Abstract
Plastics are an important usable energy resource. The energy stored in them can be used as fuel in modified high-energy materials. Such use makes it possible to simultaneously dispose of waste plastics and improve the energy properties of modified explosives. The simplest material that is used for this purpose is ammonium nitrate fuel oil (ANFO)—commonly used in rock mining. Tests carried out in the Trauzl lead block test assessing the strength of explosive have shown that, with the right amount of certain polymers, similar or even better properties—energy performance—can be achieved by replacing the fuel oil in ANFO. In turn, tests conducted on the impact of plastic comminution on the assessment of the strength of high-energy material showed that a 1.0% addition of polypropylene, polyethylene or polyurethane significantly increases the strength with reference to ANFO. The test results are of practical importance and, for a more complete assessment of the suitability of polymers for use, it will be advisable to conduct further studies of the properties of high-energy materials with selected plastics.
Keywords: High-energy materials, Innovative method of waste management, Trauzl lead block test, Grain-size analysis
Subject terms: Environmental sciences, Materials for energy and catalysis, Energy
Introduction
The primary blasting agents used in the mining industry are ammonium nitrate fuel oil explosive—ANFO—and emulsion explosives—EE. The main component in these materials (ammonium nitrate(V)) has been used, in various forms, for more than 150 years, and yet tests are still being conducted into its impact on certain properties of explosives1–6. In addition to assessing the technical aspects of the use of explosives (diameter of loads, method of hole loading, sequence of firing, etc.)7–11, tests are also being carried out related to the influence of individual components (comminution, type and quality of raw material, density, etc.) on performance properties12–14. The possibility of influencing the end result is achieved by the way the composition of the explosives is modified. A very high potential for modifying properties is shown by the two-component explosive, ANFO15–21. Much work has been devoted to both theoretical considerations of the impact of changing thermodynamic parameters on the properties of ANFO22–31, as well as to modifying the composition by including various additives32–49.
It is possible to convert, by pyrolysis, waste plastics of polyethylene (PE) and polypropylene (PP), among others, into a usable fuel with properties similar to conventional transportation fuel (diesel), which is also the combustible component in ANFO50–54. These plastics are a good raw material for the production of liquid fuels. The largest volumes of waste from the packaging, construction, electronics and toy industries contain most of these types of plastics55.
All plastics, except polyvinyl chloride (PVC) and polystyrene (PS), have a similar ratio of hydrogen and carbon atoms (Fig. 1), which is important for determining the potential as a good fuel feedstock.
Figure 1.
Distribution of hydrogen and carbon in various plastics82.
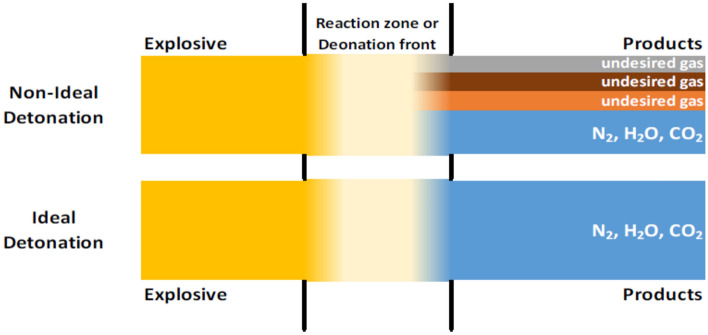
The mentioned ANFO explosive is a non-ideal explosive, which also means that it does not detonate perfectly. It has been found56 that ideal detonations do not occur in practice—explosives approach the ideal detonation state but do not achieve it. The phenomenon of ideal and non-ideal detonation is illustrated in Fig. 2.
Figure 2.
Ideal and non-ideal detonation56.
The presence of nitrogen and oxygen in ammonium nitrate(V) (NH4NO3), the oxidizing component of ANFO, poses a risk of nitrogen oxides (NOx: N2O, NO, N2O3, N2O4, N2O5) in shot gases. They can also contain nitrogen dioxide (NO2) and carbon monoxide (CO). The most harmful nitrogen dioxide generates orange/brown smoke57. The non-ideal detonation reaction follows Eqs. (1), (2):
| 1 |
On contact with atmospheric oxygen, nitric oxide is rapidly converted to dioxide:
| 2 |
Ongoing studies of the effects of various factors on the type of detonation gases44,58–60 have shown that under certain conditions toxic aerosols can also be formed: CO, NH3, HCN and CH4, as well as simple hydrocarbons (CxHy)61.
The optimal composition of ANFO (94.5% ammonium nitrate and 5.5% diesel fuel) minimizes the formation of toxic gases during the detonation process and, under ideal conditions, products that threaten human health and safety should not be formed62.
In practice, the ratio of ammonium nitrate(V) to diesel fuel is 94% and 6%, respectively, which ensures the complete reaction of ammonium nitrate(V). With less than 94% oxidizer, a deficiency of oxygen is created, leading to the formation of carbon monoxide and little (or no) nitrogen oxides. In turn, excess oxygen increases the production of nitrogen oxides and decreases carbon monoxide63. This is also associated with a decrease in the heat of explosion, which is another reason to avoid such compositions. The ideal detonation reaction—(zero oxygen balance) follows Eqs. (3), (4):
| 3 |
Simplified version:
| 4 |
When modifying an explosive by introducing various additives, it is assumed, at the stage of designing the composition, that the elemental balance of oxygen (oxygen balance) is zero, ensuring the formation of products of complete and total combustion during detonation, when the shooting technique is properly carried out64.
Another solution is the introduction of ammonium nitrate additives, which have an effect similar to the afterburning technique. Coal dust with a particle diameter of 74 μm reduces total NOx emissions by 10–50%44. Comparable emission-reduction effects have been obtained with the addition of, for example, sodium salts, potassium salts, calcium salts, sulfates, phosphates, urea, and the urea/MnO2 system65,66. There have also been attempts to use waste polymers—polyethylene67–69.
The addition of small amounts, about 50 g, of shredded recycled rubber or dried hay to ANFO resulted in non-ideal detonation; these were laboratory-scale studies70. In turn, the contribution of, for example, perlite or polystyrene in ANFO had the effect of reducing the density of this explosive71.
Powdered plastic waste along with the addition of wood flour constituted the composition of modified ANFO improving the detonation velocity and reducing the formation of toxic gases after detonation72.
It has been shown23–25 that the use of certain plastics interchangeably with diesel fuel does not cause significant changes in the thermodynamic parameters of modified ANFO. There is also no change in the qualitative composition of shot gases compared to standard ANFO.
The use of a controlled system of quantitative dosing and homogenization using MEMU-type equipment has been in use for some time, ensuring that the set parameters are maintained during detonation and having a significant impact on improving the environment when conducting blasting operations73.
A significant parameter when conducting blasting operations is the detonation velocity of the explosive, which depends on: chemical composition, density, structure, and diameter and type of envelope7,74–78. Also, the type of rock in which blasting is carried out is an integral part of assessing the environmental impact of the resulting gases and dust.
Given the numerous works devoted to modifying the composition of ANFO by introducing additives, the authors of the article also carried out tests in this area. The work consisted in the assessment of the strength of the explosive expressed in terms of its blasting capacity by the Trauzl lead block test79 after introducing an additive of plastics as a property modifier of this explosive. Preliminary tests carried out proved the suitability of the plastics and the modified ANFO had better parameters than the base material for several types of these property modifiers. Taking into account the different granularity of the plastics used, the authors carried out tests on the properties of the explosive after the modifiers were comminuted, obtaining a similar grain-size distribution for all plastics.
