human dispersals into Europe.
Keywords: hyena, Middle Paleolithic, mitochondrial DNA, mobility
Discussions of the biobehavioral emergence of modern humans in Europe have become increasingly focused on the period between ≈40,000 and ≈30,000 14C years before present (BP), as it has become increasingly documented that it was during these millennia that both late Neandertals and early modern humans occupied this peninsula of the northwestern Old World, and that there were complex interrelationships between human biological forms and the technotypological units of the late Middle Paleolithic and earlier Upper Paleolithic. Yet, late archaic human remains are scarce and largely fragmentary for this time period, and new paleontological discoveries and radiocarbon dating have seriously reshuffled the sample of pre-30,000-BP early modern humans in Europe. It is therefore important, for our understanding of the complex processes involved in the replacement of Neandertal biology by that of early modern humans, that additional pertinent human remains be documented and integrated into the paleoanthropological record. With these considerations in mind, we present here a human femoral diaphysis from the site of Les Rochers-de-Villeneuve in central western France.
Les Rochers-de-Villeneuve
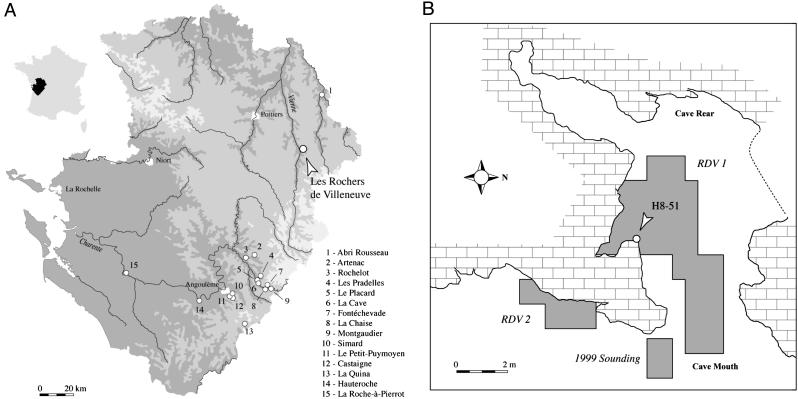
The cave of Les Rochers-de-Villeneuve (Lussac-les-Chāteaux, Vienne, France; 46°24′54″N, 0°44′27″E), between the valleys of the Vienne and Gartempe (Fig. 1A), faces east ≈20 m above the brook of Les Grands Moulins, a tributary of the Vienne River. The cavity is part of a karstic system formed in a Dogger limestone formation, ≈10 m long and ≈6 m wide (Fig. 1B). A north-south fissure crosses the cave on the west; at the northern end of this fissure, the cave opens onto the plateau by an opening of ≈1.0 m × ≈0.6 m. At its southern extremity, the cave continues as a tunnel for ≈10 m. The mouth of the cave is 4 m wide and 1.6 m high in its middle. In the southeast of this small cavity, a tunnel overhung by a subsided vault communicates with the outside. The topography that existed during the Middle Paleolithic is difficult to reconstruct because of karstic collapses.
Fig. 1.
Location of Les Rochers-de-Villeneuve and plan of the site. (A) Position of Les Rochers-de-Villeneuve (Vienne, France) and other Lower and Middle Paleolithic sites delivering human remains in Poitou-Charentes, France. (B) Map of the cave with the main excavated areas. H8-51 marks the position of the RdV 1 human femur.
An archeological level in front of the cave has been known since 1969, when P. Boutin and A. Chollet archeologically tested the entrance of the cavity. They identified Mousterian lithics and bovine, equid, and hyaenid bones and interpreted the deposit as principally a hyena den. The 1999-2003 excavations under the direction of C. Beauval included almost 30 m2 in two loci: the main chamber and the cave terrace (Fig. 1B). The main archeological zone is the southern half of the principal chamber, which yielded ≈6,300 plotted items in three stratigraphic levels.
The lower part of the stratigraphy (level SJ) is a yellow sandy level with a few bone fragments and no flint. The middle layer (level N) is a dark brown silt very rich with animal bones and a denticulate Mousterian with a discoid débitage. The superficial layer (level J) is a clear brown silt rich with abundant faunal remains and few lithic artifacts related to a Mousterian with denticulates and very large scrapers from a discoid and Levallois débitage. This form of scraper often is discovered regionally associated with mixed carnivore and human assemblages (1). Most of the archeological remains and the human femur came from level J.
