Abstract
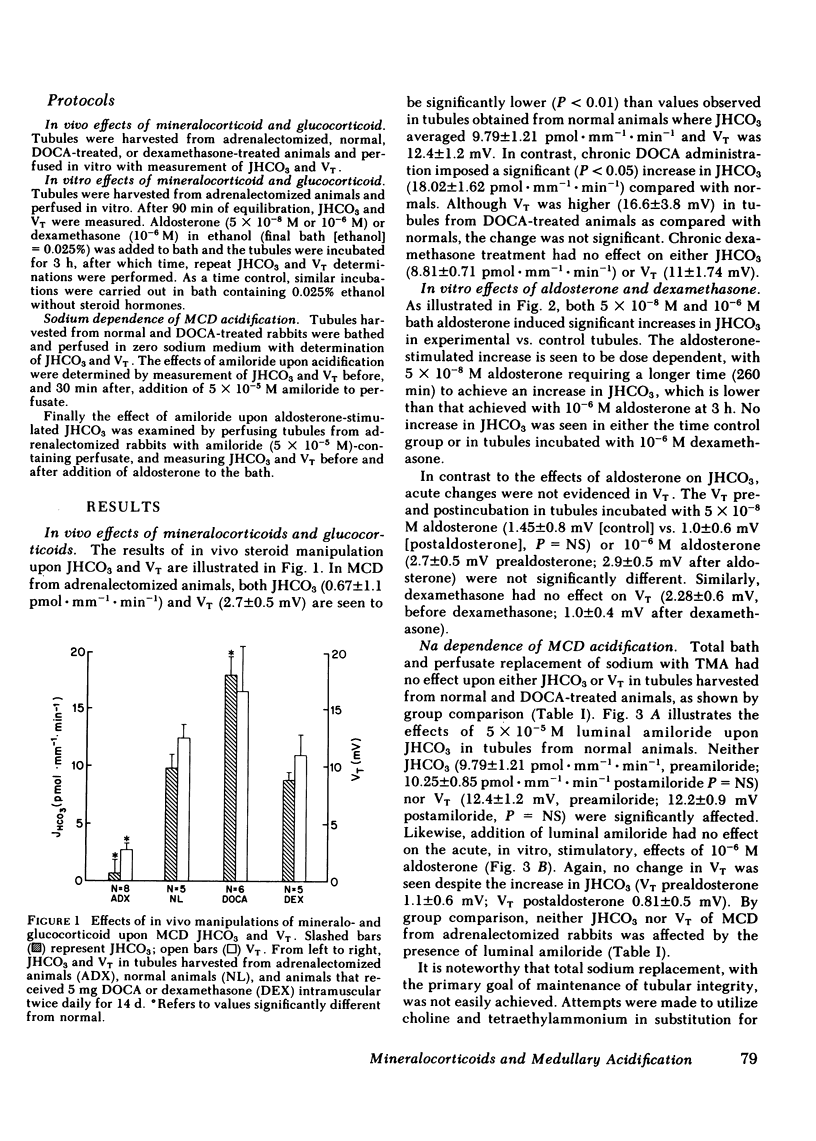
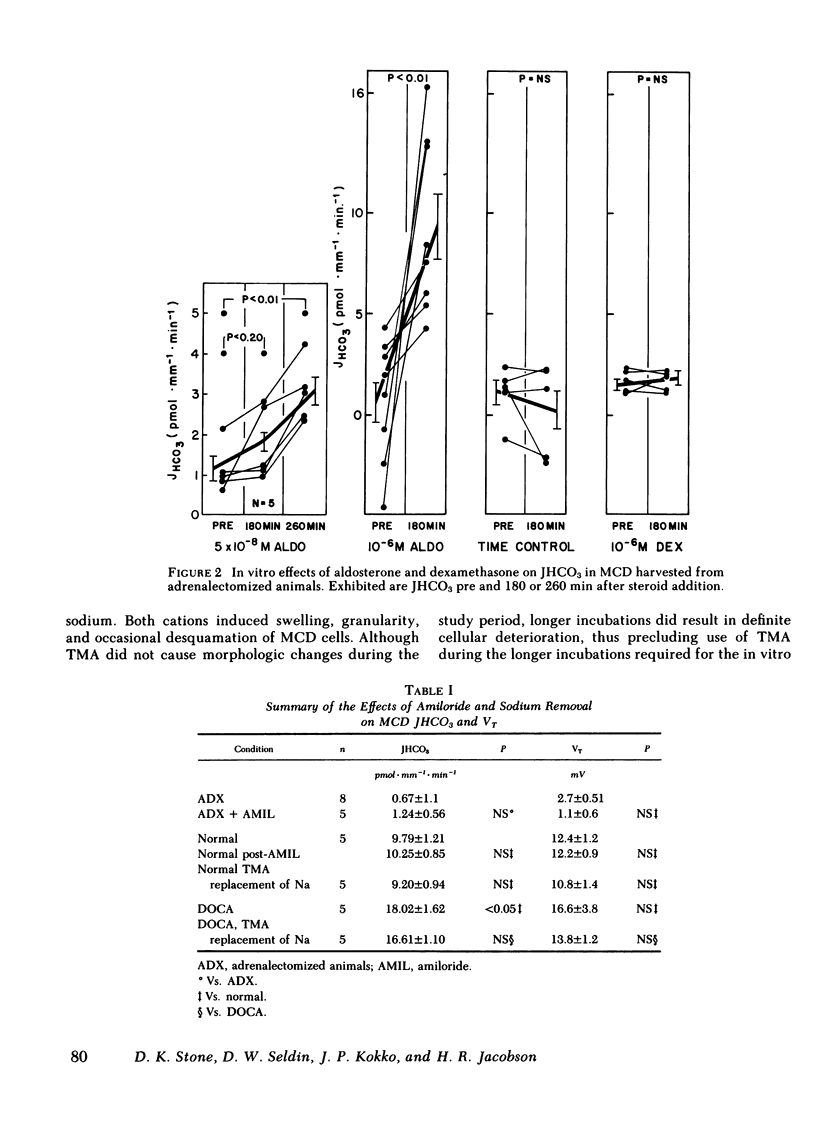
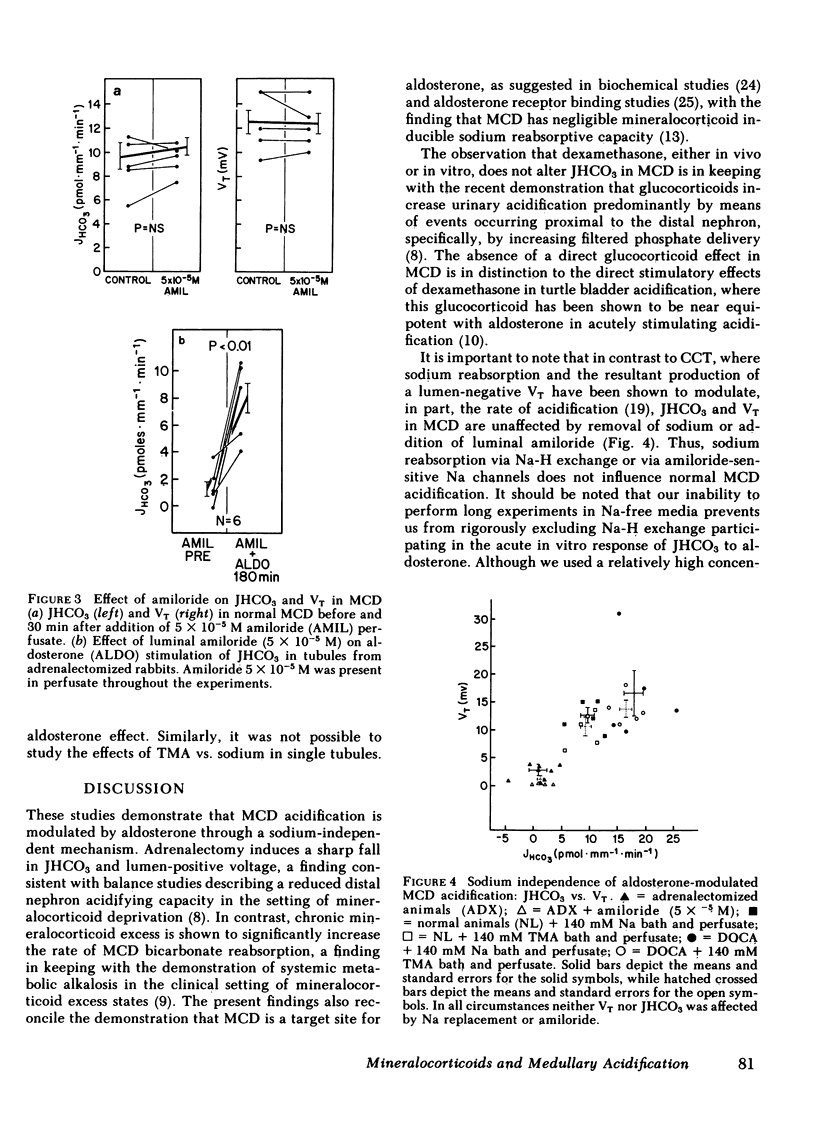
Rabbit medullary collecting duct (MCD) from inner stripe of outer medulla has been identified as a major distal nephron acidification site. The isolated, perfused tubule technique was used to examine the roles of mineralocorticoid and glucocorticoid in regulation of MCD acidification. Surgical adrenalectomy reduced bicarbonate reabsorptive rate (JHCO3, pmol X mm-1 X min-1) from the normal of 9.79 +/- 1.21 to 0.67 +/- 1.1. Chronic administration of deoxycorticosterone acetate (DOCA) increased JHCO3 of MCD significantly to 18.02 +/- 1.62 whereas chronic dexamethasone administration did not affect JHCO3. The direct effects of aldosterone and dexamethasone upon MCD acidification were examined by perfusing tubules harvested from adrenalectomized rabbits in the presence of aldosterone or dexamethasone. Aldosterone, at 5 X 10(-8) M, increased JHCO3 significantly from 1.27 +/- 0.28 to 3.09 +/- 0.34. At 10(-6) M, aldosterone produced a greater increase in JHCO3 from 0.67 +/- 1.1 to 9.39 +/- 1.59. In vitro dexamethasone treatment had no effect on JHCO3. Studies examining the sodium dependence of aldosterone-stimulated acidification demonstrated that JHCO3 in tubules harvested from normal and deoxycorticosterone acetate-treated animals was unaffected by total replacement of sodium with tetramethylammonium. Likewise, luminal amiloride (5 X 10(-5) M) had no effect on JHCO3 in tubules harvested from adrenalectomized and normal animals. Moreover, the acute, in vitro stimulatory effect of aldosterone was seen to occur in the presence of luminal amiloride. These studies define a