Abstract
We previously showed that prostatic stem cells are concentrated in the proximal regions of prostatic ducts. We now report that these stem cells can be purified from isolated proximal duct regions by virtue of their high expression of the cell surface protein stem cell antigen 1 (Sca-1). In an in vivo prostate reconstitution assay, the purified Sca-1-expressing cell population isolated from the proximal region of ducts was more effective in generating prostatic tissue than a comparable population of Sca-1-depleted cells (203.0 ± 83.1 mg vs. 11.9 ± 9.2 mg) or a population of Sca-1-expressing cells isolated from the remaining regions of ducts (transit-amplifying cells) (31.9 ± 24.1 mg). Almost all of the proliferative capacity of the proximal duct Sca-1-expressing cell population resides within the fraction of cells that express high levels of Sca-1 (top one-third), with the proximal region of prostatic ducts containing 7.2-fold more Sca-1high cells than the remaining regions. More than 60% of the high-expressing cells coexpress α6 integrin and the antiapoptotic factor Bcl-2, markers that are also characteristic of stem cells of other origins. Further stratification of the phenotype of the stem cells may enable the development of rational therapies for treating prostate cancer and benign prostatic hyperplasia.
Keywords: prostate, α6 integrin, Bcl-2
Stem cell biology and tumorigenesis may be closely linked, and stem cells may have a role in the etiology of cancer (1-5). Stem cells and tumor cells have many common features, including self-renewal, multidrug resistance, telomerase expression, and, in the case of the prostate, androgen independence. Prostatic stem cells do not require androgens for survival, as evidenced by normal prostatic regeneration after >30 cycles of androgen ablation and supplementation, which results in involution and normal regeneration of this gland (6). Because prostatic carcinoma usually progresses to an androgen-independent tumor (which may reflect a stem cell-like phenotype), an understanding of prostate stem cell biology is important for devising preventative or therapeutic approaches for prostate cancer. In addition to being a source of carcinomas, stem cells may also give rise to benign prostatic hyperplasia (7). The isolation and characterization of these stem cells is likely to increase our understanding of normal prostate physiology, and it may also lead to new therapeutic approaches for two of the most common diseases afflicting men.
The murine prostate consists of a branched ductal network with each duct consisting of proximal (adjacent to the urethra), intermediate, and distal regions. Actively proliferating cells (transit-amplifying cells) are located in the distal region of the ducts (8), whereas cells with stem cell features are concentrated in the proximal ductal region (9). Thus, cells in the proximal region are quiescent and have high proliferative potential, and isolated single cells from this region can give rise to branched glandular ductal structures in vitro (9). In addition, cell suspensions derived from the proximal region form significantly more prostatic tissue in an in vivo transplantation model than those obtained from other prostatic regions. Furthermore, cells obtained from this transplanted tissue are again able to give rise to prostatic tissue when reinoculated into other animals (unpublished data), confirming the presence in the proximal region of stem cells with regenerative capacity.
Because stem cells in other organs have been identified by their expression of specific antigens, such as a cell surface protein known as stem cell antigen 1 (Sca-1), α6 integrin, and Bcl-2, we determined whether these antigens could be used to identify the stem cell population in the proximal region of ducts. Sca-1 is expressed by stem/progenitor cells from a variety of tissues, such as hematopoietic tissue (10), cardiac tissue (11), mammary gland (12), skin (13), muscle (14), and testis (15). α6 integrin (CD49f) is expressed by primitive cells in the liver (16) and skin (17), and anti-α6 integrin antibodies have been used to enrich for spermatogonial stem cells from mouse testis (18). Bcl-2, an antiapoptotic protein (19), may protect primitive cells from death and is expressed by hematopoietic, keratinocyte, and colon stem cells (20-22).
We have identified a candidate population of prostatic stem cells in the proximal region of murine prostatic ducts that expresses high levels of Sca-1, in conjunction with α6 integrin and Bcl-2. Cells with these properties are almost absent from the remaining regions of ducts. We show that Sca-1-expressing cells isolated from the proximal region regenerate abundant normal functional prostatic ducts in an in vivo transplantation assay, whereas cells that do not express this antigen form very little tissue. These results establish that prostatic stem cells reside within the Sca-1-expressing population in the proximal region of ducts and provide a means of isolating the stem cells for further characterization.
Materials and Methods
Animals. C57BL/6 mice, athymic nude mice, and CDIGS rats were housed in the animal research facilities of the University of Cape Town or New York University, and all experiments were performed in compliance with institutional review board requirements.
Antibodies and Control Immunoglobulins (IgGs). Fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse Sca-1, rat anti-mouse α6 integrin (CD49f) FITC, and rat IgG 2a FITC were obtained from BD Biosciences, Bedford, MA. Phycoerythrin (PE)-conjugated rat anti-mouse Sca-1, rat IgG-2a PE, rat IgG, mouse anti-mouse CD16/32, rat anti-mouse Sca-1 biotin, rat IgG2a biotin, and streptavidin-conjugated allophycocyanin (APC) were from Caltag Laboratories, Burlingame, CA. Mouse anti-Bcl-2 PE was purchased from Santa Cruz Biotechnology, and IgG1 PE was obtained from DAKO.