Level J
The human occupation in level J of Les Rochers-de-Villeneuve corresponds to a series of short episodes during which humans mainly exploited carcasses of animals, principally bison, horse, and reindeer. They primarily used the flint available nearby to make and then discard Middle Paleolithic scrapers. No living structures are preserved, but the presence of burnt bones indicates the former presence of hearths. The cavity also was used as a den by cave hyenas (Crocuta crocuta spelaea), who hunted and/or scavenged the same prey as the humans, accumulating numerous bones (often broken or etched from digestion). Hyena activity and denning also are documented by their shed deciduous teeth and abundant fragments of their coprolites. The importance of the hyena occupation is supported by the relatively rarity of lithic remains, <5% of the 3,510 plotted items in level J; this value approaches those for other sites dominated by the remains of carnivore activity (2-4).
The close temporal succession of human and hyaenid occupations is attested by several bones (Equus and Bison) bearing both cutmarks and hyena damage, with one (an equid vertebra) having the tooth marks superimposed on the lithic damage. The faunal remains must have been reasonably fresh for the hyenas to exploit them after the humans had departed. In addition, the human femoral diaphysis was extensively gnawed by carnivores (see below), probably by hyenas. It is not known whether the person involved was prey or merely had its remains scavenged by the carnivores, but either scenario indicates close proximity of these two species who would have competed for prey animals and, as in the cave at Les Rochers-de-Villeneuve, for space.
Other carnivores, including wolves (Canis lupus), cave lion (Panthera leo spelaea), bear (Ursus cf. arctos), and especially fox (Vulpes vulpes and possibly Alopex lagopus) also are represented. Even though some of their bones have hyena damage, we do not know whether these carnivores were hunted by the hyenas or died in the cavity before or between the hyena occupations.
In addition to the mixing of human and carnivore debris in the deposit by the very close succession in time of the activities of these different species, the microsedimentology indicates that the deposits were moderately affected by several periglacial freeze-thaw cycles; this cyroturbation displaced some of the archeological and paleontological material, principally moving the largest pieces superiorly. However, of the 278 bone sections refit into 113 bones (of which 215 and 85, respectively, are in level J), none of them are between level J and other levels, and only one was between the two deeper levels (SJ and N); this pattern of refits indicates that the geological movement of items was essentially within levels, and it supports the integrity of the deposits of level J.
Consequently, remains belonging to different human and carnivore occupations became mixed into a single archeologicostratigraphic accumulation within level J. However, the homogeneity of the bone preservation (generally excellent, in contrast with that of the underlying level N), the lack of stratigraphic distinctions within level J, and the evidence of both humans and hyenas processing the same bones indicate the near contemporaneity of use of the cave by humans and carnivores and the short-term formation of level J.
To determine the age of the deposits, a 145-mm-long section of a hyena radius (J8-61) was sampled for accelerator MS 14C dating. The excavation coordinates of the hyena bone (square J8: x: 12, y: 40, z: 131) and those of the human femur (square H8: x: 59, y: 68, z: 105) place them 173 cm apart near the top of level J, the difference in the vertical (z) coordinates being due to the slope of the deposits. The hyena bone is therefore from the same stratigraphic depth as the human femur in an adjacent square (J8 vs. H8). The similar overall size of the bones (see below) suggests that they would have been affected by vertical displacement, if any, within level J in a similar manner. In any case, the short-term alternation of humans and hyenas in level J and homogeniety of the stratigraphic level make it unlikely that the bones would be significantly different in age. Any difference in age would most likely have been well within the error range of 14C dates of this antiquity.
The accelerator MS 14C result is 40,700 ± 900 BP (Beta-177765; δ13C, -18.6%; sample weight, 3.5 g; collagen weight, 17.4 mg) or 44,152 ± 817 cal years BP (calpal, Version 1.2, www.calpal.de). This age for level J is in agreement with the presence of a distinctive denticulate Mousterian in the underlying level N, a Middle Paleolithic assemblage composition most commonly found in the region <45,000 BP (5-7). It also conforms with the morphology of the horse (Equus caballus) remains, which are intermediate between those associated with the oxygen isotope stage (OIS) 4 and early OIS 3 Middle Paleolithic in France and those found in later OIS 3 Upper Paleolithic deposits of the same region (8). The date for level J therefore places it and its contents late in the Middle Paleolithic of western Europe, close in age to the Neandertal partial skeletons from Feldhofer (9) and Le Moustier (5), and a few millennia older than the Châtelperronian Neandertal from La Roche-à-Pierrot (Saint-Césaire) (6).