In this way, the impact of comminution on the strength of modified ANFO was assessed using the aforementioned test.
Five—the most common and widely used—plastics were used in these tests: polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), and polyurethane (PU).
This stage of testing facilitated the comparison of the plastic-modified explosive with the reference material and, due to the satisfactory results obtained, indicated the need for testing of other performance properties (detonation velocity, air blast wave and qualitative and quantitative analysis of the blast gases).
The basic research carried out was intended to test the assumption of the possibility of using as much plastic as possible in the ANFO structure without significant loss of energy parameters. The research was basic in scope. It was hypothesized that the degree of fragmentation of the introduced component into the structure of the explosive would improve its ability to perform in the Trauzla block.
Materials and methods
To modify the explosive, an ANFO mixture material was used with ammonium nitrate(V) as the oxidiser and the fuel oil. An oxidiser/fuel ratio of 94/6, the most commonly used by explosive manufacturers, was adopted. In the tests, the explosive selected in this way was the base material, which was modified with the addition of comminuted plastics. The basic component, ammonium nitrate (trade name “UltrAN 70”), was produced by Yara. The fuel oil produced by Silesia Oil sp. z o.o. was used in the tests. Detailed characteristics of the fuel oil can be found in80,81. The same production batch of oxidiser and fuel was used during the tests.
The tests were carried out at the Conformity Assessment Body, Central Mining Institute. The ANFO explosive was modified with the addition of five types of plastic: polyethylene (PE), polystyrene (PS), polypropylene (PP), polyvinyl chloride (PVC)—two variations, and polyurethane (PU).The plastics were comminuted to obtain a form suitable for good incorporation into the mixture, as shown schematically in Fig. 3. The finer material fills the empty spaces between the granules more accurately. This way of homogenising the mixture can significantly impact the density of the explosive and thus improve certain performance parameters. A higher density (up to the limit value) of the high-energy mixture material leads to an increase in one of the energy parameters—the detonation velocity. It is expected that the capacity to perform work in the Trauzla block will increase.
Figure 3.
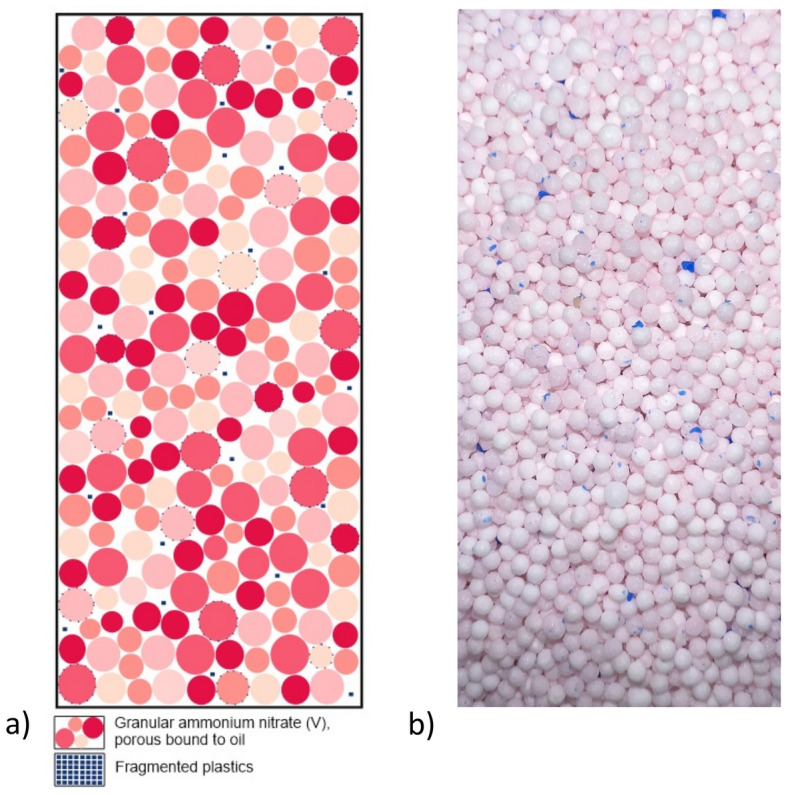
Arrangement of explosive components in the ANFO cartridge. (a) Schematic cross-section of plastic dispersion in the explosive cartridge. (b) Picture of the PS 2.0 used in the tests.
Table 1 shows pictures of the comminuted plastics used in the tests; pictures of the product (before comminution) are also included for comparison.
Table 1.
Types and varieties of plastics used in “in situ” tests.
The plastics were comminuted using a Fritsch Pulverisette 19 cutting mill. The material was poured through the funnel directly into the chamber of the machine on the knives. The short cutting time of the doze did not cause the plastic to heat up and thus clump into larger aggregates. The grain size distribution was tested with a Fritsch Analysette 22 NanoTec plus laser particle sizer using the laser diffraction method. Wet dispersion method was carried out.
Based on the results of theoretical calculations of various mixtures24, the compositions shown in Table 2 were selected for testing with the Trauzl test method.
Table 2.
List of calculated thermodynamic parameters24.
| Parameter | ANFO | PE 1.0 | PE 2.0 | PP 1.0 | PP 2.0 | PS 2.0 | PVC 1.0 | PU 1.0 | PU 1.5 |
|---|---|---|---|---|---|---|---|---|---|
| Explosion pressure (MPa) | 3900 | 3807 | 3834 | 3824 | 3837 | 3808 | 3741 | 3879 | 3835 |
| Explosion temperature (K) | 2968 | 2946 | 2939 | 2961 | 2964 | 2969 | 2909 | 2957 | 2928 |
| Explosion heat at constant volume (kJ/kg) | 3914 | 3875 | 3860 | 3902 | 3914 | 3886 | 3761 | 3834 | 3758 |
| Detonation velocity (m/s) | 4410 | 4369 | 4370 | 4377 | 4384 | 4355 | 4312 | 4370 | 4356 |
| Gas volume in standard conditions (l/kg) | 1057 | 1059 | 1061 | 1059 | 1061 | 1049 | 1049 | 1052 | 1052 |
| Oxygen balance (%) | − 0.99 | − 1.12 | − 1.24 | − 1.12 | − 1.24 | − 0.54 | 1.03 | 0.05 | 0.57 |
The following symbols were adopted: PE1.0 (94.0% ammonium nitrate, 5.0% fuel oil, 1.0% polyethylene), PE2.0 (94.0% ammonium nitrate, 4.0% fuel oil, 2.0% polyethylene), PP1.0 (94.0% ammonium nitrate, 5.0% fuel oil, 1.0% polypropylene), PP2.0 (94.0% ammonium nitrate, 4.0% fuel oil, 2.0% polypropylene), PS2.0 (94.0% ammonium nitrate, 4.0% fuel oil, 2.0% polystyrene), PVC1.0 (94.0% ammonium nitrate, 5.0% fuel oil, 1.0% polyvinyl chloride), PU1.0 (94.0% ammonium nitrate, 5.0% fuel oil, 1.0% polyurethane), PU1.5 (94.0% ammonium nitrate, 4.5% fuel oil, 1.5% polyurethane),
In the applied calculations, the amount of ammonium nitrate remained unchanged and fuel oil was replaced with plastics, as appropriate.
The compositions were formulated in such a way as to obtain an oxygen balance equal to or close to zero, assuming a constant rate of change in component content of at least 0.5% by weight. This level of accuracy in mixing components is attainable under production conditions. This is in line with the design of explosives for civilian uses. In the case of a non-ideal ANFO explosive, a zero oxygen balance allows optimal energy performance31.