Cell Preparation and FACS Analysis. The dorsal, ventral, and lateral prostates were removed from C57BL/6 mice (6 weeks old) and dissected into two regions: (i) the proximal region, which includes those ducts nearest the urethra, and (ii) the remaining region, which includes the intermediate and distal ducts. Cell digests (9) were resuspended in FACS buffer [PBS containing BSA (0.1%), sodium azide (0.01%), and aprotinin (20 μg/ml)]. Fc receptors were blocked with mouse CD16/32 antibodies and rat IgG, and the cells were incubated with antibody or control IgG for 30 min on ice and washed with FACS buffer. In some experiments, the dye 7-aminoactinomycin D (1 μg/ml) was added 5 min before analysis, so that dead cells could be excluded. Bcl-2 expression was determined in paraformaldehyde-fixed cells, permeabilized with Tween 20 (Merck-Schuchardt, Hohenbrunn, Germany). Antibodies to Sca-1, conjugated to PE, FITC, or biotin, were used in conjunction with antibodies to α6 integrin conjugated to FITC or antibodies to Bcl-2 conjugated to PE, to determine the incidence of coexpression of Sca-1 and these antigens. Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson), using cellquest software (Becton Dickinson). Sca-1+ cells with fluorescent intensities in the upper one-third were defined as Sca-1high cells.
Implantation of Grafts Under the Renal Capsule. Cells (1 × 105 or 3 × 104) from different regions of prostatic ducts were combined with urogenital sinus mesenchyme (UGM) cells (2.5 × 105) and resuspended in 30 μl of type 1 collagen (BD Biosciences). The collagen grafts were inserted under the renal capsule (23). Each experiment contained grafts of UGM alone to ensure that tissue growth did not result from contaminating urogenital sinus epithelial cells. Grafts were harvested and weighed after 8-10 weeks. UGM was isolated from the urogenital sinus of embryos (18 days old) from CDIGS rats (23-25).
Isolation of Sca-1-Expressing Cells. Prostatic duct digests were enriched for Sca-1-expressing cells by immunomagnetic separation, using magnetically activated cell sorter microbeads coated with antibodies to Sca-1 and the MiniMACS magnetically activated cell sorter system (Miltenyi Biotec, Auburn, CA). In some experiments, cells were sorted by FACS into various fractions (Sca-1high, Sca-1med/lo or Sca-1neg) according to the mean fluorescence intensity (MFI) of Sca-1 expression by the cells.
Statistical Analysis. The results are depicted as the means and standard deviations of each set of data. Comparisons between groups were made by using the two-tailed, paired Student t test, or in the case of different sized samples, the Mann-Whitney U test. A P value of <0.05 is considered statistically significant.
Results
Cells in the Proximal Region of Murine Prostatic Ducts Coexpress High Levels of Sca-1, α6 Integrin, and Bcl-2. We have shown that cells with stem cell features (quiescence and high proliferative potential) are concentrated in the proximal region of prostatic ducts (9). By using FACS analysis, we determined whether the expression of three antigens, Sca-1, α6 integrin, and Bcl-2, known to be expressed by stem cells of other origins (10-18, 20-22), differs between the proximal and remaining ductal regions.
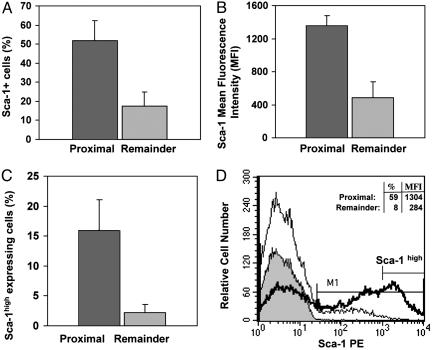
We found that these three antigens are expressed by at least some cells in all regions of the ducts, but significant differences were noted in their distribution. They were expressed by a substantially higher proportion of cells in the proximal region than in the remaining regions (Table 1), and the levels of expression of each antigen (MFI) were higher in proximal cells than in cells of the remaining ductal regions. The proximal region contained a 2.9-fold (P < 0.0001) higher proportion of Sca-1-expressing cells that had a 2.8-fold-higher MFI (P < 0.01) than cells from the remaining ductal regions (Fig. 1 A and B and Table 1). Because high levels of Sca-1 are found on purified populations of other types of stem cells (14, 15, 26, 27), we determined the location of cells with high MFI for Sca-1. The proximal region of ducts contained 7.2-fold more cells with high levels of Sca-1 (Sca-1+ cells with fluorescence intensities in the upper one-third) than the remaining regions (Table 1; P < 0.00001; Fig. 1 C and D), indicating that Sca-1high cells are concentrated proximally.