The Rochers-de-Villeneuve 1 (RdV 1) Human Femur
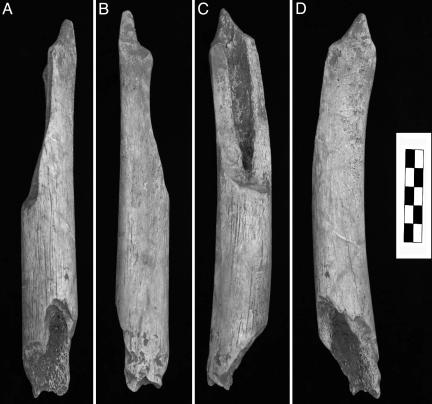
The human partial femoral diaphysis (RDV02-H8-51, or RdV 1) was discovered in situ in level J, square H8 (Fig. 1B) on June 25, 2002, by Charlène Bouyssou and recognized as human by D.P. RdV 1 is a midproximal and middle portion of a femoral diaphysis, 201.1 mm long (Fig. 2). Proximally, the femur is present laterally from the distal gluteal buttress, but the medial half was removed in an oblique break 84.3 mm long to near midshaft. There is a section 60.8 mm long near midshaft in which the full circumference of the diaphysis is preserved, but distally, it is broken obliquely anteroproximal to posterodistal 56.0 mm long. A proximal portion of the popliteal surface is evident by the beginning of the lateral deviation of the distolateral branch of the linea aspera. The anterior and medial convexities of the shaft, the distal end of the gluteal buttress swelling, and the left deviation of the linea aspera identify it as a left femur. The diaphysis presents no pathological lesions (even after radiography) or anthropic marks.
Fig. 2.
RdV 1 left femur fragment. Shown are anterior (A), posterior (B), medial (C), and lateral (D) areas.
The split ends of the femur exhibit several pits (10), or circular depressions, of the cortical surface. On the distal extremity, two depressions are very clear on the posterior surface, along with damage associated with removal of the bony surface near the distal fracture (Fig. 3). At the proximal end, the depressions are more difficult to discern because the cortical surface is partially exfoliated postdepositionally. However, there are three pits similar to those observed near the distal end, as well as some removal of the cortical surface. These pits indicate a modest intensity of carnivore action, similar to that on most of the bones from the level.
Fig. 3.
Details of the carnivore toothmarks on the distal end of the RdV 1 human femur diaphysis.
Comparative analyses of Late Pleistocene human femora (11-15) have shown that Neandertal femora, as with archaic Homo femora in general (16-21), have largely subcircular midshaft subperiosteal contours, variable development of a medial buttress, a variably prominent linea aspera, and no evidence of a pilaster. In addition, it is sometimes difficult to distinguish both lips of the linea aspera on Neandertal femora, because they are united in a single relief forming a soft crest. Although some Late Pleistocene modern human femora approach this cross-sectional morphology (22, 23), they usually have tear-drop shaped cross-sections, prominent pilasters, and flat or especially concave surfaces adjacent to the linea aspera. The RdV 1 femur exhibits most of the archaic Homo femoral diaphyseal features and contrasts with the femora of early modern humans; its midshaft cross-section is almost round with a continuously convex contour; there is little projection of the linea aspera, no flattening adjacent to the linea aspera, and, hence, no pilaster. Even though one could find a gracile recent human femur with a similarly round cross-section, in an OIS 3 European context the specimen is morphologically well within Neandertal ranges of variation and distinct from the femora of early modern humans.
Ancient DNA Analysis
To further assess the affinities of the RdV 1 femur and contribute to the sample of Late Pleistocene human ancient DNA (aDNA), 300 mg of bone was removed from the RdV 1 femur for aDNA analysis. All of the work was done in a laboratory dedicated exclusively to aDNA work.