mammalian distal nephron segment that possesses major acidifying capacity, which is modulated by mineralocorticoid but independent of luminal sodium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Awqati Q., Norby L. H., Mueller A., Steinmetz P. R. Characteristics of stimulation of H+ transport by aldosterone in turtle urinary bladder. J Clin Invest. 1976 Aug;58(2):351–358. doi: 10.1172/JCI108479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I. Mineralcorticoid receptors along the nephron: [3H]aldosterone binding in rabbit tubules. Am J Physiol. 1981 Dec;241(6):F605–F611. doi: 10.1152/ajprenal.1981.241.6.F605. [DOI] [PubMed] [Google Scholar]
- Gluck S., Kelly S., Al-Awqati Q. The proton translocating ATPase responsible for urinary acidification. J Biol Chem. 1982 Aug 25;257(16):9230–9233. [PubMed] [Google Scholar]
- Grantham J. J., Kurg M. B., Obloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest. 1970 Oct;49(10):1815–1826. doi: 10.1172/JCI106399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. B., Imai M., Kokko J. P. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest. 1975 Jun;55(6):1284–1294. doi: 10.1172/JCI108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulter H. N., Ilnicki L. P., Harbottle J. A., Sebastian A. Impaired renal H+ secretion and NH3 production in mineralocorticoid-deficient glucocorticoid-replete dogs. Am J Physiol. 1977 Feb;232(2):F136–F146. doi: 10.1152/ajprenal.1977.232.2.F136. [DOI] [PubMed] [Google Scholar]