Table 1. Expression of Sca-1, α6 integrin, and Bcl-2 by cells from the proximal region of prostatic ducts compared with cells from the remaining ductal regions.
| Phenotype | No. of experiments | Proximal expression, % | Remainder expression, % | Increase, fold | P |
|---|---|---|---|---|---|
| Sca-1+ | 16 | 51.8 ± 10.5 | 17.7 ± 7.2 | 2.9 | <0.0001 |
| α6 integrin+ | 13 | 40.8 ± 10.0 | 21.1 ± 11.4 | 1.9 | <0.0001 |
| Bcl-2+ | 12 | 42.1 ± 7.0 | 27.5 ± 8.2 | 1.5 | <0.0001 |
| Sca-1+ α6 integrin+ Bcl-2+ | 3 | 27.5 ± 4.4 | 1.4 ± 0.8 | 19.6 | <0.01 |
| Sca-1high* | 12 | 15.9 ± 5.2 | 2.2 ± 1.4 | 7.2 | <0.00001 |
| Sca-1high* α6 integrin+ Bcl-2+ | 3 | 9.8 ± 1.2 | 0.1 ± 0.06 | 98.0 | <0.01 |
Sca-1 + cells with fluorescence intensities in the upper one-third were defined as Sca-1high cells.
Fig. 1.
Sca-1 is highly expressed by cells in the proximal region of prostatic ducts. The expression of Sca-1 by cell digests from the proximal and remaining regions of prostatic ducts was determined. (A) The proximal region contained 2.9-fold more Sca-1-expressing cells than the remaining ductal regions (P < 0.0001). (B) Cells from the proximal region expressed 2.8-fold more molecules of Sca-1 per cell (higher MFI) than cells from the remaining regions (P < 0.01). (C) Cells with high levels of Sca-1 expression (cells with fluorescence intensities in the upper one-third) were 7.2-fold more prevalent in the proximal region than in the remaining regions (P < 0.00001). Results in A-C are means of at least three experiments. (D) A representative histogram (from one of five experiments) of Sca-1 expression by viable (7-aminoactinomycin D-negative) cells from the proximal region (thick line) and the remaining regions (thin line) of ducts shows the difference in Sca-1 expression between these two regions. The gray-filled histogram represents the appropriate IgG control. The marker M1 is placed so that <1% of control cells are positive. A second marker denotes Sca-1high cells.
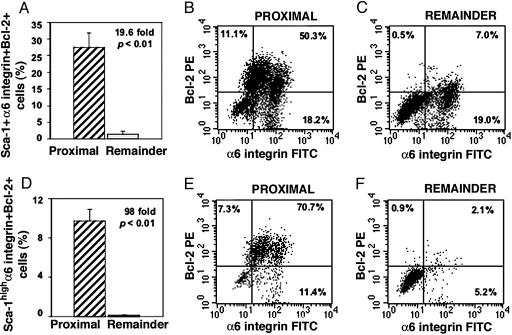
Determination of the coexpression of all three antigens indicated that cells from the proximal region contain significantly more (19.6-fold; P < 0.01) Sca-1+ α6 integrin+ Bcl-2+ cells (27.5 ± 4.4%) than those from the remaining regions (1.4 ± 0.8%) (Fig. 2 A-C and Table 1). Analysis of the proximal region for cells expressing high levels of Sca-1 together with α6 integrin and Bcl-2 (Sca-1high α6 integrin+ Bcl-2+ cells) revealed that 98-fold more triple-labeled cells reside in the proximal compared with the other regions of ducts (9.8 ± 1.2% vs. 0.1 ± 0.06%, P < 0.01; Fig. 2 D-F and Table 1). In addition, each antigen alone was expressed by more cells (Table 1) and with a higher MFI (data not shown) in the proximal region compared with remaining regions.
Fig. 2.
The proximal region is considerably enriched in Sca-1-expressing cells that coexpress α6 integrin and Bcl-2. Three-color FACS analysis was performed to determine the incidence of Sca-1+ α6 integrin+ Bcl-2+ cells (A-C) and Sca-1high α6 integrin+ Bcl-2+ cells (D-F) in the proximal and remaining regions of ducts. (A) The proximal region contained 19.6-fold more Sca-1+ α6 integrin+ Bcl-2+ cells than the remaining regions (P < 0.01). (B and C) In these representative dot plots, 50.3% of proximal Sca-1+ cells coexpressed both α6 integrin and Bcl-2 (B), whereas 7.0% of cells from the remaining regions coexpressed these antigens (C). (D) Analysis of triple-labeled cells expressing high levels of Sca-1 showed that the proximal region contained 98-fold more Sca-1high α6 integrin+ Bcl-2+ cells than the remaining regions (P < 0.01). (E and F) For these dot plots, 70% of proximal Sca-1high cells coexpressed both α6 integrin and Bcl-2 (E), whereas 2% of cells from the remaining regions were Sca-1high α6 integrin+ Bcl-2+ (F). The results are the mean of three experiments.
These results show that there are striking differences in the distribution of cells expressing Sca-1, α6 integrin, and Bcl-2 in different ductal regions. Cells with high levels of Sca-1 are predominantly confined to the proximal region and triple-labeled cells with high levels of Sca-1 are almost exclusively confined to this region.