Initially, to assess the bone's amino acid composition (9), the proteins were hydrolyzed and amino acids labeled with o-phtaldialdehyde/N-acetyl-l-cysteine, and the respective concentrations of eight amino acids (d- and l-alanine, glycine, d- and l-aspartic acid, serine, glutamic acid, valine, d- and l-leucine, and isoleucine) were measured by using high-performance liquid chromatography (Shimadzu) under conditions that separate the amino acids as well as some of their stereoisomers. To estimate the macromolecular preservation of RdV 1, the amino acid composition and the extent of amino acid racemization were analyzed (24). The bone fragment showed a d-/l ratio for aspartic acid of 0.045 and a total amount of 75,901 ppm for the eight amino acids measured. These values fulfill the criteria compatible with retrieval of endogenous DNA in other Paleolithic remains (25), and we therefore proceeded with DNA extraction.
RdV 1 bone was extracted three times independently, each time by using 100-120 mg of bone powder as described in refs. 25 and 26. Mitochondrial DNA (mtDNA) sequences were amplified by PCR using 5 μl of extract and 60 cycles. A minimum of four PCR blanks were used in each amplification set to detect possible lab contamination. In these cases, the amplification reactions are performed without adding any source of DNA (i.e., extract or modern DNA). Thus, a positive amplification of one of these blank indicates the presence of modern contaminant DNA in the laboratory environment. If this positive amplification occurs, the whole amplification set (the blanks and extracts) is discarded. In the RdV 1 analysis, all blanks remained negative, and no lab contamination could be detected. The first extraction used a “hominoid” amplification with primers L16022/H16095 (26) and an annealing temperature of 54°C. The hominoid amplification of 72 base pairs of mtDNA was performed under reaction conditions that amplify the homologous hypervariable region of human, Neandertal, and African great ape mtDNA. A second amplification was carried out under “Neandertal-specific” conditions by using primers NL16230/NH16262 (26) and an annealing temperature of 60°C. The Neandertal-specific amplification of 31 bp of mtDNA was performed under conditions for which only Neandertal mtDNA sequences can be amplified, even in the presence of a large amount of modern human mtDNA. All PCR products were cloned into Escherichia coli by using the TOPO TA cloning kit (Invitrogen), and 10-30 clones from each amplification were sequenced on an ABI 3700 DNA sequencer (Applied Biosystems).
One of the extractions did not yield any PCR products by using either hominoid or Neandertal-specific amplification conditions. We tested whether this extract contained PCR inhibitors by adding either 5 μl of the extract or 5 μl of water, respectively, to a PCR containing 1,000 copies of modern human mtDNA in a separate experiment outside of the aDNA laboratory. Although the reaction performed with the water yielded a strong PCR product, no product was detected in the reaction containing the extract, confirming the presence of amplification inhibitors in this extract. A 10-fold dilution of the extract allowed the amplification of the 1,000 copies of modern human mtDNA and therefore, both hominoid and Neandertal-specific amplifications were performed again for this extraction on a 10-fold dilution. All three extracts yielded PCR products by using hominoid conditions. Multiple mtDNA sequences were retrieved from each extract, and all of these DNA sequences were identical to modern mtDNA sequences present in human databases. By using Neandertal-specific amplification conditions, two of three extracts (including the extract diluted 10-fold) yielded a PCR product.
After cloning and sequencing, a single mtDNA sequence was observed (Table 1), identical to the homologous fragment of mtDNA sequence retrieved from Mezmaiskaya 1, Feldhofer 2, Engis 2, and La Chapelle-aux-Saints 1; it differed from the Feldhofer 1, Sidrón 441, and Vindija remains by a single substitution. Unfortunately, the DNA preservation was not sufficient to allow us to retrieve additional fragments of the mtDNA sequence. This mtDNA sequence differs from all modern humans sequenced so far by carrying a unique combination of two substitutions not seen in any modern humans analyzed to date (25). We believe that this mtDNA sequence represents endogenous DNA from RdV 1, because it cannot be explained as contamination from a known modern source. This result supports previous results that suggest that the mtDNA gene pool of Neandertals was genetically distinct from those of extant human populations and early modern humans, although only a few of the latter group have been analyzed so far (25). These aDNA results therefore conform with the external morphology to show that RdV 1 derives from the same regional lineage as the other Neandertal individuals analyzed so far.