- Hulter H. N., Licht J. H., Glynn R. D., Sebastian A. Renal acidosis in mineralocorticoid deficiency is not dependent on NaCl depletion or hyperkalemia. Am J Physiol. 1979 Mar;236(3):F283–F294. doi: 10.1152/ajprenal.1979.236.3.F283. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R., Kokko J. P. Intrinsic differences in various segments of the proximal convoluted tubule. J Clin Invest. 1976 Apr;57(4):818–825. doi: 10.1172/JCI108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassirer J. P., London A. M., Goldman D. M., Schwartz W. B. On the pathogenesis of metabolic alkalosis in hyperaldosteronism. Am J Med. 1970 Sep;49(3):306–315. doi: 10.1016/s0002-9343(70)80021-3. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Helman S. I. Acidification of luminal fluid by the rabbit cortical collecting tubule perfused in vitro. Am J Physiol. 1982 May;242(5):F521–F531. doi: 10.1152/ajprenal.1982.242.5.F521. [DOI] [PubMed] [Google Scholar]
- Kurtzman N. A., White M. G., Rogers P. W. Aldosterone deficiency and renal bicarbonate reabsorption. J Lab Clin Med. 1971 Jun;77(6):931–940. [PubMed] [Google Scholar]
- Loeb R. F. CHEMICAL CHANGES IN THE BLOOD IN ADDISON'S DISEASE. Science. 1932 Nov 4;76(1975):420–421. doi: 10.1126/science.76.1975.420. [DOI] [PubMed] [Google Scholar]
- Ludens J. H., Fanestil D. D. Aldosterone stimulation of acidification of urine by isolated urinary bladder of the Colombian toad. Am J Physiol. 1974 Jun;226(6):1321–1326. doi: 10.1152/ajplegacy.1974.226.6.1321. [DOI] [PubMed] [Google Scholar]
- MILLS J. N., THOMAS S., WILLIAMSON K. S. The acute effect of hydrocortisone, deoxycorticosterone and aldosterone upon the excretion of sodium, potassium and acid by the human kidney. J Physiol. 1960 May;151:312–331. doi: 10.1113/jphysiol.1960.sp006440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am J Physiol. 1978 Feb;234(2):F141–F145. doi: 10.1152/ajprenal.1978.234.2.F141. