Abstract
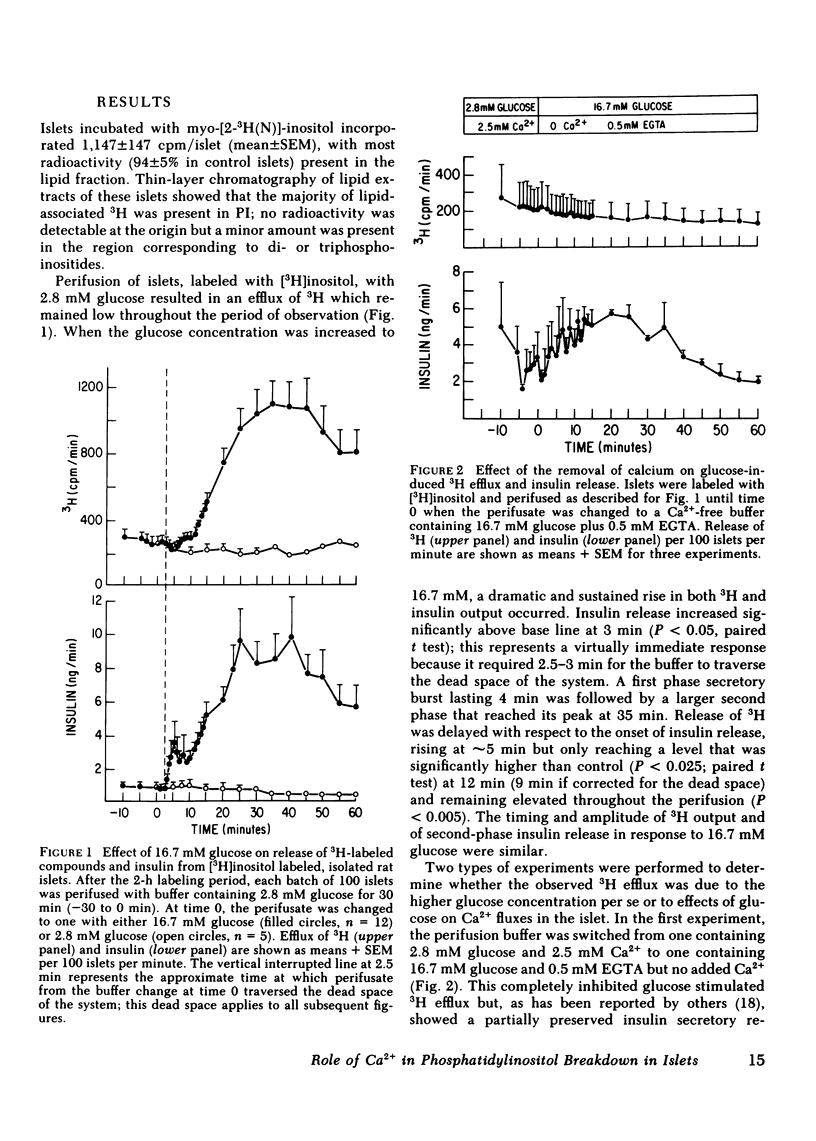
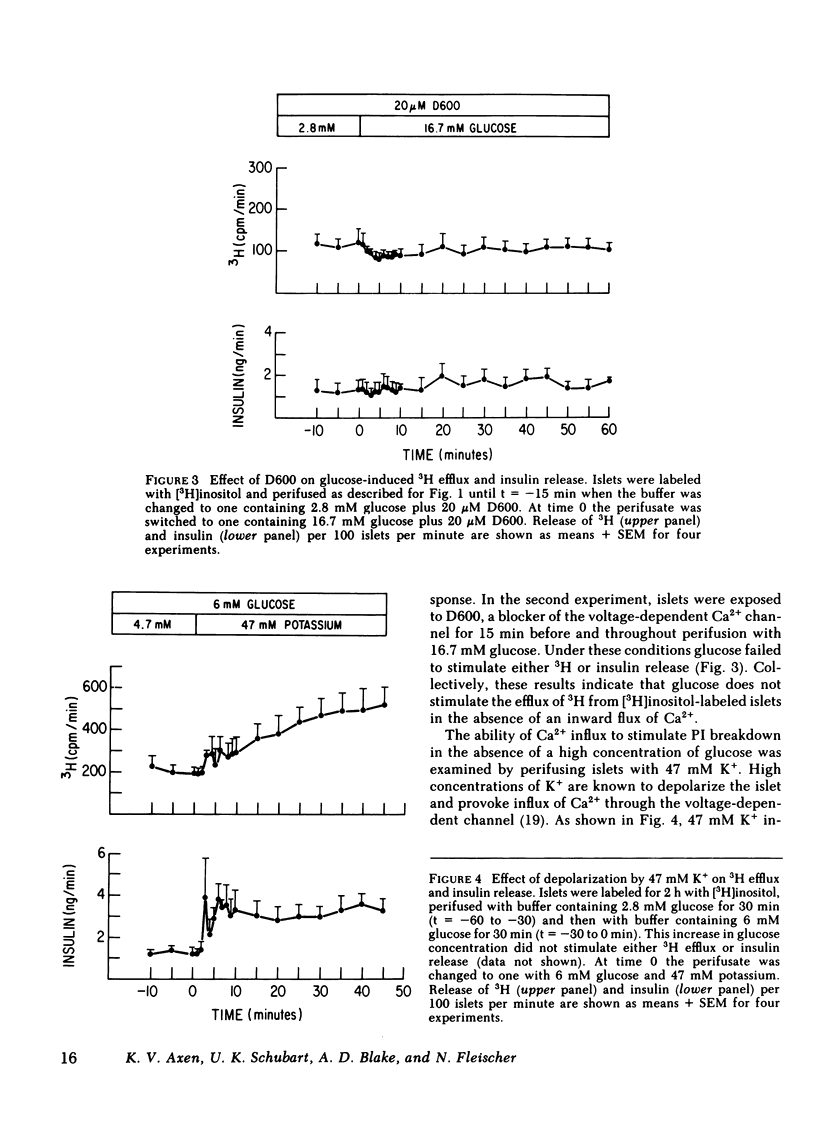
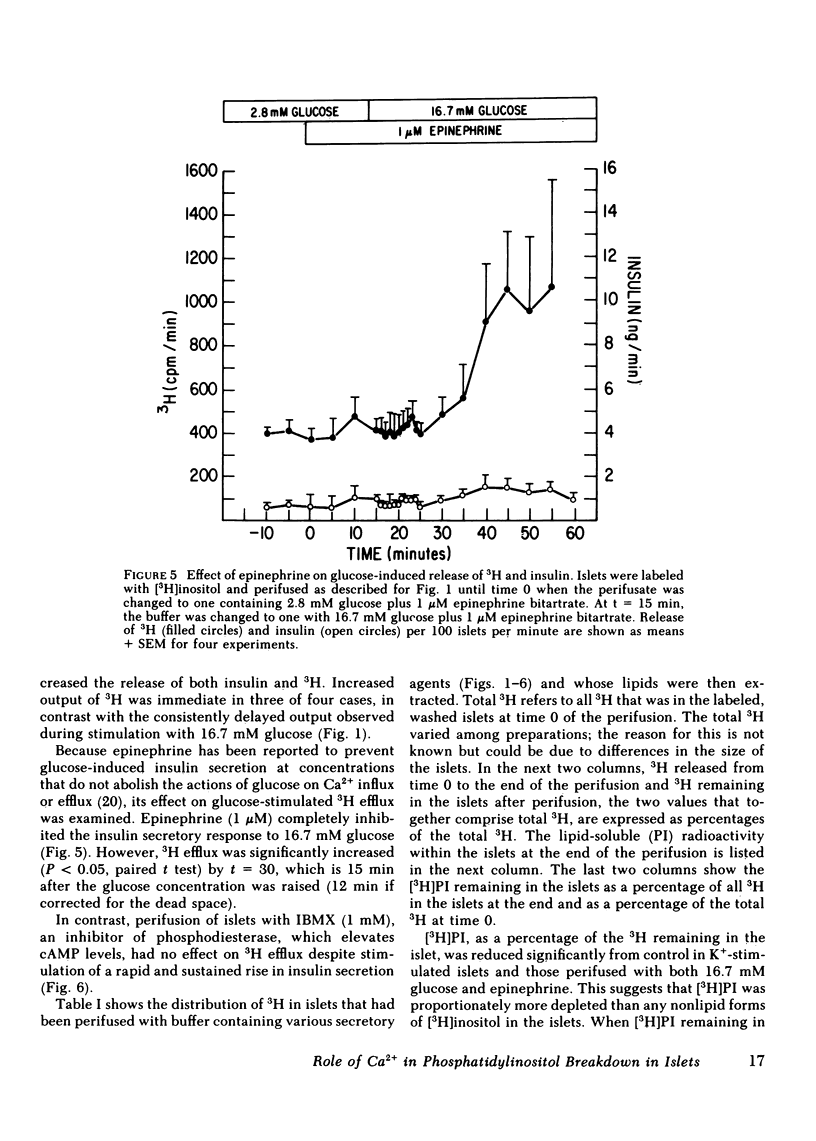
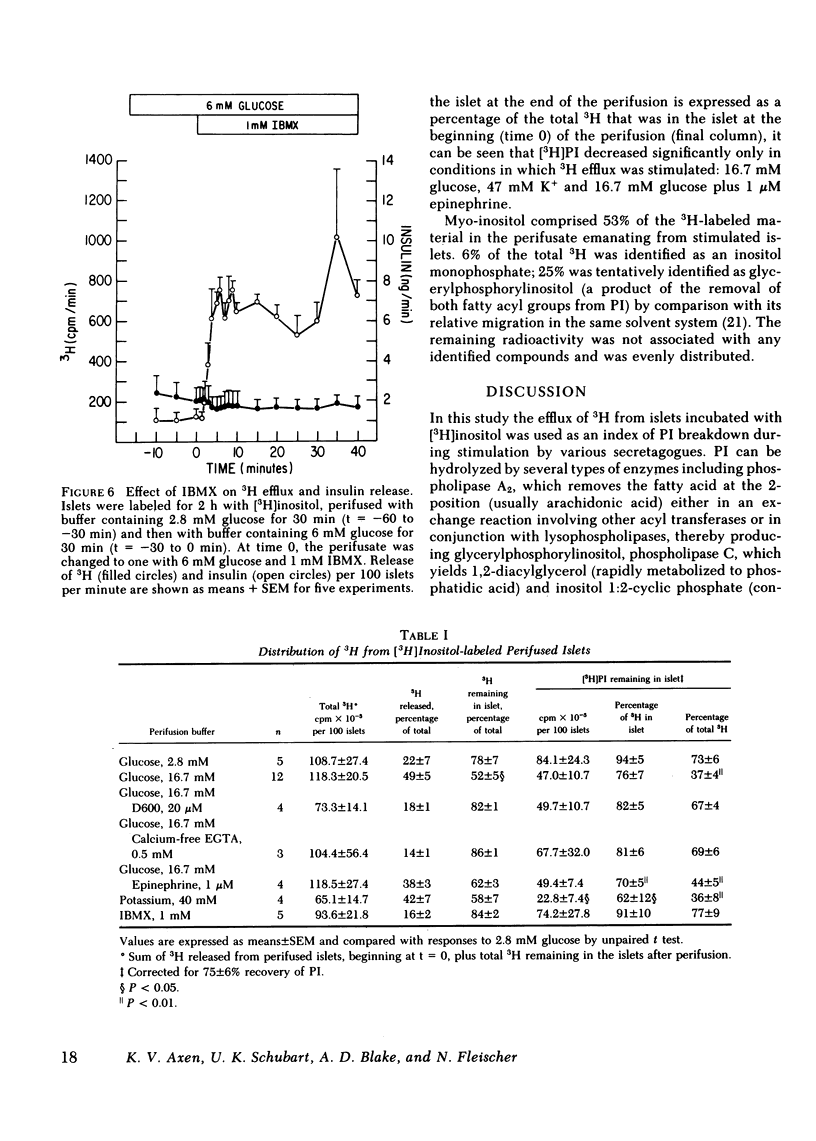
Breakdown of phosphatidylinositol (PI) has been shown to be increased during Ca2+-mediated stimulation of cellular responses in many systems and has been proposed to be involved in stimulus-secretion coupling. The effects on PI breakdown of insulin secretagogues that alter cellular Ca2+ or cyclic (c)AMP levels were investigated in perifused rat islets of Langerhans. Isolated islets were labeled with myo-[2-3H(N)]inositol and the efflux of 3H-labeled metabolites was monitored. Glucose (16.7 mM) greatly increased 3H release in a manner that paralleled the second phase of the insulin secretory response; by 60 min, the amount of [3H]PI in the islet decreased by 50%. Removal of Ca2+ from the perifusate or blockade of Ca2+ entry through the voltage-dependent channels by D600 (20 microM) abolished the glucose-induced increase in 3H efflux. Depolarization with 47 mM K+, which increases Ca2+ entry, stimulated protracted 3H and insulin release. Glucose-stimulated output of 3H was not prevented by epinephrine (1 microM) even though the insulin response was abolished. In contrast, 3H output was not affected by isobutylmethylxanthine (1 mM), known to raise cellular levels of cAMP, although insulin release was stimulated. These findings indicate that PI breakdown is not related to the exocytotic process since stimulation of insulin release and