Table 1. Consensus sequence of RdV 1 obtained by using the Neandertal-specific primers.
| Human reference sequence (27) | T C A C A C A T C A A C T G C A A C T C C A A A G C C A C C C |
| RdV 1 | ... T......... A........... A..... |
| La Chapelle-aux-Saints (25) | ... T......... A........... A..... |
| Engis 2 (25) | ... T......... A........... A..... |
| Feldhofer 1 (26) | ... T......... A........... A. G... |
| Feldhofer 2 (9) | ... T......... A........... A..... |
| Mezmaiskaya 1 (28) | ... T......... A........... A..... |
| Sidrón 441 (29) | ... T......... A........... A. G... |
| Vindija-Vi75 (30) | ... T......... A........... A. G... |
| Vindija-Vi77 (25) | ... T......... A........... A. G... |
| Vindija-Vi80 (25) | ... T......... A........... A. G... |
Dots indicate identity to the human reference sequence displayed above. DNA sequences determined from the previously amplified Neandertals are shown.
Comparative Femoral Morphology
The femur is insufficiently preserved to indicate whether it is fully mature, because it lacks its metaphyseal ends, but the dense and smooth subperiosteal surface, the marked cortical thickness, and its diaphyseal dimensions (Fig. 2 and Table 2) suggest that it derives from a late adolescent or, more likely, an adult. The incompleteness of the bone makes it inappropriate to estimate its original length, even though comparisons with other Neandertal femora suggest that it was similar in length to smaller Neandertal specimens. It is therefore difficult to precisely locate the section of complete diaphyseal cross-section as a percentage of its original length, but the cross-sectional shape and the left distal deviation of the linea aspera make it likely that the proximal end of the complete subperiosteal contour is close to midshaft. Because human femoral diaphyses vary little in diaphyseal size or proportions for several centimeters proximodistally near midshaft, the uncertainty in this positioning will have little effect on morphological assessment of the bone.
Table 2. Comparative morphometrics and diaphyseal cross-sectional parameters of the RdV 1 femoral midshaft.
| Measure | RdV 1 | Roche-à-Pierrot | Neandertals | Mladeč 27 | Early Moderns |
|---|---|---|---|---|---|
| Anteroposterior diameter, mm | 29.9 | 35.7 | 29.5 ± 2.1 (9) | 28.3 | 32.4 ± 4.0 (27) |
| Mediolateral diameter, mm | 27.9 | 29.5 | 29.7 ± 0.8 (9) | 24.0 | 27.7 ± 2.2 (27) |
| Pilastric index | 107.2 | 121.1 | 99.3 ± 7.3 (9) | 117.9 | 117.0 ± 10.4 (27) |
| Total area, mm2 | 646.0 | 740.4 | 661.0 ± 54.3 (6) | 497.7 | 606.8 ± 95.4 (21) |
| Cortical area, mm2 | 544.9 | 623.7 | 523.5 ± 53.6 (6) | 390.7 | 459.5 ± 89.3 (21) |
| % cortical area | 84.3 | 84.2 | 79.1 ± 2.7 (6) | 78.5 | 75.6 ± 7.3 (21) |
| Anteroposterior second moment of area (Ix), mm4 | 34,007 | 47,905 | 32,413 ± 5,476 (6) | 23,588 | 35,652 ± 13,068 (21) |
| Mediolateral second moment of area (Iy), mm4 | 30,948 | 38,670 | 35,782 ± 5,831 (6) | 15,493 | 23,911 ± 7,264 (21) |
| Ix/Iy | 1.10 | 1.24 | 0.91 ± 0.10 (6) | 1.52 | 1.48 ± 0.21 (21) |
Data [X ± SD (N)] are provided for Roche-à-Pierrot (Saint-Césaire) 1, Middle Paleolithic European Neandertals, Mladeč 27, and European Middle Upper Paleolithic (28,000-22,000 BP) early modern humans.
Therefore, in the context of its morphological and genetic affinities and its geological age, the midshaft cross-sectional morphology of RdV 1 was analyzed morphometrically and compared with European OIS 3 and OIS 4 human femora. This analysis was done by using external diameters (31) to maximize comparative samples sizes and by employing cross-sectional parameters (32) to maximize information on the bone distribution. To reconstruct the midshaft cross-section noninvasively, the subperiosteal contour was transferred by using polysiloxane putty (Cuttersil Putty Plus, Heraeus), and the endosteal contour was interpolated by using parallax-corrected cortical thicknesses from biplanar radiography. The anteroposterior plane was taken to be through the mediolateral midpoint and the linea aspera. The resultant cross-section was digitized, and the parameters were computed by using a PC version (33) of slice (34) (Table 2). Comparative samples consist of Late Pleistocene Middle Paleolithic European Neandertals, the Roche-à-Pierrot 1 Châtelperronian Neandertal, the Aurignacian Mladeč 27 early modern human, and European Middle Upper Paleolithic (Gravettian) early modern humans.