The effect of the proposed modifications was assessed by measuring the strength of the explosive based on the Trauzl block test. The method of measurement and how the cm3 volume of the expansion volume (volume increase after detonation) of the hole in the lead block was calculated was as described in79.
The Trauzl lead block test method consists of measuring the volume in cm3 of the expansion (increase in volume after detonation) in a lead block of standardized dimensions (Fig. 3) caused by the detonation of a 10 g charge of explosive. The resulting volume is converted to conditions that eliminate the effect of temperature on the determination. This is a comparative method using picric acid as a standard, in which the detonation of a 10 g charge with a density of 0.85 g/cm3 at 15 °C results in a 310 cm3 expansion defined as a ‘normal expansion in a lead block’. The increase in the volume of the hole in the lead block caused by the detonation of the explosive under test is defined as 'lead block expansion. The blocks with high-energetic material prepared for testing are shown in Fig. 4.
Figure 4.

A series of Trauzl blocks prepared for testing.
Each series of determinations was carried out in lead blocks from a single melt. The blocks to be tested were set up in an enclosed blasting room and held until their temperature equilibrated with ambient temperature. Before blasting, the original volume of the holes in the blocks was measured using a calibrated burette and a cylinder. Charges of the tested and reference explosive were armed with 0.65 g PETN detonator. The armed explosive charge was then placed in the hole of the block and the remainder of the hole was filled with dry sand (grain size less than 100 µm). After firing the charge, the inside of the hole was cleaned and the volume increase measured.
The expansion volume (V0) and (Ve) in [cm3] obtained under test conditions was calculated by subtracting the primary lead block hole volume (VP) from the measured volume of the hole resulting from the detonation of the explosive (Ve) according to the following formulas (5) and (6):
| 5 |
| 6 |
where V0—volume of expansion in the block resulting from detonation of the reference explosive [cm3], VX—volume of expansion in the block resulting from detonation of the tested explosive [cm3], VP—primary lead block hole volume [cm3], Ve—final volume of the hole (after firing) in the lead block [cm3].
The results of the measurements were converted to normal lead block conditions according to formula (7), obtaining the relative work capacity of the tested explosive:
| 7 |
where VY—arithmetic mean (VX) from expansion in the block resulting from detonation of the tested explosive [cm3], VZ—arithmetic mean (V0) expansion in the block resulting from detonation of the reference explosive [cm3].
Results
The results of the specific particle size values obtained from the laser particle sizer test for the individual samples are shown in Table 3.
Table 3.
Results of grain size distribution for the plastics used in the tests.
| No. | Name of plastic | Grain size distribution | |||
|---|---|---|---|---|---|
| d(10) µm | d(50) µm | d(90) µm | d(4.3) µm | ||
| 1 | PE HDPE regranulate | 637.7 | 907.3 | 1213.1 | 917.0 |
| 2 | PP regranulate | 393.4 | 795.6 | 1083.6 | 756.9 |
| 3 | PS 7135 K blue | 651.9 | 908.4 | 1214.4 | 904.2 |
| 4 | PVC ShD (hard) HEZ0177 natural colour | 596.2 | 888.2 | 1195.9 | 864.3 |
| 5 | PVC ShA (soft) EPA71/1 T colour black hardness 71 ShA | 679.3 | 926.3 | 1225.5 | 918.3 |
| 6 | PU Wezcrystal (shore: 95A/46D) | 700.0 | 919.1 | 1215.2 | 942.7 |
Detailed grain size distributions for the samples tested are shown in Supplementary material.
The similar values of d(10), d(50), d(90) and d(4.3) for the individual plastics confirm the reproducibility of the results of the comminution process and the similar grain size distribution of all samples.
The Trauzl block test was performed in three trials. Three measurements of expansion volume were carried out in each of the trials for picric acid and the baseline ANFO. Three expansion volume measurements were also taken for each explosive with the addition of the selected plastic. The results were converted to normal conditions. The difference in expansion volume for the tested explosive as compared to ANFO for each particular trial was calculated.
A summary of the results of the first trial is shown in Table 4. A statistical analysis of the results of the first series is shown in Table 5.
Table 4.
Results of the first trial of the test in the Trauzl lead block test for the explosive with added plastics after comminution.
| Trial no | Explosive name (composition) |
Primary volume Vp [cm3] | End volume Ve [cm3] | Expansion volume V0/Vx [cm3] | Arithmetic mean on volume [cm3] | Statistical distribution of results [cm3] | Expansion volume (converted) V [cm3] | Expansion difference with regard to ANFO [cm3] | Expansion difference with regard to ANFO [%] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Reference explosive (picric acid) | 67.8 | 368.4 | 300.6 | 300.06 | 0.4 | 300.6 | Not applicable | Not applicable |
| 2 | 67.6 | 368.8 | 301.2 | ||||||
| 3 | 68.2 | 368.3 | 300.1 | ||||||
| 1 | PS 2.0 (94% AN + 4% FO + 2% PS 7135 K) | 67.7 | 324.4 | 256.7 | 255.8 | 7.8 | 263.8 | 17.4 | 7.1 |
| 2 | 64.5 | 329.4 | 264.9 | ||||||
| 3 | 65.6 | 311.4 | 245.8 | ||||||
| 1 | PU 1.0 (94% AN + 5% FO + 1% PU Wezrystal) | 68.2 | 352.2 | 284.0 | 277.1 | 5.2 | 285.7 | 39.3 | 16.0 |
| 2 | 67.6 | 339.0 | 271.4 | ||||||
| 3 | 67.8 | 343.6 | 275.8 | ||||||
| 1 | PVC 1.0 (94% AN + 5% FO + 1% PVC EPA 71/1 T) | 65.5 | 329.6 | 264.1 | 265.6 | 1.0 | 273.8 | 27.5 | 11.1 |
| 2 | 65.5 | 332.0 | 266.5 | ||||||
| 3 | 65.6 | 331.7 | 266.1 | ||||||
| 1 | PVC 1.0 (94% AN + 5% FO + 1% PVC ShD HEZ0177) | 66.2 | 317.4 | 251.2 | 262.0 | 8.3 | 270.1 | 23.8 | 9.6 |
| 2 | 66.3 | 337.6 | 271.3 | ||||||
| 3 | 66.5 | 329.9 | 263.4 | ||||||
| 1 | ANFO 94% AN + 6% FO | 67.8 | 304.0 | 236.2 | 238.9 | 5.3 | 246.4 | 0.0 | 0.0 |
| 2 | 67.4 | 301.6 | 234.2 | ||||||
| 3 | 66.4 | 312.8 | 246.4 |
Significant values are given in bold.
AN ammonium nitrate(V), FO diesel.
Table 5.
Results of statistical parameters of variation for data from the first series.
| Explosive name (composition) | Expansion volume (converted) V [cm3] | Arithmetic mean of the result and ANFO [cm3] | Statistical distribution of results relative to ANFO [cm3] | Variance of the result relative to ANFO | Standard deviation relative to ANFO | Coefficient of variation relative to ANFO [%] |
|---|---|---|---|---|---|---|
| PS 2.0 (94% AN + 4% FO + 2% PS 7135 K) | 263.8 | 255.1 | 17.4 | 75.62 | 8.70 | 3.41 |
| PU 1.0 (94% AN + 5% FO + 1% PU Wezrystal) | 285.7 | 266.0 | 39.3 | 386.54 | 19.66 | 7.39 |
| PVC 1.0 (94% AN + 5% FO + 1% PVC EPA 71/1 T)) | 273.8 | 260.1 | 27.5 | 188.56 | 13.73 | 5.28 |
| PVC 1.0 (94% AN + 5% FO + 1% PVC ShD HEZ0177) | 270.1 | 258.3 | 23.8 | 141.03 | 11.88 | 4.60 |
| ANFO | 246.4 | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
Significant value is given in bold.