PI breakdown could be uncoupled, and that it is not associated with cAMP-mediated regulation of insulin release. PI breakdown in islets differs from the immediate, transient phenomenon reported in other systems in both its timing and requirement for Ca2+. It appears to result from the entry of Ca2+ and not to be the mechanism by which glucose initiates Ca2+ influx.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Brisson G. R., Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. VII. A proposed site of action for adenosine-3',5'-cyclic monophosphate. J Clin Invest. 1972 Feb;51(2):232–241. doi: 10.1172/JCI106808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements R. S., Jr, Evans M. H., Pace C. S. Substrate requirements for the phosphoinositide response in rat pancreatic islets. Biochim Biophys Acta. 1981 Apr 17;674(1):1–9. doi: 10.1016/0304-4165(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Rhoten W. B. Phosphoinositide metabolism and insulin secretion from isolated rat pancreatic islets. J Clin Invest. 1976 Mar;57(3):684–691. doi: 10.1172/JCI108325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. The dependence on Ca2+ of phosphatidylinositol breakdown and enzyme secretion in rabbit neutrophils stimulated by formylmethionyl-leucylphenylalanine or ionomycin. Biochem J. 1981 Dec 15;200(3):501–508. doi: 10.1042/bj2000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Clarke N. D-myoinositol 1:2-cyclic phosphate 2-phosphohydrolase. Biochem J. 1972 Mar;127(1):113–118. doi: 