The subperiosteal and cortical dimensions of the RdV 1 midshaft are close to the Neandertal mean values, moderately greater than the early modern human means (except for the anteroposterior diameter), and below those of Roche-à-Pierrot 1. The percent cortical areas of both RdV 1 and Roche-à-Pierrot 1 are moderately high but remain within two standard deviations of the values for both of the (insignificantly different, P = 0.085) comparative samples. The primary contrasts, as with the morphological distinctions presented above, are with respect to the anteroposterior vs. mediolateral distributions of midshaft cortical bone.
The Middle Paleolithic Neandertal and Gravettian early modern human samples are highly significantly (P < 0.001) different in the two ratios of anteroposterior to mediolateral midshaft bone distribution, and the one Middle Aurignacian femur, Mladeč 27, falls completely with the more recent Upper Paleolithic sample. However, as noted in ref. 15, the Roche-à-Pierrot 1 femur, despite presenting an archaic Homo, non-pilastric femoral diaphysis, has midshaft morphometric values that place it among the early modern humans. The RdV 1 femur, although within two standard deviations of both comparative sample means, has relatively high values for a Neandertal. Its pilastric index of 107.2 is exceeded only by the undated Fond-de-Forêt 1 femur (108.9) and approached by those of La Chapelle-aux-Saints 1 (106.9) and Feldhofer 1 (105.1). However, the Ix/Iy ratio is more appropriate because it reflects cortical bone distribution rather than merely external dimensions. The value of 1.10 for RdV 1 places it above all of the other European Neandertals except Roche-à-Pierrot 1. In this measure, it is approached by the approximately contemporaneous Feldhofer 1 (1.07), but the next highest value is 0.98 for Fond-de-Forêt 1. Consequently, the RdV 1 femur, joined by the Feldhofer 1 femora, documents a shift in cross-sectional morphology in the direction of the anteroposteriorly reinforced femoral diaphysis of the Châtelperronian Roche-à-Pierrot 1 skeleton.
Discussion and Conclusion
The small cave of Les Rochers-de-Villeneuve in central western France reinforces the pattern, evident in a variety of other Middle Paleolithic sites (2, 4, 35, 36), of a close interaction between human populations and large carnivores, especially in the utilization of natural shelters. It is not possible to determine, as in many cases, the degree to which humans and carnivores directly competed for resources (including space) at Les Rochers-de-Villeneuve, but the data from this site document that this pattern of proximity continued through the Middle Paleolithic in western Europe. Whether the Neandertal individual, represented only by a femoral diaphysis, was prey or merely scavenged after death cannot be determined. However, its taphonomic history only serves to reinforce the pattern evident in the other faunal remains.
At the same time, the RdV 1 femur supports previous evidence of changing loading patterns of the human lower limb during the waning millennia of the Middle Paleolithic and into the initial Upper Paleolithic. The shift in loading patterns might be seen as due to a change in body proportions, because the hyperarctic body proportions of the Neandertals (37) should tend to increase mediolateral hypertrophy of the femoral diaphysis (38), and a reduction in mediolateral diaphyseal hypertrophy might be perceived as an increase in anteroposterior reinforcement. However, there is little evidence of changing body proportions in Middle Paleolithic Neandertal-associated skeletons (37). Moreover, the level of diaphyseal hypertrophy of the Roche-à-Pierrot 1 femur and tibia can only be reasonably explained if that Châtelperronian individual maintained the hyperarctic body proportions of earlier OIS 3 Neandertals (15). It is therefore likely that the RdV 1 Neandertal possessed similarly broad body proportions, and therefore the changes in femoral diaphyeal shape and inferred loading patterns cannot be explained as a product of more linear body proportions.
It is therefore likely that the shifting femoral midshaft cross-sectional proportions in these late Neandertals were due to an increase in locomotion-induced habitual anteroposterior loading of the femur, a pattern associated with increased levels of mobility (39, 40). Because mobility becomes an important component of Middle Upper Paleolithic hunter-gatherer adaptive patterns across Europe, these late Middle Paleolithic and initial Upper Paleolithic biological data suggest that this biobehavioral shift had its roots a dozen millennia earlier, in final stages of the Middle Paleolithic.