Sca-1-Expressing Cells Have High in Vivo Proliferative Potential. The ability to regenerate tissue in vivo is a characteristic of stem cells, and this property has been used to identify various antigens, including Sca-1, as stem cell markers. For example, Sca-1-expressing cells isolated from bone marrow are able to reconstitute all blood cell types (10), and mammary epithelial cells enriched for Sca-1 can reconstitute the mammary gland in vivo and have greater growth potential than Sca-1-depleted cells (12).
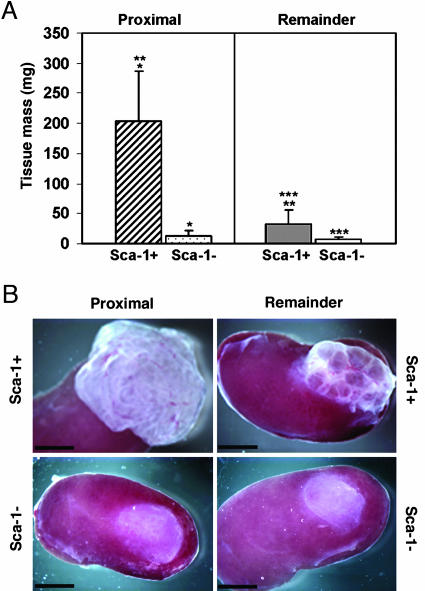
We therefore determined the growth potential of Sca-1-expressing cells isolated from the proximal and the remaining ductal regions and compared their proliferative potential in vivo with cells that did not express this antigen. Sca-1-expressing (Sca-1+) and Sca-1-depleted (Sca-1-) populations were isolated from digests of the proximal and the remaining ductal regions by using antibodies to Sca-1 and magnetic microbeads. These populations were combined with cells isolated from the UGM [inductive mesenchyme for prostatic tissue (23-25)] and inserted under the renal capsule of recipient male animals, and the amount of prostatic tissue generated was measured after 8 weeks. Sca-1+ cells isolated from the proximal region formed significantly more prostatic tissue (203.0 ± 83.1 mg; 17.1-fold) than was obtained from the Sca-1- proximal population (11.9 ± 9.2 mg; P < 0.001) (Fig. 3 A and B). Sca-1+ cells isolated from the remaining ductal regions also formed prostatic tissue under the renal capsule (31.9 ± 24.1 mg) but formed far less tissue than observed for Sca-1+ cells isolated from the proximal region (203.0 ± 83.1 mg), indicating that these two Sca-1+ populations differ markedly in their in vivo growth potential (P < 0.001). Sca-1- cells isolated from the remaining regions of ducts formed very little subrenal capsule tissue (6.6 ± 5.0 mg).
Fig. 3.
Sca-1+ cells have greater in vivo proliferative capacity than Sca-1- cells. (A) The growth of Sca-1+ and Sca-1- cells (105 cells) that were isolated from either the proximal region or the remaining regions of ducts and transplanted under the renal capsule was measured after 8 weeks. Sca-1+ cells obtained from the proximal region formed 17.1-fold more prostatic tissue than Sca-1- cells (*, P < 0.001). Sca-1+ cells obtained from the remaining ductal regions had far less growth potential than Sca-1+ proximal cells (**, P < 0.001). Sca-1- cells from the remaining regions showed less growth than Sca-1+ cells from this region (***, P < 0.001). The results are the means of two experiments, using the data obtained from the inoculation of a total of 7, 11, 14, and 12 kidneys with Sca-1+ and Sca-1- cells from the proximal and remaining regions, respectively. (B) Prostate tissue under the renal capsule initiated with 105 Sca-1+ or Sca-1- cells from either the proximal region or the remaining regions. (Scale bars: 3 mm.)
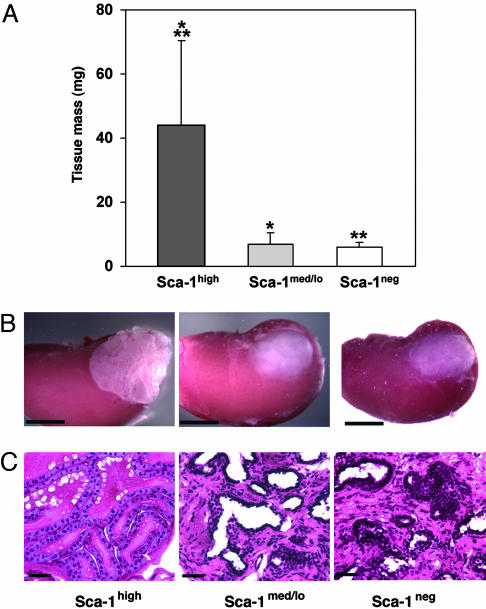
Because our FACS data showed that cells expressing high levels of Sca-1 were confined predominantly to the proximal region of ducts, we determined whether Sca-1high cells had greater growth potential than cells with medium/low Sca-1 expression. Proximal cell populations were FACS-sorted into fractions containing cells with high MFI (Sca-1high), medium to low MFI (Sca-1med/lo) and no Sca-1 expression (Sca-1neg) and inserted under the renal capsule of recipient animals. Sca-1high cells formed significantly more prostatic tissue (43.7 ± 26.8 mg; 6.3-fold) than Sca-1med/lo cells (6.9 ± 3.6 mg; P < 0.001) and 7.5-fold more tissue than Sca-1neg cells (5.8 ± 1.6 mg; P = 0.001) (Fig. 4 A-C). Although the tissue growth obtained was less than in experiments using magnetic beads (because of the stress that the cells undergo during FACS sorting), these results show that almost all of the in vivo growth potential is confined to cells that express high levels of Sca-1. The prostatic tissue obtained from Sca-1high cells had normal prostatic histology comprising basal and luminal cells lining prostatic ducts. The lumens of the ducts contained abundant amounts of secretory material (Fig. 4C). In contrast, the tissue arising from Sca-1med/lo and Sca-1neg cells contained more stroma with less of an epithelial component, and little secretory material was generally noted within the ducts (Fig. 4C).