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- RELMAN A. S., SCHWARTZ W. B. The effect of DOCA on electrolyte balance in normal man and its relation to sodium chloride intake. Yale J Biol Med. 1952 Jun;24(6):540–558. [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Hansen U. P., Slayman C. L. Role of the plasma membrane proton pump in pH regulation in non-animal cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5903–5907. doi: 10.1073/pnas.78.9.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Schwartz M. J., Kokko J. P. Urinary concentrating defect of adrenal insufficiency. Permissive role of adrenal steroids on the hydroosmotic response across the rabbit cortical collecting tubule. J Clin Invest. 1980 Aug;66(2):234–242. doi: 10.1172/JCI109849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A., Sutton J. M., Hulter H. N., Schambelan M., Poler S. M. Effect of mineralocorticoid replacement therapy on renal acid-base homeostasis in adrenalectomized patients. Kidney Int. 1980 Dec;18(6):762–773. doi: 10.1038/ki.1980.195. [DOI] [PubMed] [Google Scholar]
- Stokes J. B., Ingram M. J., Williams A. D., Ingram D. Heterogeneity of the rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int. 1981 Sep;20(3):340–347. doi: 10.1038/ki.1981.144. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Anion dependence of rabbit medullary collecting duct acidification. J Clin Invest. 1983 May;71(5):1505–1508. doi: 10.1172/JCI110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]
- Wilcox C. S., Cemerikic D. A., Giebisch G. Differential effects of acute mineralo- and glucocorticosteroid administration on renal acid elimination. Kidney Int. 1982 Apr;21(4):546–556. doi: 10.1038/ki.1982.61. [DOI] [PubMed] [Google Scholar]