Acknowledgments
We thank Jean-François Baratin and Jean Airvaux (Service Régional de l'Archéologie Poitou-Charentes) and the Ministry of Culture and Communication of France for general support and permission to excavate, C. Boutin (Gradignan, France) for allowing excavations on her property, and the Laboratoire d'Anthropologie des Populations du Passé and the Institut de Préhistoire et de Géologie du Quaternaire of the Université de Bordeaux 1 for their support. S. E. Churchill, T. W. Holliday, and B. Holt provided helpful comments on an earlier version of the paper.
Author contributions: C.B., B.M., D.S., S. Pääbo, and E.T. designed research; C.B., B.M., F.L.-C., D.S., D.P., J.-G.B., D.C., I.C., D.D., V.L., A.L., J.-B.M., S. Pasty, J.P., N.R., S. Pääbo, and E.T. performed research; C.B., B.M., D.S., D.P., J.-G.B., and E.T. analyzed data; and C.B., B.M., D.S., S. Pääbo, and E.T. wrote the paper.
Abbreviations: BP, 14C years before present; OIS, oxygen isotope stage; aDNA, ancient DNA; mtDNA, mitochondrial DNA; RdV 1, Rochers-de-Villeneuve 1.
References
- 1.Airvaux, J., Duport, L. & Lévêque, F. (1999) Un Siècle de Recherches Préhistoriques en Charente: La Charente Paléolithique dans son Contexte Régional (Association pour la Valorisation du Patrimoine Préhistorique de la Charente, La Rochefoucauld, France).
- 2.Clot, A., ed. (1987) La Grotte de Gerde (Haute-Pyrénées) (Société Ramond, Bagnères-de-Bigorre, France).
- 3.Fosse, P. (1994) Ph.D. thesis (Université d'Aix-Marseille, France).
- 4.Villa, P. & Soressi, M. (2000) J. Anthropol. Res. 56, 187-215. [Google Scholar]
- 5.Valladas, H., Geneste, J. M., Joron, J. L. & Chadelle, J. P. (1986) Nature 322, 452-454. [Google Scholar]
- 6.Mercier, N., Valladas, H., Joron, J. L., Reyes, J. L., Lévêque, F. & Vandermeersch, B. (1991) Nature 351, 737-739. [DOI] [PubMed] [Google Scholar]
- 7.Valladas, H., Mercier, N., Falguères, C. & Bahain, J. J. (1999) Gallia Préhist. 41, 153-166. [Google Scholar]
- 8.Prat, F. (1976) in La Préhistoire Française, ed. de Lumley, H. (Éditions du Centre National de la Recherche Scientifique, Paris), pp. 409-415.
- 9.Schmitz, R. W., Serre, D., Bonani, G., Feine, S., Hillgruber, F., Krainitzki, H., Pääbo, S. & Smith, F. H. (2002) Proc. Natl. Acad. Sci. USA 99, 13342-13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binford, L. R. (1981) Bones: Ancient Men and Modern Myths (Academic, New York).
- 11.Twiesselmann, F. (1961) Mém. Inst. Roy. Sci. Nat. Belg. 149, 1-164. [Google Scholar]
- 12.Endo, B. & Kimura, T. (1970) in The Amud Man and His Cave Site, eds. Suzuki, H. & Takai, F. (Academic, Tokyo), pp. 231-406.
- 13.Trinkaus, E. (1976) Z. Morphol. Anthropol. 67, 291-319. [PubMed] [Google Scholar]
- 14.Trinkaus, E. (1983) The Shanidar Neandertals (Academic, New York).