Fig. 4.
Sca-1high cells have greater in vivo proliferative capacity than cells that express lower levels of Sca-1. Cells were isolated from the proximal region and sorted by FACS into Sca-1high, Sca-1med/lo and Sca-1neg fractions according to the level of Sca-1 expression. The cell populations (3 × 104) were transplanted under the renal capsule and the growth of prostatic tissue was measured after 10 weeks. (A) Sca-1high cells formed 6.3-fold more prostatic tissue than Sca-1med/lo cells (*, P < 0.001) and 7.5-fold more prostatic tissue than Sca-1neg cells (**, P = 0.001). These results are the means of two experiments, using the data obtained from the inoculation of a total of 10, 9, and 10 kidneys with cell populations containing Sca-1high, Sca-1med/lo and Sca-1neg cells, respectively. (B) Prostate tissue initiated with 3 × 104 Sca-1high, Sca-1med/lo, or Sca-1neg cells isolated by FACS from the proximal region of ducts. (Scale bars: 3 mm.) (C) Paraffin sections stained with hematoxylin and eosin showing the morphology of prostatic tissue arising from Sca-1high, Sca-1med/lo or Sca-1neg cells. The prostatic tissue obtained from Sca-1high cells had normal prostatic histology comprising basal and luminal cells lining prostatic ducts. The lumens of the ducts were filled with secretory material. The tissue arising from Sca-1med/lo and Sca-1neg cells contained increased stroma with less of an epithelial component, and little secretory material was noted within the ducts. (Scale bars: 40 μm.)
These results show that cells expressing Sca-1 have considerably more growth potential than those lacking this antigen, and that the proliferative ability within the Sca-1-expressing proximal cells resides in cells that express high levels of this antigen. They also show that Sca-1-expressing cells residing in the proximal region are more primitive than those Sca-1-expressing cells in the remaining ductal regions, as they have far higher proliferative capacity. These data indicate that stem cells reside within the Sca-1-expressing population in the proximal region, whereas the transit-amplifying cells, with more limited growth potential, reside within the Sca-1-expressing cells in the remaining ductal regions.
Discussion
We show that Sca-1-expressing cells, isolated from the proximal region of ducts, form significantly more prostatic tissue in vivo than cells that lack this antigen and that most of the proliferative ability resides in cells that express high levels of this antigen. In addition, Sca-1-expressing cells isolated from the proximal region have far greater proliferative potential than Sca-1-expressing cells isolated from the remaining ductal regions (which express lower levels of Sca-1 per cell than proximal cells). The Sca-1high cells that coexpress α6 integrin and Bcl-2 are almost exclusively confined to the proximal region of ducts, indicating that prostate stem cells express high levels of Sca-1 together with α6 integrin and Bcl-2.
Sca-1 is expressed by stem cells from a variety of origins including hematopoietic tissue, heart, mammary gland, skin, muscle, and testis (10-15). Although a ligand for Sca-1 has not been identified, Sca-1 appears to be important for the self-renewal of mesenchymal (28) and hematopoietic (29) stem cells. Sca-1-/- mice have greatly reduced bone mass resulting from a primary defect in the self-renewal capacity of early mesenchymal progenitor cells (28). In addition, hematopoietic stem cells from Sca-1-/- mice have a decreased repopulation potential and manifest a lower engraftment of secondary transplants than cells from wild-type mice (29), indicating that Sca-1 is required for self-renewal. These findings are consistent with our data showing that Sca-1neg cells have little capacity to generate prostatic tissue when implanted under the renal capsule, and indicate that Sca-1 may also be involved in the self-renewal of stem cells in the prostate.
Stem cells are rare cells, and, because large numbers of cells isolated from prostatic ducts express Sca-1, it is unlikely that all Sca-1-expressing cells are stem cells. Our data, in fact, indicate that prostatic stem cells reside in the Sca-1high population that also expresses α6 integrin and Bcl-2. The presence of α6 integrin together with high levels of Sca-1 is also characteristic of spermatogonial stem cells (15). Stem cells from other origins also express α6 integrin. The gene for this integrin was the only common gene identified in a study using transcriptional profiling to identify genes expressed by stem cells of embryonic, neural, hematopoietic, and retinal origin (30). Keratinocyte stem cells also express high levels of α6 integrin (31), and these cells have enhanced long-term proliferative potential (32).