A summary of the results of the second trial is shown in Tables 6 and 7.
Table 6.
Results of the second trial of the test in the Trauzl lead block test for the explosive with added plastics after comminution.
| Trial no | Explosive name (composition) | Primary volume Vp [cm3] | End volume Ve [cm3] | Expansion volume V0/Vx [cm3] | Arithmetic mean on volume [cm3] | Statistical distribution of results [cm3] | Expansion volume (converted) V [cm3] | Expansion difference with regard to ANFO [cm3] | Expansion difference with regard to ANFO [%] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Reference explosive (picric acid) | 65.0 | 388.0 | 323.0 | 315.8 | 6.0 | 315.8 | Not applicable | Not applicable |
| 2 | 66.0 | 374.4 | 308.4 | ||||||
| 3 | 64.3 | 380.2 | 315.9 | ||||||
| 1 | PU 1.5 (94% AN + 4.5% FO + 1.5% PU Wezcrystal) | 65.4 | 310.0 | 244.6 | 247.7 | 2.4 | 243.2 | 8.6 | 3.7 |
| 2 | 65.8 | 316.2 | 250.4 | ||||||
| 3 | 64.7 | 312.8 | 248.1 | ||||||
| 1 | PE 2.0 (94% AN + 4% FO + 2% PE HDPE regranulate) | 65.8 | 331.0 | 265.2 | 255.6 | 7.1 | 250.9 | 16.4 | 7.0 |
| 2 | 66.0 | 314.2 | 248.2 | ||||||
| 3 | 66.1 | 319.5 | 253.4 | ||||||
| 1 | PP 2.0 (94% AN + 4% FO + 2% PP regranulate) | 67.0 | 336.4 | 269.4 | 275.7 | 22.0 | 270.6 | 36.1 | 15.4 |
| 2 | 66.8 | 372.0 | 305.2 | ||||||
| 3 | 65.8 | 318.2 | 252.4 | ||||||
| 1 | ANFO (94% AN + 6% FO) | 67.8 | 304.0 | 236.2 | 238.9 | 5.3 | 234.6 | 0.0 | 0.0 |
| 2 | 67.4 | 301.6 | 234.2 | ||||||
| 3 | 66.4 | 312.8 | 246.4 |
Significant values are given in bold.
AN ammonium nitrate(V), FO diesel.
Table 7.
Results of statistical parameters of variation for data from the second series.
| Explosive name (composition) | Expansion volume (converted) V [cm3] | Arithmetic mean of the result and ANFO [cm3] | Statistical distribution of results relative to ANFO [cm3] | Variance of the result relative to ANFO | Standard deviation relative to ANFO | Coefficient of variation relative to ANFO [%] |
|---|---|---|---|---|---|---|
| PU 1.5 (94% AN + 4.5% FO + 1.5% PU) | 243.2 | 238.9 | 8.6 | 18.52 | 4,.30 | 1.80 |
| PE 2.0 (94% AN + 4% FO + 2% PE HDPE regranulate) | 250.9 | 242.8 | 16.4 | 66.93 | 8.18 | 3.37 |
| PP 2.0 (94% AN + 4% FO + 2% PP regranulate) | 270.6 | 252.6 | 36.1 | 325.13 | 18.03 | 7.14 |
| ANFO | 234.6 | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
Significant value is given in bold.
A summary of the results of the third trial is shown in Tables 8 and 9.
Table 8.
Results of the third trial of the test in the Trauzl lead block test for the explosive with added plastics after comminution.
| Trial no | Explosive name (composition) | Primary volume Vp [cm3] | End volume Ve [cm3] | Expansion volume V0/Vx [cm3] | Arithmetic mean on volume [cm3] | Statistical distribution of results [cm3] | Expansion volume (converted) V [cm3] | Expansion difference with regard to ANFO [cm3] | Expansion difference with regard to ANFO [%] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Reference explosive (picric acid) | 64.8 | 366.2 | 301.4 | 298.0 | 2.5 | 298.0 | Not applicable | Not applicable |
| 2 | 66.0 | 361.4 | 295.4 | ||||||
| 3 | 65.3 | 362.5 | 297.2 | ||||||
| 1 | PP 1.0 (94% AN + 5% FO + 1% PP regranulate) | 65.6 | 376.0 | 310.4 | 327.3 | 12.0 | 340.5 | 70.5 | 28.1 |
| 2 | 65.6 | 402.8 | 337.2 | ||||||
| 3 | 66.1 | 400.5 | 334.4 | ||||||
| 1 | PE 1.0 (94% AN + 5% FO + 1% PE HDPE regranulate) | 62.4 | 300.4 | 238.0 | 274.3 | 26.1 | 285.3 | 34.7 | 13.9 |
| 2 | 67.6 | 366.2 | 298.6 | ||||||
| 3 | 68.1 | 354.3 | 286.2 | ||||||
| 1 | ANFO (94% AN + 6% FO) | 66.8 | 308.0 | 241.2 | 240.9 | 3.4 | 250.6 | 0.0 | 0.0 |
| 2 | 65.4 | 301.9 | 236.5 | ||||||
| 3 | 66.6 | 311.5 | 244.9 |
Significant values are given in bold.
AN ammonium nitrate(V), FO diesel.
Table 9.
Results of statistical parameters of variation for data from the third series.
| Explosive name (composition) | Expansion volume (converted) V [cm3] | Arithmetic mean of the result and ANFO [cm3] | Statistical distribution of results relative to ANFO [cm3] | Variance of the result relative to ANFO | Standard deviation relative to ANFO | Coefficient of variation relative to ANFO [%] |
|---|---|---|---|---|---|---|
| PP 1.0 (94% AN + 5% FO + 1% PP regranulate) | 340.5 | 295.5 | 89.9 | 2022.69 | 44.97 | 15.22 |
| PE 1.0 (94% AN + 5% FO + 1% PE HDPE regranulate) | 285.3 | 267.9 | 34.7 | 301.80 | 17.37 | 6.48 |
| ANFO | 250.6 | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
Discussion
First series of the test
Both the obtained average expansion and expansion sizes after conversion to normal conditions for all plastics are higher compared to ANFO (Table 4). The standard deviations calculated from single measurements for the individual explosives are in the range of 0.4–8.3 cm3. The standard deviation result for the base ANFO was 5.3 cm3. These values indicate the high repeatability of the obtained results. The model explosive—picric acid was characterized by the lowest value of this indicator − 0.4. The largest variation in the results was the PVC 1.0 ShD HEZ0177 − 8.3 cm3. The largest difference in the size of the expansion between ANFOs was shown by PU 1.0–39.3 cm3 which is 16.0% difference.
Analysis of statistical parameters showed the highest values of variance and standard deviation for PU 1.0 (386.53 and 19.66, respectively) and PVC 1.0 EPA (188.56 and 13.73, respectively). PVC 1.0 EPA’s standard deviation from the individual excursion results (Table 4a) was low at 1.0. The calculated coefficient of variation for PU 1.0 was 7.39% while for PVC 1.0 EPA it was 5.28%. These results indicate the greater potential of these explosives to perform work (strength) relative to ANFO.
Second series of the test
The average expansion volumes (before converting the results) for each explosive ranged from 247.7–275.7 cm3. The base ANFO in the second trial of the test had an average result of 238.9 cm3. The standard deviations for the results of measuring the expulsions for individual explosives were in the range of 2.4–22.0. The largest scatter of results was shown by the results made for PP 2.0. The highest repeatability of measurements was observed for PU1.5. The normal expansion in the lead block were higher than the standard ANFO by 3.7–15.4%. The highest difference was calculated for PP 2.0.