- 15.Trinkaus, E., Churchill, S. E., Ruff, C. B. & Vandermeersch, B. (1999) J. Archaeol. Sci. 26, 753-773. [Google Scholar]
- 16.Day, M. H. (1971) Nature 232, 383-387. [DOI] [PubMed] [Google Scholar]
- 17.Geraads, D. & Tchernov, E. (1983) L'Anthropol. (Paris) 87, 138-141. [Google Scholar]
- 18.Trinkaus, E. (1984) Am. J. Phys. Anthropol. 64, 137-139. [Google Scholar]
- 19.Belli, G., Belluomini, G., Cassoli, P. F., Cecchi, S., Cucarzi, M., Delitala, L., Fornaciari, G., Mallegni, F., Piperno, M., Segre, A. G. & Segre-Naldini, E. (1991) L'Anthropol. (Paris) 95, 47-88. [Google Scholar]
- 20.Hublin, J. J. (1992) C. R. Acad. Sci. Paris Série II 314, 975-980. [Google Scholar]
- 21.Grimaud-Hervé, D., Valentin, F., Sémah, F., Sémah, A. M., Djubiantono, T. & Widianto, H. (1994) C. R. Acad. Sci. Paris Série II 318, 1139-1144. [Google Scholar]
- 22.Matiegka J. (1938) Homo Předmostensis. Fosilní človĕk z Předmostí na Moravĕ II (Česká Akademie Vĕd a Umĕní, Prague).
- 23.Trinkaus, E. & Ruff, C. B. (1999) J. Archaeol. Sci. 26, 409-424. [Google Scholar]
- 24.Poinar, H. N., Hoss, M., Bada, J. L. & Pääbo, S. (1996) Science 272, 864-866. [DOI] [PubMed] [Google Scholar]
- 25.Serre, D., Langaney, A., Chech, M., Teschler-Nicola, M., Paunović, M., Mennecier, P., Hofreiter, M., Possnert, G. G. & Pääbo, S. (2004) PLoS Biol. 2, 313-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krings, M., Stone, A., Schmitz, R.W., Krainitzki, H., Stoneking, M. & Pääbo, S. (1997) Cell 90, 19-30. [DOI] [PubMed] [Google Scholar]
- 27.Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H., Coulson, A. R., Drouin, J., Eperon, I. C., Nierlich, D. P., Roe, B. A., Sanger, F., et al. (1981) Nature 290, 457-465. [DOI] [PubMed] [Google Scholar]
- 28.Ovchinnikov, I. V., Gotherstrom, A., Romanova, G. P., Kharitonov, V. M., Liden, K. & Goodwin, W. (2000) Nature 404, 490-493. [DOI] [PubMed] [Google Scholar]
- 29.Lalueza-Fox, C., Sampietro, M. L., Caramelli, D., Puder, Y., Lari, M., Calafell, F., Martínez-Maza, C., Bastir, M., Fortea, J., de la Rasilla, M., et al. (2005) Mol. Biol. Evol. 22, 1077-1081. [DOI] [PubMed] [Google Scholar]
- 30.Krings, M., Capelli, C., Tschentscher, F., Geisert, H., Meyer, S., von Haeseler, A., Grossschmidt, K., Possnert, G., Paunović, M. & Pääbo, S. (2000) Nat. Genet. 26, 144-146. [DOI] [PubMed] [Google Scholar]
- 31.Bräuer, G. (1988) in Anthropologie, ed. Knussman R. (Fischer, Stuttgart), pp. 160-232.
- 32.Ruff, C. B. (2000) in Biological Anthropology of the Human Skeleton, eds. Katzenberg, M. A. & Saunders, S. R. (Wiley-Liss, New York), pp. 71-102.
- 33.Eschman, P. N. (1992) slcomm 1.6 (Eschmann Archaeological Services, Albuquerque, NM).
- 34.Nagurka, M. L. & Hayes, W. C. (1980) J. Biomech. 13, 195-202. [DOI] [PubMed] [Google Scholar]
- 35.Tournepiche, J. F. (1994) Paléo 6, 319-321. [Google Scholar]
- 36.Straus, L. G. (1982) J. Anthropol. Res. 38, 75-96. [Google Scholar]
- 37.Holliday, T. W. (1997) Am. J. Phys. Anthropol. 104, 245-258. [DOI] [PubMed] [Google Scholar]
- 38.Weaver, T. D. (2003) Proc. Natl. Acad. Sci. USA 100, 6926-6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruff, C. B. (1999) in Understanding Prehistoric Lifeways in the Great Basin Wetlands: Bioarchaeological Reconstruction and Interpretation, eds. Hemphill, H. & Larsen, C. (Univ. Utah Press, Salt Lake City), pp. 290-320.
- 40.Holt, B. (2003) Am. J. Phys. Anthropol. 122, 200-215. [DOI] [PubMed] [Google Scholar]