Abstract
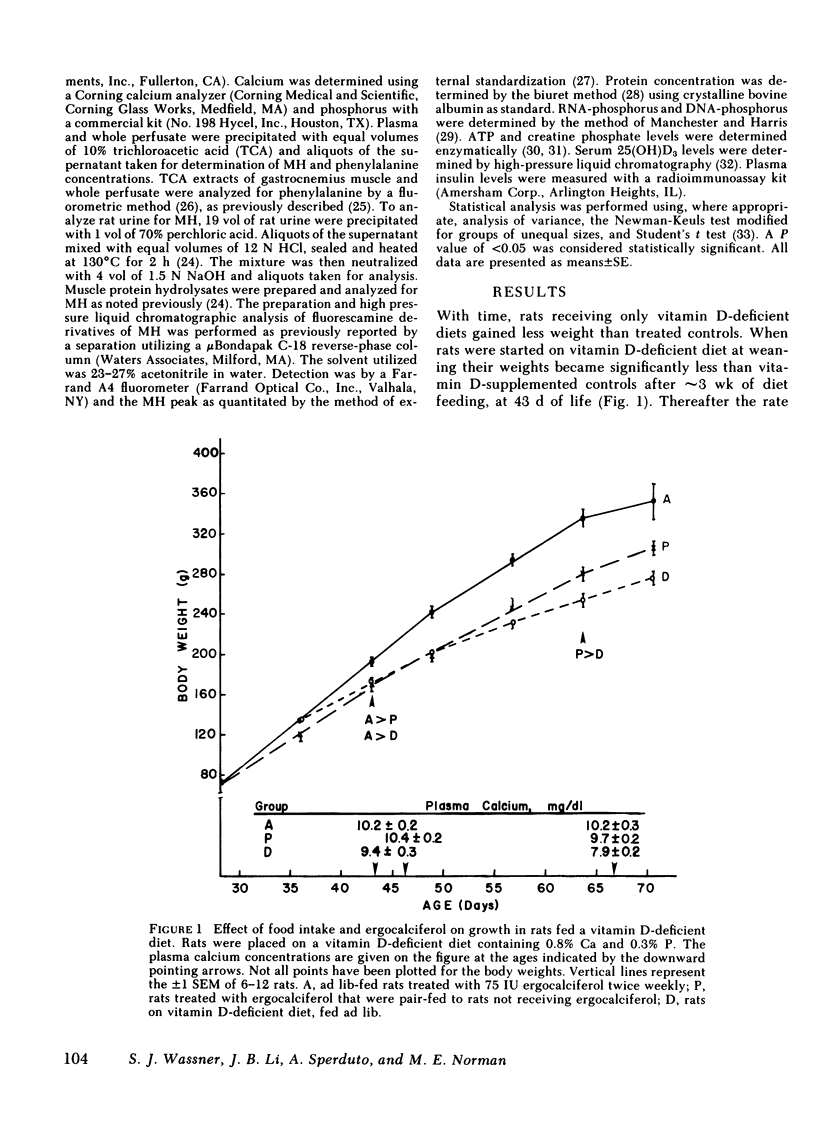
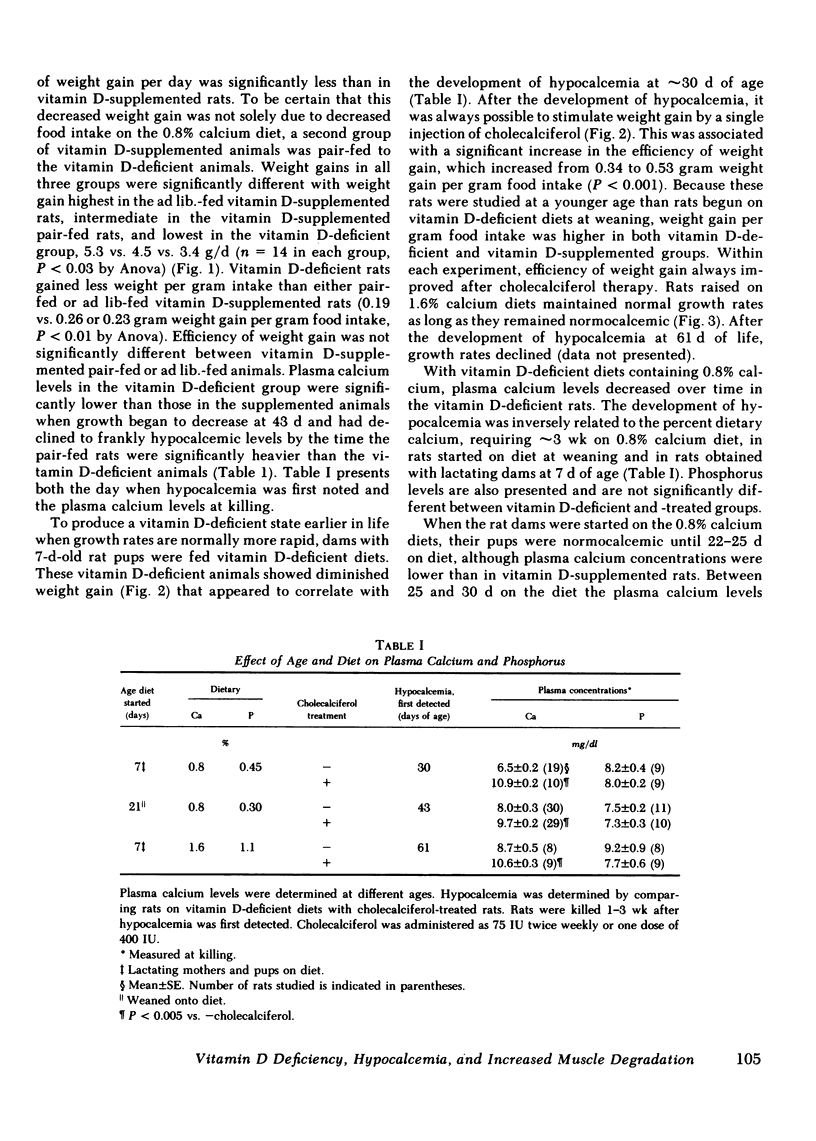
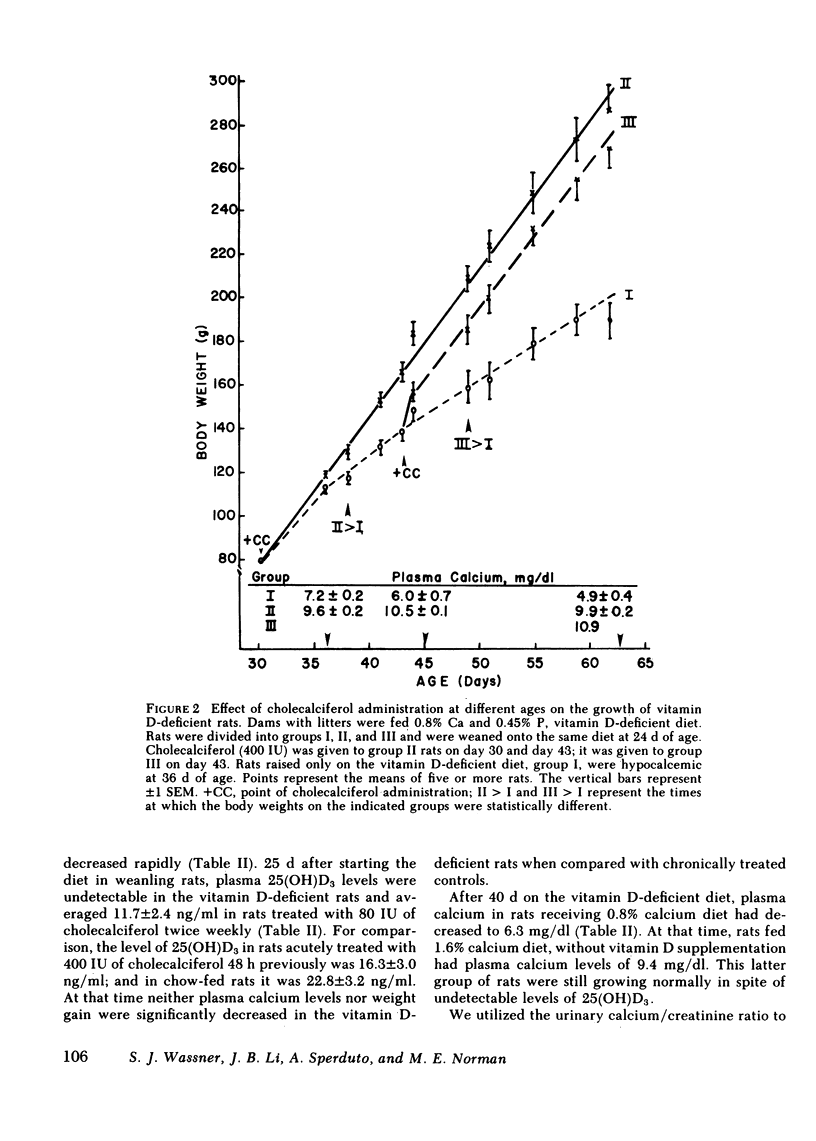
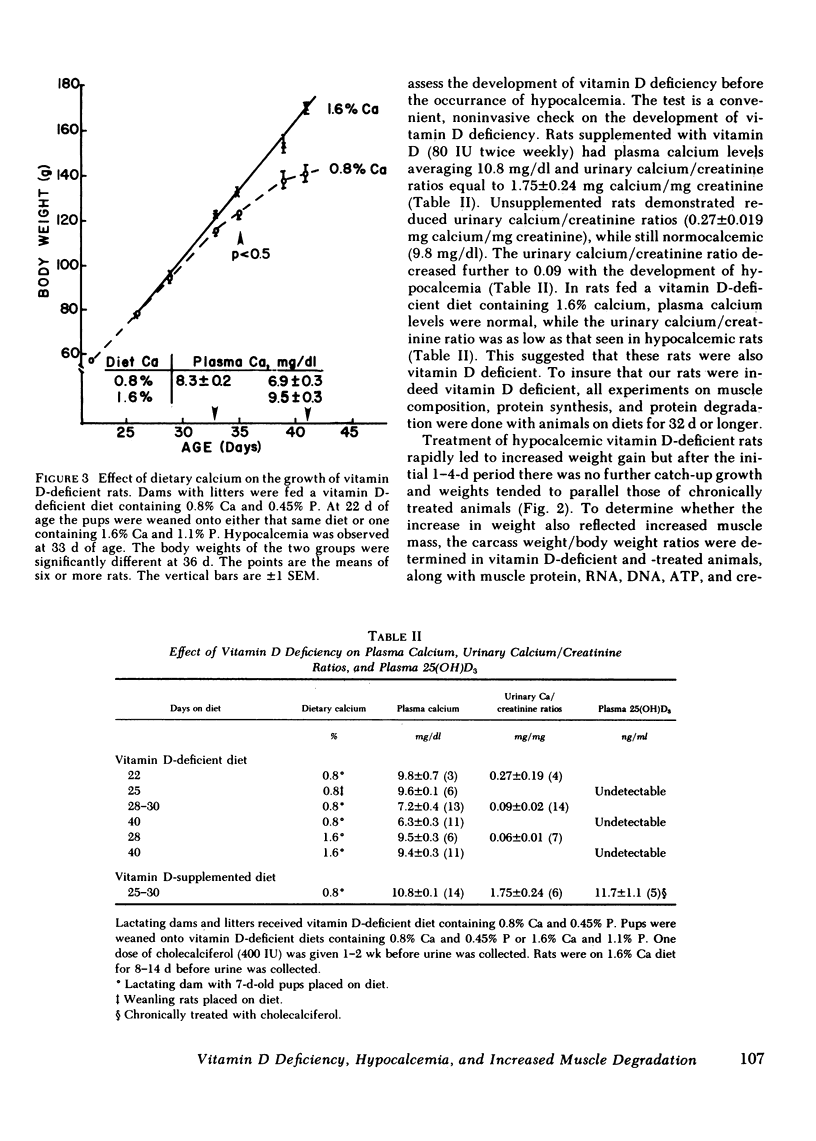
The myopathy associated with vitamin D deficiency was examined in vitamin D-deficient and vitamin D-supplemented rats. When compared with either vitamin D-supplemented ad lib. or pair-fed rats, weight gain and muscle mass were decreased in vitamin D-deficient hypocalcemic animals. With the exception of a modest decrease in muscle creatine phosphate levels, muscle composition was unchanged by vitamin D deficiency. Muscle protein turnover rates were determined in both in vivo and in vitro studies and demonstrated that myofibrillar protein degradation was increased in vitamin D deficiency. Normal growth rates could be maintained be feeding the rats vitamin D-deficient diets containing 1.6% calcium, which maintained plasma calcium within the normal range. In addition to its role in maintaining plasma calcium, vitamin D-supplemented rats had significantly higher levels of the anabolic hormone insulin. Vitamin D supplementation may affect muscle protein turnover by preventing hypocalcemia, as well as directly stimulating insulin secretion, rather than by a direct effect within skeletal muscle.