The variance and standard deviation showed the highest values for PP 2.0 at 325.13 and 18.03, respectively. This allowed a coefficient of variation of 7.14.
Third series of the test
The average expansion results in the lead block for individual explosives had a standard deviation in the range of 2.5–26.1. Similarly to the first series, picric acid had the highest repeatability of single measurements (standard deviation 2.5) and PE1.0 had the lowest repeatability (standard deviation 26.1). Very high average blowout results were observed for PP1.0 − 327.3 cm3 with a coefficient of variation of individual results of 12.00. After conversion to normal condition, this explosive showed the highest normal expansion value in a lead block of 340.5 cm3.
Due to the large difference in normal expansion in the lead block, the highest value of variance and standard deviation was calculated for PP1.0 (2022.69 and 44.97, respectively). This explosive had the highest coefficient of variation of 15.22%, which indicates a greater ability to perform work than other explosives.
The use of the adopted methodology and essential components for the production of ANFO with appropriate technical parameters and purity allowed a high similarity of expansion volume results for both the reference explosive—picric acid (result range: 298.0–315.8 cm3) and the baseline explosive—ANFO (result range: 234.6–250.6 cm3) in all trials.
For most of the tested explosives with the addition of comminuted plastics (with the exception of PU 1.5), an increase can be found in expansion volume of the hole in the lead block as compared to the baseline ANFO. The values of expansion volume converted to normal conditions are in the range of 243.2–340.5 cm3.
PP 1.0 showed the highest blasting capacity in the lead block test. The achieved value of 340.5 cm3 is 89.9 cm3 higher than the baseline ANFO, representing an improvement of 35.9% with respect to this explosive. Increasing the amount of polypropylene in the structure of the explosive to 2.0% for PP 2.0 still yielded a higher energy potential as compared to ANFO (36.1 cm3 increase in expansion volume, 15.4% improvement in performance) but the values obtained are lower compared to PP 1.0. The use of 1.0% polyurethane admixture in the structure of the explosive resulted in an increase of 39.3 cm3 in the block expansion volume for PU 1.0, representing a 16.0% improvement as compared to ANFO. However, the admixture of 1.5% of this component deteriorates the performance of the explosive—PU 1.5 (expansion volume of 243.2 cm, similar to ANFO). Energetically favourable results were obtained for PE 1.0. Expansion volume improved in this case by 34.7 cm3 (13.9%) compared to the conventional ANFO. However, as in the case of the explosive with the addition of polypropylene, the addition of 2.0% polyethylene resulted in a deterioration of the performance of PE 2.0 (an expansion volume of 250.9 cm3, slightly higher than that obtained with ANFO).
As part of preliminary tests, the volume of the expansion in the lead block was measured for selected plastics in the form supplied by the manufacturer, i.e. pellets and granules. A comparative summary of the results is shown in Table 10.
Table 10.
Comparisons of expansion volumes for individual explosives with added plastics in comminuted and uncomminuted forms.
| Group of plastics | Explosive name (composition) | Uncomminuted plastic2 | Comminuted plastic | ||
|---|---|---|---|---|---|
| Difference in expansion volume as compared to ANFO1 [cm3] | Difference in expansion volume as compared to ANFO1 [%] | Difference in expansion volume as compared to ANFO1 [cm3] | Difference in expansion volume as compared to ANFO1 [%] | ||
| PE | PE 1.0 (94% AN + 5% FO + 1% PE HDPE regranulate) | – | – | 34.7 | 13.9 |
| PE 2.0 (94% AN + 4% FO + 2% PE HDPE regranulate) | – | – | 16.4 | 7.0 | |
| PP | PP 1.0 (94% AN + 5% FO + 1% PP regranulate) | − 66.7 | − 20.4 | 89.9 | 35.9 |
| PP 2.0 (94% AN + 4% FO + 2% PP regranulate) | − 44.7 | − 13.7 | 36.1 | 15.4 | |
| PS | PS 2.0 (94% AN + 4% FO + 2% PS 7135 K) | 11.4 | 3.5 | 17.4 | 7.1 |
| PVC | PVC 1.0 (94% AN + 5% FO + 1% PVC ShD HEZ0177) | 9.3 | 2.8 | 23.8 | 9.6 |
| PVC 1.0 (94% AN + 5% FO + 1% PVC EPA 71/1 T) | − 6.9 | − 2.1 | 27.5 | 11.1 | |
| PU | PU 1.0 (94% AN + 5% FO + 1% PU Wezrystal) | 21.0 | 6.4 | 39.3 | 16.0 |
| PU 1.5 (94% AN + 4.5% FO + 1.5% PU Wezcrystal) | KunstPur 95A PU for moulds, flexible (− 7.6) | KunstPur 95A PU for moulds, flexible (− 2.3) | 8.6 | 3.7 | |
AN ammonium nitrate(V), FO diesel.
1Values provided in relation to the result obtained for ANFO from the individual trials.
2Results of preliminary tests carried out on plastics in the form provided by the manufacturer or supplier.
Comminuted polyethylene, which had not been used in the preliminary tests, was additionally introduced in the actual tests. For all explosives with the addition of comminuted plastics, higher values of expansion volume were obtained as compared to the baseline ANFO. The largest difference in value was obtained for PP 1.0 when using powdered polypropylene, with an increase of 159.9 cm3 in expansion volume compared to ANFO.
Conclusions
The results obtained after the Trauzl test in the lead block showed a high energy potential of the plastics used in correcting the blasting capacity of the ANFO explosive.
The use of comminuted plastics in amounts of 1–2% by weight of the explosive allows, in most cases (with the exception of PU 1.5), higher lead block expansion volume values compared to ANFO. The values obtained are much higher than the thermodynamic simulation results would suggest. The expansion volumes for explosives with the addition of comminuted plastics are higher than for the same formulation compositions containing plastic granules or pellets.
The much better comminution of the plastics used compared to the oxidiser results in better homogeneity of the added components in the mixture. This is particularly important when modifying the properties of non-ideal explosives with small amounts of corrector. Obtaining similar results in the three expansion volume measurements for the Trauzl test with each polymer indicates that the components are properly mixed. The powder disperses more evenly in the structure of the explosive resulting in stable and reproducible explosive performance. The finer form of the modifier of properties also increases its specific surface area and thus the chemical reactivity of the plastic. Therefore, the use of plastics in the form of powder seems reasonable and desirable.
The most favourable blasting capacity results were obtained for explosives containing 1.0%: polypropylene (PP 1.0), polyurethane (PU 1.0) and polyethylene (PE 1.0), in their respective order. In the case of PP 2.0 and PE 2.0, the use of a 2.0% admixture of these oil-replacement plastics further allows obtaining a higher blasting capacity potential compared to the baseline ANFO.
The lead block test allows determining the relative ability of a high-energy material to performance work (strength). Since it is standardized, the results obtained can be compared between different high-energy materials. However, it does not allow to determine the influence of the diameter, length, different ways of initiating charges, the type of boosters and igniters used on the efficiency of the performance of a non-ideal high-energy material. The obtained results constitute a basis for conducting additional tests of the detonation velocity for small and large diameter charges in various charge configurations.
The proposed conceptual, unconventional method of waste disposal, if commercialized, could complement existing methods. Despite the existence of many methods for disposing of plastic waste, its recovery is low. The large number of open-pit mining operations in the country and around the world, as well as the availability and prevalence of waste plastics, is logistically advantageous and offers potential for industrial-scale application in the future.