Members of the integrin family are important regulators of stem cell function (33). Keratinocyte and putative prostate stem cells are more adhesive than the more mature transit-amplifying cells, and putative human prostate stem cells express high levels of α2 integrin (34-36). It is possible that a number of members of the integrin family are expressed by stem cells because there is recent evidence that the adhesive properties of integrins may be involved in maintaining stem cells within their niche (37, 38). Because stem cells and cancer cells have many similar properties (1-5), it is of interest that changes in the expression of integrins, particularly α6β4 integrin, are implicated in tumorigenesis and invasion and that the α6 integrins play a role in the progression of cancer (39-41).
The prostate cells from the proximal region that express high levels of Sca-1 also coexpress the antigen Bcl-2. The presence of Bcl-2 in Sca-1-expressing prostate stem cells may protect these cells from apoptotic death. Stem cells are needed for the lifetime of their host, and mechanisms to protect them from death are important to ensure their long-term survival. The Bcl-2 protein suppresses apoptosis (19) and is present in many long-lived cells (42). Bcl-2 protects hematopoietic and keratinocyte stem cells from apoptotic death (21, 43), and over-expression of Bcl-2 increases the numbers of hematopoietic stem cells in vivo (20) and protects hematopoietic stem cells from the harmful effects of a number of chemotherapeutic agents, thus ensuring their survival (44). The expression of Bcl-2 by the prostate stem cell population that has high levels of Sca-1 and significant in vivo proliferative potential is therefore likely to ensure the long-term survival of this cell population.
High levels of Bcl-2 in the proximal stem cell region may also be required to protect these cells from apoptosis that accompanies androgen withdrawal. Castration results in an increase in TGF-β levels (45), leading to apoptosis and involution of the more distal regions of the gland, whereas the proximal region is relatively unchanged (46, 47). We find a TGF-β signaling gradient in prostatic ducts, with high levels of signaling in the quiescent proximal region (high Bcl-2 expression) and low levels of signaling in the distal region (low Bcl-2 expression) (S.N.S., P.E.B., S.C., K.G., D.M., and E.L.W., unpublished data). The proximal region is therefore protected from TGF-β-mediated apoptosis by high Bcl-2 expression. Aberrant regulation of Bcl-2 expression may contribute to the etiology of prostatic diseases such as benign prostatic hyperplasia (48), proliferative inflammatory atrophy, which is a regenerative lesion that may give rise to prostate cancer (49), and prostate cancer itself (50). In addition, the over-expression of Bcl-2 is implicated in the formation of hormone-independent prostate tumors because it inhibits the apoptotic effect of TGF-β and androgens (51). The identification of the phenotype of prostatic stem cells that express high levels of Bcl-2 may therefore aid in identifying the target cells from which these lesions originate.
The identification of other antigens expressed by the population of cells that express Sca-1, α6 integrin, and Bcl-2 may result in the definition of a more comprehensive phenotype for prostate stem cells. For example, the expression of antigens such as CD133 (prominin), which has been found on human putative prostatic stem cells (52), signaling molecules, such as Wnt, Notch, and Hedgehog, that are involved in stem cell renewal and maintaining stem cell niches (53, 54), and members of the Polycomb family, such as Bmi1 and EZH2 (55, 56), may further stratify the prostatic stem cell phenotype. Because cancers may arise from mutations in stem cells (2, 4, 5) and because benign prostatic hyperplasia may result from aberrant proliferation of these cells (7), the identification of the stem cell phenotype of prostate cells may permit the development of rational targeted therapies for treating both conditions.
Acknowledgments
We thank Drs. Dan Rifkin, Milton Adesnik, and David Sabatini for general discussion and helpful comments on the manuscript. This work was supported by the University of Cape Town Staff Research Fund, the Medical Research Council of South Africa, National Institutes of Health Grant DK52634, and Department of Defense Grants W81XWH-04-1-0255 and DAMD-17-02-1-0115.
Author contributions: P.E.B., D.M., and E.L.W. designed research; P.E.B., X.X., S.C., S.N.S., K.G., and E.L.W. performed research; P.E.B., D.M., and E.L.W. analyzed data; and P.E.B. and E.L.W. wrote the paper.
Abbreviations: MFI, mean fluorescence intensity; PE, phycoerythrin; Sca-1, stem cell antigen 1; UGM, urogenital sinus mesenchyme.
References
- 1.Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. (2003) Proc. Natl. Acad. Sci. USA 100, 3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Hajj, M. & Clarke, M. F. (2004) Oncogene 23, 7274-7282. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot, T., Sirard, C., Vormoor, J., Murdoch, B., Hoang, T., Caceres-Cortes, J., Minden, M., Paterson, B., Caligiuri, M. A. & Dick, J. E. (1994) Nature 367, 645-648. [DOI] [PubMed] [Google Scholar]
- 4.Pardal, R., Clarke, M. F. & Morrison, S. J. (2003) Nat. Rev. Cancer 3, 895-902. [DOI] [PubMed] [Google Scholar]
- 5.Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. (2001) Nature 414, 105-111. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs, J. T. (1985) in Benign Prostatic Hyperplasia, eds. Rodgers, C. H., Coffey, D. S., Cunha, G., Grayshack, J. T., Henman, R., Jr., & Horton, R. (Natl. Inst. of Health, Washington, DC), DHHS Publ. No. 87-2881, pp. 85-94.