10.1042/bj1270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa K., Sacktor B., Takenawa T. Ca2+-dependent and Ca2+-independent degradation of phosphatidylinositol in rabbit vas deferens. Biochem J. 1981 Jan 15;194(1):129–136. doi: 10.1042/bj1940129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg F., Jr D-myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J Biol Chem. 1967 Apr 10;242(7):1375–1382. [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Sabir M. A. Effects of Ca2+ ionophore A23187 and Ca2+ deficiency on pancreatic phospholipids and amylase release in vitro. Biochim Biophys Acta. 1980 Dec 15;633(3):479–484. doi: 10.1016/0304-4165(80)90205-6. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Sabir M. A. Insulin and its secretagogues activate Ca2+-dependent phosphatidylinositol breakdown and amylase secretion in rat pancreas in vitro. Diabetes. 1981 May;30(5):396–401. doi: 10.2337/diab.30.5.396. [DOI] [PubMed] [Google Scholar]
- Fex G., Lernmark A. Effects of insulin secretagogues on phospholipid metabolism in pancreatic beta-cells. Biochim Biophys Acta. 1975 Apr 18;388(1):1–4. doi: 10.1016/0005-2760(75)90055-7. [DOI] [PubMed] [Google Scholar]
- Freinkel N., El Younsi C., Dawson M. C. Inter-relations between the phospholipids of rat pancreatic islets during glucose stimulation, and their response to medium inositol and tetracaine. Eur J Biochem. 1975 Nov 1;59(1):245–252. doi: 10.1111/j.1432-1033.1975.tb02448.x. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Relative importance of extracellular and intracellular calcium for the two phases of glucose-stimulated insulin release: studies with theophylline. Endocrinology. 1978 Mar;102(3):723–730. doi: 10.1210/endo-102-3-723. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hui D. Y., Harmony J. A. Phosphatidylinositol turnover in mitogen-activated lymphocytes. Suppression by low-density lipoproteins. Biochem J. 1980 Oct 15;192(1):91–98. doi: 10.1042/bj1920091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Ui M. Islet-activating protein. Enhanced insulin secretion and cyclic AMP accumulation in pancreatic islets due to activation of native calcium ionophores. J Biol Chem. 1979 Jan 25;254(2):469–479. [PubMed] [Google Scholar]
- Kikuchi M., Wollheim C. B., Cuendet G. S., Renold A. E., Sharp G. W. Studies on the dual effects of glucose on 45Ca++ efflux from isolated rat islets. Endocrinology. 1978 May;102(5):1339–1349. doi: 10.1210/endo-102-5-1339. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Laychock S. G. Fatty acid incorporation into phospholipids of isolated pancreatic islets of the rat. Relationship to insulin release. Diabetes. 1983 Jan;32(1):6–13. doi: 10.2337/diab.32.1.6. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Oberleas D. The determination of phytate and inositol phosphates. Methods Biochem Anal. 1971;20:87–101. doi: 10.1002/9780470110393.ch3. [DOI] [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. Phosphatidylinositol breakdown induced by vasopressin and epinephrine in hepatocytes is