The cutting mill used in the study is dedicated to comminuting plastics in the quantities used in laboratory conditions; however, there are devices available on the market that are suited to handling industrial quantities of plastics. The comminution process takes a relatively short time, does not require large amounts of energy input and results in a reproducible grain size distribution, which is important in the production of explosives due to the standardisation of the resulting products and their energy parameters.
Mobile explosive manufacturing unit (MEMU) adapted for the production of ANFO explosives and emulsion explosives using the in situ method features a set of chambers with essential components of explosives and additives in bulk form, e.g. aluminium, acting as property correctors. Thus, the use of another corrector of properties in bulk form (plastic) is technically possible and does not require significant design or software changes to such systems. This offers great potential for the industrial use of plastics to produce ANFO in the future or to conduct experimental research under “in situ” conditions at an open-pit mining facility.
Supplementary Information
Acknowledgements
The authors would like to thank the Company "EURO-WTÓR Kamila Kogut" Prusice (Poland) for providing plastics: PE, PS and PP for the research.
Author contributions
J.B. Conceptualizing the article, Obtaining funding, Writing part of the manuscript K. B, Methodology, Writing part of the manuscript, K.H. Research, Revisions of maunscript M.P. Research, Revisions of maunscript G. T. Acquisition of materials, Revisions of maunscript.
Funding
Research project supported/partly supported by the “Excellence Initiative—Research University” programme for the AGH University of Krakow (grant number 1553/2021).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-67823-y.
References
- 1.Bohanek, V., Dobrilovič, M. & Škrelec, V. Influence of the initiation energy on the velocity of detonation of ANFO explosive. Cent. Eur. J. Energ. Mater.10(4), 555–568 (2013). [Google Scholar]
- 2.Dobrilovič, M., Bohanek, V. & Žganec, S. Influence of explosive charge temperature on the velocity of detonation of ANFO explosives. Cent. Eur. J. Energ. Mater.11(2), 191–197 (2014). [Google Scholar]
- 3.Maranda A, Paszula J, Zawadzka-Małota I, Kuczyńska B, Witkowski W, Nikolczuk K, Wilk Z. Aluminum Powder Influence on ANFO Detonation Parameters. In Proceedings of the 14th Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic. (2011).
- 4.Paszula, J., Maranda, A., Nikolczuk, K. & Giercuszkiewicz, A. Modification of the detonation parameters of mining explosives containing hydrogen peroxide and aluminium powder. Cent. Eur. J. Energ. Mater.18(4), 279–292 (2011). [Google Scholar]
- 5.Sitkiewicz-Wołodko, R., Maranda, A. & Paszula, J. M. Modification of ANFO detonation parameters by addition of ground of ammonium nitrate(V) and aluminium powder. Cent. Eur. J. Energ. Mater.16(1), 122–134 (2019). 10.22211/cejem/104700 [DOI] [Google Scholar]
- 6.Žganec S., Dobrilovič M., Bohanek V. Borehole Velocity of Detonation of ANFO and Heavy ANFO Explosives. In Proceedings of the 17th Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 9–11. (2014). https://www.ntrem.com/download/NTREM_2014_abstracts.pdf, https://www.ntrem.com/index.html
- 7.Arai, H. et al. Detonation behaviour of ANFO in resin tubes. Sci. Tech. Energ. Mater.65(6), 201–205 (2004). [Google Scholar]
- 8.Kabongo KK. Low-Density Ammonium Nitrate Fuel Oil to Improve Gold Recovery. The Journal of The South African Institute of Mining and Metallurgy. https://www.saimm.co.za. (1995).
- 9.Miyake, A., Takahara, K., Ogawa, T., Ogata, Y. & Arai, H. Detonation velocity and pressure of the non-ideal explosive ANFO. J. Jap. Explos. Soc.63, 279–282 (2002). [Google Scholar]
- 10.Miyake, A. et al. Influence of physical properties of ammonium nitrate on the detonation behaviour of ANFO. J. Loss Prev. Proc. Ind.14, 533–538 (2001). 10.1016/S0950-4230(01)00041-9 [DOI] [Google Scholar]
- 11.Vuillaume M, Bouvet JM. What Really is ANFO? Explosive Performance and Blasting Advantages of Microporous and Low Density Ammonium Nitrate-Based ANFO. Proceedings of the Third Summer School Conference on Rock Blasting, 83–97. (1993).
- 12.Catanach, R. A. & Hill, L. G. Diameter effect curve and detonation front curvature measurements for ANFO. AIP Conf. Proc.620, 906–909 (2002). 10.1063/1.1483684 [DOI] [Google Scholar]
- 13.Zawadzka-Małota, I. & Maranda, A. Comparison of the methods used in Poland for the determination of the composition of blasting fumes after the detonation of mining explosives. Prz. Gór.65(3), 27–32 (2008). [Google Scholar]
- 14.Zygmunt, B. & Buczkowski, D. Influence of ammonium nitrate prills on detonation velocity of ANFO. Propellants Explos. Pyrotech.32(5), 411–414 (2007). 10.1002/prep.200700045 [DOI] [Google Scholar]
- 15.Biessikirski, A. et al. Application of silicon dioxide as the inert component or oxide component enhancer in ANFO. Energies14(8), 2152 (2021). 10.3390/en14082152 [DOI] [Google Scholar]
- 16.Biessikirski, A. et al. Badanie ciekłych składników palnych stosowanych w materiałach wybuchowych opartych na azotanie amonu. Przem. Chem.97(3), 457–462 (2018). [Google Scholar]
- 17.Biessikirski, A. Research on the Physicochemical Properties of Non-Ideal Explosives Based on Various Types of Ammonium Nitrate(V) and Fuel Oils (AGH-UST, 2020). [Google Scholar]
- 18.Dobrilović M, Škrlec V, Bohanek V. Velocity of Detonation of Low Density ANFO Mixture. Proceeding of 16th seminar on New Trends in Research of Energetic Materials, 543–554. (2013).
- 19.Hurley C. Development of Ammonium Nitrate Based Explosives to Optimize Explosive Properties and Explosive Welding Parameters used During Explosion Cladding. Mines Theses & Dissertations 2013. https://hdl.handle.net/11124/77790, https://repository.mines.edu/bitstream/handle/11124/77790/Hurley_mines_0052N_10118.pdf?sequence=1&isAllowed=y. (2013).
- 20.Jackson, S. I. The dependence of ammonium-nitrate fuel-oil (ANFO) detonation on confinement. Proc. Combust. Inst.36(2), 2791–2798 (2017). 10.1016/j.proci.2016.09.027 [DOI] [Google Scholar]
- 21.Sugihara H., Noguchi K., Yoshihara K., Sato Y. Development and Field-Blasting Test of Water-Resistant Granular Explosive. Third EFEE World Conf. on Explosives and Blasting. Proc. Conf., 3rd, Brighton. 557–561. (2005).
- 22.Akers SA, Weed R, Rickman DD, Danielson K. Numerical Simulations of Explosive Wall Breaching, Proceedings of the Users Group Conference. Computer Society. (2005).