- 7.De Marzo, A. M., Nelson, W. G., Meeker, A. K. & Coffey, D. S. (1998) J. Urol. 160, 2381-2392. [DOI] [PubMed] [Google Scholar]
- 8.Cunha, G. R., Donjacour, A. A., Cooke, P. S., Mee, S., Bigsby, R. M., Higgins, S. J. & Sugimura, Y. (1987) Endocr. Rev. 8, 338-362. [DOI] [PubMed] [Google Scholar]
- 9.Tsujimura, A., Koikawa, Y., Salm, S., Takao, T., Coetzee, S., Moscatelli, D., Shapiro, E., Lepor, H., Sun, T. T. & Wilson, E. L. (2002) J. Cell Biol. 157, 1257-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spangrude, G. J., Heimfeld, S. & Weissman, I. L. (1988) Science 241, 58-62. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura, K., Nagai, T., Nishigaki, N., Oyama, T., Nishi, J., Wada, H., Sano, M., Toko, H., Akazawa, H., Sato, T., et al. (2004) J. Biol. Chem. 279, 11384-11391. [DOI] [PubMed] [Google Scholar]
- 12.Welm, B. E., Tepera, S. B., Venezia, T., Graubert, T. A., Rosen, J. M. & Goodell, M. A. (2002) Dev. Biol. 245, 42-56. [DOI] [PubMed] [Google Scholar]
- 13.Montanaro, F., Liadaki, K., Volinski, J., Flint, A. & Kunkel, L. M. (2003) Proc. Natl. Acad. Sci. USA 100, 9336-9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asakura, A. (2003) Trends Cardiovasc. Med. 13, 123-128. [DOI] [PubMed] [Google Scholar]
- 15.Falciatori, I., Borsellino, G., Haliassos, N., Boitani, C., Corallini, S., Battistini, L., Bernardi, G., Stefanini, M. & Vicini, E. (2004) FASEB J. 18, 376-378. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki, A., Zheng, Y., Kondo, R., Kusakabe, M., Takada, Y., Fukao, K., Nakauchi, H. & Taniguchi, H. (2000) Hepatology 32, 1230-1239. [DOI] [PubMed] [Google Scholar]
- 17.Tani, H., Morris, R. J. & Kaur, P. (2000) Proc. Natl. Acad. Sci. USA 97, 10960-10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinohara, T., Avarbock, M. R. & Brinster, R. L. (1999) Proc. Natl. Acad. Sci. USA 96, 5504-5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams, J. M. & Cory, S. (1998) Science 281, 1322-1326. [DOI] [PubMed] [Google Scholar]
- 20.Domen, J., Cheshier, S. H. & Weissman, I. L. (2000) J. Exp. Med. 191, 253-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiberio, R., Marconi, A., Fila, C., Fumelli, C., Pignatti, M., Krajewski, S., Giannetti, A., Reed, J. C. & Pincelli, C. (2002) FEBS Lett. 524, 139-144. [DOI] [PubMed] [Google Scholar]
- 22.Potten, C. S., Wilson, J. W. & Booth, C. (1997) Stem Cells 15, 82-93. [DOI] [PubMed] [Google Scholar]
- 23.Cunha, G. R. & Donjacour, A. (1987) Prog. Clin. Biol. Res. 239, 273-282. [PubMed] [Google Scholar]
- 24.Norman, J. T., Cunha, G. R. & Sugimura, Y. (1986) Prostate 8, 209-220. [DOI] [PubMed] [Google Scholar]
- 25.Xin, L., Ide, H., Kim, Y., Dubey, P. & Witte, O. N. (2003) Proc. Natl. Acad. Sci. USA 100, 11896-11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodell, M. A., Brose, K., Paradis, G., Conner, A. S. & Mulligan, R. C. (1996) J. Exp. Med. 183, 1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gussoni, E., Soneoka, Y., Strickland, C. D., Buzney, E. A., Khan, M. K., Flint, A. F., Kunkel, L. M. & Mulligan, R. C. (1999) Nature 401, 390-394. [DOI] [PubMed] [Google Scholar]
- 28.Bonyadi, M., Waldman, S. D., Liu, D., Aubin, J. E., Grynpas, M. D. & Stanford, W. L. (2003) Proc. Natl. Acad. Sci. USA 100, 5840-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito, C. Y., Li, C. Y., Bernstein, A., Dick, J. E. & Stanford, W. L. (2003) Blood 101, 517-523. [DOI] [PubMed] [Google Scholar]
- 30.Fortunel, N. O., Otu, H. H., Ng, H. H., Chen, J., Mu, X., Chevassut, T., Li, X., Joseph, M., Bailey, C., Hatzfeld, J. A., et al. (2003) Science 302, 393. [DOI] [PubMed] [Google Scholar]