calcium-dependent. J Biol Chem. 1982 Oct 10;257(19):11323–11331. [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. myo-Inositol uptake and metabolism in isolated rat liver cells. J Biol Chem. 1982 Oct 10;257(19):11315–11322. [PubMed] [Google Scholar]
- Siegel E. G., Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. Dependency of cyclic AMP-induced insulin release on intra- and extracellular calcium in rat islets of Langerhans. J Clin Invest. 1980 Feb;65(2):233–241. doi: 10.1172/JCI109665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. G., Wollheim C. B., Sharp G. W. Glucose-induced first phase insulin release in the absence of extracellular Ca2+ in rat islets. FEBS Lett. 1980 Jan 14;109(2):213–215. doi: 10.1016/0014-5793(80)81089-1. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. Somatostatin- and epinephrine-induced modifications of 45Ca++ fluxes and insulin release in rat pancreatic islets maintained in tissue culture. J Clin Invest. 1977 Nov;60(5):1165–1173. doi: 10.1172/JCI108869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. The roles of intracellular and extracellular Ca++ in glucose-stimulated biphasic insulin release by rat islets. J Clin Invest. 1978 Aug;62(2):451–458. doi: 10.1172/JCI109146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Siegel E. G., Kikuchi M., Renold A. E., Sharp G. W. The role of extracellular Ca++ and islet calcium stores in the regulation of biphasic insulin release. Horm Metab Res Suppl. 1980;Suppl 10:108–115. [PubMed] [Google Scholar]