- 23.Biegańska, J. & Barański, K. Wpływ dodatków odpadowych tworzyw sztucznych na parametry energetyczne materiałów wybuchowych typu ANFO. Mater. Wysokoenergetyczne9, 145–158 (2017). 10.22211/matwys/0153 [DOI] [Google Scholar]
- 24.Biegańska, J., Barański, K., Hebda, K. & Pytlik, M. Thermodynamic assessment of the impact of selected plastics on the energy parameters of explosives. Energies15, 9583 (2022). 10.3390/en15249583 [DOI] [Google Scholar]
- 25.Biegańska, J., Barański, K. & Tumen-Ulzii, G. Wpływ dodatku tworzyw sztucznych na skład produktów gazowych powstałych po detonacji materiału wybuchowego typu ANFO. Mater. Wysokoenergetyczne10, 5–12 (2018). 10.22211/matwys/0164 [DOI] [Google Scholar]
- 26.Filler, W. S. Post Detonation and Thermal Studies of Solid High Explosives in a Closed Chamber. 6th International Symposium on Coal Combustion, Yale Unversiity, New Haven 1956, 648–657. Accessed 15 Mar 2023. https://www.sciencedirect.com/science/article/abs/pii/S0082078457800903 (2023).
- 27.Jitea, I. C. et al. Computational study in the civil use explosives area. In 14th Geo Conference on Science and Technologies in Geology 139–145 (Exploration and Mining (SGEM), 2014). [Google Scholar]
- 28.Louw, M. J., Sarracino, R. S. & Vather, S. M. A comparison of the theoretical and measured velocities of detonation for selected explosives. J. S. Afr. Inst. Min. Metall. Jun.93(6), 147–153 (1993). [Google Scholar]
- 29.Lu, M. & Lu, C. A computer model for formulation of ANFO Explosives. Sci. Tech. Energ. Mater.68(4), 117–119 (2007). [Google Scholar]
- 30.Rus, D.-C., Miron, C., Miclea, O., Ilici, C. & Grece, M. Computerized simulations and modelling for evaluation of ballistic and security parameters of explosives for civil use. MATEC Web Conf.373, 00047 (2022). 10.1051/matecconf/202237300047 [DOI] [Google Scholar]
- 31.Sochet, I., Gardebas, D., Calderara, S., Marchal, Y. & Longuet, B. Blast wave parameters for spherical explosives detonation in free air. Open J. Saf. Sci. Technol.1(2), 31–42 (2011). 10.4236/ojsst.2011.12004 [DOI] [Google Scholar]
- 32.Biessikirski, A. et al. Research on the possible application of polyolefin waste-derived pyrolysis oils for ANFO manufacturing. Energies14(1), 172 (2021). 10.3390/en14010172 [DOI] [Google Scholar]
- 33.Hussain, G. & Rees, G. Combustion of NH4NO3 and carbon-based mixtures. Fuel72, 1475–1479 (1993). 10.1016/0016-2361(93)90003-K [DOI] [Google Scholar]
- 34.Izato, Y., Miyake, A. & Date, S. Combustion characteristics of ammonium nitrate and carbon mixtures based on thermal decomposition mechanism. Propellants Explos. Pyrotech.38(1), 129–135 (2013). 10.1002/prep.201100106 [DOI] [Google Scholar]
- 35.Lurie, B. A. & Lianshen, C. Kinetics and mechanism of thermal decomposition of ammonium nitrate powder under the action of carbon black. Combust. Explos. Shock Waves36, 607–617 (2000). 10.1007/BF02699524 [DOI] [Google Scholar]
- 36.Maranda, A., Papliński, A. & Gałęzowski, D. Investigation on detonation and thermochemical parameters of aluminized ANFO. J. Energ. Mater.21, 1–13 (2003). 10.1080/07370650305585 [DOI] [Google Scholar]
- 37.Martel, R. et al. Carbon monoxide poisoning associated with blasting operations close to underground enclosed spaces. Part 1. CO production and migrating mechanisms. Can. Geotech. J.41, 371–382 (2004). 10.1139/t04-001 [DOI] [Google Scholar]
- 38.Miyake, A. et al. Detonation characteristics of ammonium nitrate and activated carbon mixtures. J. Loss Prev. Process Ind.20(4–6), 584–588 (2007). 10.1016/j.jlp.2007.04.026 [DOI] [Google Scholar]
- 39.Miyake, A., Kobayashi, H., Echigoya, H. & Ogawa, T. Combustion and ignition properties of ammonium nitrate and activated carbon mixtures. Int. J. Energ. Mater. Chem. Propuls.8, 411–419 (2009). [Google Scholar]
- 40.Oxley, J. C., Smith, J. L. & Wang, W. Compatibility of ammonium nitrate with monomolecular explosives 2. Nitroarenes12. J. Phys. Chem.98, 3901 (1994). 10.1021/j100065a054 [DOI] [Google Scholar]
- 41.Pat. U.S. No. 5,041,177. Hajto EA, Christopher J, Reckzin ED. Ammonium Nitrate/Fuel Oil Blasting Explosive Having Decreased Oil Segregation. https://patents.google.com/patent/US5041177A/en (1991).
- 42.Pat. US No. 4,736,683. Bachman HE, Totman RS. Dry Ammonium Nitrate Blasting Agents. https://patents.google.com/patent/US4736683A/en (1988).
- 43.Revey, G. F. Practical methods to control explosives losses and reduce ammonia and nitrate levels in mine water. Min. Eng.48(7), 61–65 (1996). [Google Scholar]
- 44.Sapko, M., Rowland, J. H., Mainiero, R. & Zlochower, I. Chemical and physical factors that influence NOx production during blasting—Exploratory study. In Proceedings of 28th Annual Conference on Explosives and Blasting Technique 317–329 (ISEE, 2002). [Google Scholar]
- 45.Sharma PD. Priming of Explosives for Efficient Blasting, Mining and Blasting. https://miningandblasting.wordpress.com/2013/05/14/priming-of-explosives/ (2013).
- 46.Sinditskii, V. P., Egorshev, V. Y., Levshenkov, A. I. & Serushkin, V. V. Ammonium nitrate: Combustion mechanism and the role of additives. Propellants Explos. Pyrotech.30, 269–280 (2005). 10.1002/prep.200500017 [DOI] [Google Scholar]
- 47.Wang, L. et al. Conductive carbon microfibers derived from wet-spun lignin/nanocellulose hydrogels. ACS Sustain. Chem. Eng.7, 6013–6022 (2019). 10.1021/acssuschemeng.8b06081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zawadzka-Małota, I. & Maranda, A. The effect of chemical and physical properties and particle morphology of granulated ammonium nitrate on the composition of post-explosion gases and detonation velocity of ammonium nitrate fuel oils. High Energy Mater.13, 157–166 (2021). [Google Scholar]
- 49.Zygmunt, B. Detonation parameters of mixtures containing ammonium nitrate and aluminium. Cent. Eur. J. Energ. Mater.6, 57–66 (2009). [Google Scholar]
- 50.Ananthakumar, S., Jayabal, S. & Thirumal, P. Investigation on performance, emission, and combustion characteristics of variable compression engine fuelled with diesel, waste plastics oil blends. J. Braz. Soc. Mech. Sci. Eng.39, 19–28 (2017). 10.1007/s40430-016-0518-6 [DOI] [Google Scholar]
- 51.Jana, S. K., Pattanayak, S., Bhausaheb, M. S., Ruidas, BCh. & Pal, D. B. Pyrolysis of waste plastic to fuel conversion for utilization in internal combustion engine. Chem. Chem. Technol.17(2), 438–449 (2023). 10.23939/chcht17.02.438 [DOI] [Google Scholar]
- 52.Khan, M. Z. H., Sultana, M., Al-Mamun, M. R. & Hasan, M. R. Pyrolytic waste plastic oil and its diesel blend: Fuel characterization. J. Environ. Public Health8, 1–6 (2016). 10.1155/2016/7869080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar, S., Ramakrishnan, P., Murugan, S. & Singh, R. K. Performance, and emission analysis of blends of waste plastic oil obtained by catalytic pyrolysis of waste HDPE with diesel in a CI engine. Energy Convers. Manag.74, 323–331 (2013). 10.1016/j.enconman.2013.05.028 [DOI] [Google Scholar]
- 54.Owusu, P. A., Banadda, N. E., Zziwa, A., Seay, J. & Kiggundu, N. Reverse engineering of plastic waste into useful fuel products. J. Anal. Appl. Pyrolysis130, 285–293 (2018). 10.1016/j.jaap.2017.12.020 [DOI] [Google Scholar]
- 55.Geyer, R., Jambeck, J. R. & Law, K. L. Production, use and fate of all plastics ever made. Sci. Adv.3(7), e1700782 (2017). 10.1126/sciadv.1700782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DNRM (Department of Natural Resources and Mines). Queensland Guidance Note QGN 20 v 3.5. Management of Oxides of Nitrogen in Open Cut Blasting. https://www.resources.qld.gov.au/__data/assets/pdf_file/0010/212500/qld-guidance-note-20-mgmt-oxides-nitrogen.pdf. Accessed 09 Feb 2017. (2017).