- 31.Li, A., Simmons, P. J. & Kaur, P. (1998) Proc. Natl. Acad. Sci. USA 95, 3902-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur, P. & Li, A. (2000) J. Invest. Dermatol. 114, 413-420. [DOI] [PubMed] [Google Scholar]
- 33.Watt, F. M. (2002) EMBO J. 21, 3919-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, P. H. & Watt, F. M. (1993) Cell 73, 713-724. [DOI] [PubMed] [Google Scholar]
- 35.Bickenbach, J. R. & Chism, E. (1998) Exp. Cell Res. 244, 184-195. [DOI] [PubMed] [Google Scholar]
- 36.Collins, A. T., Habib, F. K., Maitland, N. J. & Neal, D. E. (2001) J. Cell Sci. 114, 3865-3872. [DOI] [PubMed] [Google Scholar]
- 37.Campos, L. S., Leone, D. P., Relvas, J. B., Brakebusch, C., Fassler, R., Suter, U. & ffrench-Constant, C. (2004) Development (Cambridge, U.K.) 131, 3433-3444. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs, E., Tumbar, T. & Guasch, G. (2004) Cell 116, 769-778. [DOI] [PubMed] [Google Scholar]
- 39.Chung, J. & Mercurio, A. M. (2004) Mol. Cells 17, 203-209. [PubMed] [Google Scholar]
- 40.Chao, C., Lotz, M. M., Clarke, A. C. & Mercurio, A. M. (1996) Cancer Res. 56, 4811-4819. [PubMed] [Google Scholar]
- 41.Cress, A. E., Rabinovitz, I., Zhu, W. & Nagle, R. B. (1995) Cancer Metastasis Rev. 14, 219-228. [DOI] [PubMed] [Google Scholar]
- 42.Hockenbery, D. M., Zutter, M., Hickey, W., Nahm, M. & Korsmeyer, S. J. (1991) Proc. Natl. Acad. Sci. USA 88, 6961-6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domen, J. & Weissman, I. L. (2000) J. Exp. Med. 192, 1707-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domen, J. & Weissman, I. L. (2003) Exp. Hematol. 31, 631-639. [DOI] [PubMed] [Google Scholar]
- 45.Kyprianou, N. & Isaacs, J. T. (1989) Mol. Endocrinol. 3, 1515-1522. [DOI] [PubMed] [Google Scholar]
- 46.Rouleau, M., Leger, J. & Tenniswood, M. (1990) Mol. Endocrinol. 4, 2003-2013. [DOI] [PubMed] [Google Scholar]
- 47.Sugimura, Y., Cunha, G. R. & Donjacour, A. A. (1986) Biol. Reprod. 34, 973-983. [DOI] [PubMed] [Google Scholar]
- 48.Colombel, M., Vacherot, F., Diez, S. G., Fontaine, E., Buttyan, R. & Chopin, D. (1998) Br. J. Urol. 82, 380-385. [DOI] [PubMed] [Google Scholar]
- 49.De Marzo, A. M., Marchi, V. L., Epstein, J. I. & Nelson, W. G. (1999) Am. J. Pathol. 155, 1985-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonnell, T. J., Troncoso, P., Brisbay, S. M., Logothetis, C., Chung, L. W., Hsieh, J. T., Tu, S. M. & Campbell, M. L. (1992) Cancer Res. 52, 6940-6944. [PubMed] [Google Scholar]
- 51.Bruckheimer, E. M., Spurgers, K., Weigel, N. L., Logothetis, C. & McDonnell, T. J. (2003) J. Urol. 169, 1553-1557. [DOI] [PubMed] [Google Scholar]
- 52.Richardson, G. D., Robson, C. N., Lang, S. H., Neal, D. E., Maitland, N. J. & Collins, A. T. (2004) J. Cell Sci. 117, 3539-3545. [DOI] [PubMed] [Google Scholar]
- 53.Molofsky, A. V., Pardal, R. & Morrison, S. J. (2004) Curr. Opin. Cell Biol. 16, 700-707. [DOI] [PubMed] [Google Scholar]
- 54.Karhadkar, S. S., Bova, G. S., Abdallah, N., Dhara, S., Gardner, D., Maitra, A., Isaacs, J. T., Berman, D. M. & Beachy, P. A. (2004) Nature 431, 707-712. [DOI] [PubMed] [Google Scholar]
- 55.Valk-Lingbeek, M. E., Bruggeman, S. W. & van Lohuizen, M. (2004) Cell 118, 409-418. [DOI] [PubMed] [Google Scholar]
- 56.Varambally, S., Dhanasekaran, S. M., Zhou, M., Barrette, T. R., Kumar-Sinha, C., Sanda, M. G., Ghosh, D., Pienta, K. J., Sewalt, R. G., Otte, A. P., et al. (2002) Nature 419, 624-629. [DOI] [PubMed] [Google Scholar]