- 57.NIOSH. Pocket Guide to Chemical Hazards: Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health—Softcover 2007. ISBN 10:1470098857. https://stacks.cdc.gov/view/cdc/21265 (2007).
- 58.Brochu S. Assessment of ANFO on the Environment. Technical Investigation 09–01. Defence R&D Canada—Valcartier Technical Memorandum DRDC Valcartier TM 2009–195. https://apps.dtic.mil/sti/tr/pdf/ADA593200.pdf (2010).
- 59.Mahadevan, E. G. Ammonium nitrate explosives for civil applications. In Slurries, Emulsions and Ammonium Nitrate Fuel Oils (Wiley-VCH, 2013). [Google Scholar]
- 60.NIOSH. Pocket Guide to Chemical Hazards: Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health—Softcover 2007. ISBN 10:1470098857. (2007).
- 61.Liu, J. Liquid Explosives (Springer-Verlag, 2015). [Google Scholar]
- 62.Oluwoye, I. et al. Thermal reduction of NOx with recycled plastics. Environ. Sci. Technol.51(13), 7714–7722 (2017). 10.1021/acs.est.6b05560 [DOI] [PubMed] [Google Scholar]
- 63.AEISG. Prevention and Management of Blast Generated NOx Gases in Surface Blasting. Code of Practice. Australian Explosives Industry and Safety Group Inc. https://aeisg.org.au/aeisg-codes-of-practice, https://www.aeisg.org.au/wp-content/uploads/aeisg_cop_nox_edition_02aug2011.pdf. (2011).
- 64.Urbański, T. Chemistry and Technology of Explosives (Pergamon Press, 1964). [Google Scholar]
- 65.Djerdjev, A. M. et al. The mechanism of the spontaneous detonation of ammonium nitrate in reactive grounds. J. Environ. Chem. Eng.6(1), 281–288 (2018). 10.1016/j.jece.2017.12.003 [DOI] [Google Scholar]
- 66.Oxley, J. C., Smith, J. L., Rogers, E. & Yu, M. Ammonium nitrate: Thermal stability and explosivity modifiers. Thermochim. Acta384, 23–45 (2002). 10.1016/S0040-6031(01)00775-4 [DOI] [Google Scholar]
- 67.Oluwoye, I., Altarawneh, M., Gore, J. & Dlugogorski, B. Z. Oxidation of crystalline polyethylene. Combust. Flame162(10), 3681–3690 (2015). 10.1016/j.combustflame.2015.07.007 [DOI] [Google Scholar]
- 68.Oluwoye, I., Altarawneh, M., Gore, J., Bockhorn, H. & Dlugogorski, B. Z. Oxidation of polyethylene under corrosive NOx atmosphere. J. Phys. Chem. C120(7), 3766–3775 (2016). 10.1021/acs.jpcc.5b10466 [DOI] [Google Scholar]
- 69.Oluwoye, I., Dlugogorski, B. Z., Gore, J., Oskierski, H. C. & Altarawneh, M. Atmospheric emission of NOx from mining explosives: A critical review. Atmos. Environ.167, 81–96 (2017). 10.1016/j.atmosenv.2017.08.006 [DOI] [Google Scholar]
- 70.Škrlec, V., Sućeska, M., Dobrilović, M. & Vincek, J. Detonability of ammonium nitrate mixtures with the addition of organic materials. Appl. Sci.14(4), 1580 (2024). 10.3390/app14041580 [DOI] [Google Scholar]
- 71.Wilson JM, Moxon NT. The Development of a Low Shock Energy Ammonium Nitrate Based Explosive. In Proceedings of the Second Large Open Pit Mining Conference, Latrobe Valley, Australia. 39–43. (1989).
- 72.Weiguo Q, Bo L, Yonghua Y, Jie Z, Yu Q, Xueyang L, Wujun L. Ammonium nitrate explosive containing plastic micropowder and preparation method thereof. Patent: CN 111302874A. https://worldwide.espacenet.com/patent/search/family/071158863/publication/CN111302874A?q=CN%20111302874%20A (2020).
- 73.Ilici, S., Gheorghiosu, E., Rus, D. & Jitea, C. Research in the field of evaluation of ANFO explosive preparation installations, tests and results. MATEC Web Conf.342, 01003 (2021). 10.1051/matecconf/202134201003 [DOI] [Google Scholar]
- 74.Bohanek, V., Sućeska, M., Dobrilović, M. & Hartlieb, P. Effect of confinement on detonation velocity and plate dent test results for ANFO explosive. Energies15(12), 4404 (2022). 10.3390/en15124404 [DOI] [Google Scholar]
- 75.Bohanek, V., Sućeska, M., Dobrilović, I. & Pleše, P. Influence of confining materials on detonation parameters of ANFO explosive. Rud. Geol. Naft. Zb.39(1), 35–44 (2024). [Google Scholar]
- 76.Esen, S. A statistical approach to predict the effect of confinement on the detonation velocity of commercial explosives. Rock Mech. Rock Eng.37(4), 317–330 (2004). 10.1007/s00603-004-0026-3 [DOI] [Google Scholar]
- 77.Jackson, S. I., Kiyanda, C. B. & Short, M. Experimental observations of detonation in ammonium-nitrate-fuel-oil (ANFO) surrounded by a high-sound-speed, shockless, aluminum confiner. Proc. Combust. Inst.33(2), 2219–2226 (2011). 10.1016/j.proci.2010.07.084 [DOI] [Google Scholar]
- 78.Short, M. & Jackson, S. I. Dynamics of high sound-speed metal confiners driven by non-ideal high-explosive detonation. Combust. Flame162(5), 1857–1867 (2015). 10.1016/j.combustflame.2014.12.007 [DOI] [Google Scholar]
- 79.PN-C-86037:2000: Explosives—Determination of Explosive Strength in a Lead Block, Polish Committee for Standardization, Warsaw (2000).
- 80.Biessikirski, A. et al. Influence of the ammonium nitrate(V) porous prill assortments and absorption index on ammonium nitrate fuel oil blasting properties. Energies13, 3763 (2020). 10.3390/en13153763 [DOI] [Google Scholar]
- 81.Pytlik, M. et al. The influence of microstructured charcoal additive on ANFO’s properties. Energies14(14), 4354 (2021). 10.3390/en14144354 [DOI] [Google Scholar]
- 82.Chandran, M., Tamilkolundu, S. & Murugesan, Conversion of plastic waste to fuel. In Plastic Waste and Recycling 385–401 (Elsevier Inc., 2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.