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge S. J., Haddad J. G. 25-hydroxycholecalciferol stimulation of muscle metabolism. J Clin Invest. 1975 Nov;56(5):1100–1107. doi: 10.1172/JCI108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. E., Tsai H. C., Morris R. C., Jr Metabolic acidosis in the vitamin D-deficient chick. Metabolism. 1977 Oct;26(10):1099–1105. doi: 10.1016/0026-0495(77)90036-1. [DOI] [PubMed] [Google Scholar]
- Brion F., Dupuis Y. Calcium and monoamine regulation: role of vitamin D nutrition. Can J Physiol Pharmacol. 1980 Dec;58(12):1431–1434. doi: 10.1139/y80-217. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- Coburn J. W. Renal osteodystrophy. Kidney Int. 1980 May;17(5):677–623. doi: 10.1038/ki.1980.79. [DOI] [PubMed] [Google Scholar]
- Floyd M., Ayyar D. R., Barwick D. D., Hudgson P., Weightman D. Myopathy in chronic renal failure. Q J Med. 1974 Oct;43(172):509–524. [PubMed] [Google Scholar]
- Gedik O., Zileli M. S. Effects of hypocalcemia and theophylline on glucose tolerance and insulin release in human beings. Diabetes. 1977 Sep;26(9):813–819. doi: 10.2337/diab.26.9.813. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem. 1969 Jun 25;244(12):3217–3222. [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Haverberg L. N., Deckelbaum L., Bilmazes C., Munro H. N., Young V. R. Myofibrillar protein turnover and urinary N-tau-methylhistidine output. Response to dietary supply of protein and energy. Biochem J. 1975 Dec;152(3):503–510. doi: 10.1042/bj1520503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Kameyama T., Etlinger J. D. Calcium-dependent regulation of protein synthesis and degradation in muscle. Nature. 1979 May 24;279(5711):344–346. doi: 10.1038/279344a0. [DOI] [PubMed] [Google Scholar]
- Li J. B., Fulks R. M., Goldberg A. L. Evidence that the intracellular pool of tyrosine serves as precursor for protein synthesis in muscle. J Biol Chem. 1973 Oct 25;248(20):7272–7275. [PubMed] [Google Scholar]
- Li J. B., Wassner S. J. Muscle degradation in uremia: 3-methylhistidine release in fed and fasted rats. Kidney Int. 1981 Sep;20(3):321–325. doi: 10.1038/ki.1981.141. [DOI] [PubMed] [Google Scholar]
- Manchester K. L., Harris E. J. Effect of denervation on the synthesis of ribonucleic acid and deoxyribonucleic acid in rat diaphragm muscle. Biochem J. 1968 Jun;108(2):177–183. doi: 10.1042/bj1080177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massry S. G., Goldstein D. A. The search for uremic toxin(s) "X" "X" = PTH. Clin Nephrol. 1979 Apr;11(4):181–189. [PubMed] [Google Scholar]
- Mehls O., Ritz E., Gilli G., Wangdak T., Krempien B. Effect of vitamin D on growth in experimental uremia. Am J Clin Nutr. 1978 Oct;31(10):1927–1931. doi: 10.1093/ajcn/31.10.1927. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Frankel J. B., Heldt A. M., Grodsky G. M. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980 Aug 15;209(4458):823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Oxidation of leucine by rat skeletal muscle. Am J Physiol. 1972 Dec;223(6):1376–1383. doi: 10.1152/ajplegacy.1972.223.6.1376. [DOI] [PubMed] [Google Scholar]
- Pleasure D., Wyszynski B., Sumner A., Schotland D., Feldman B., Nugent N., Hitz K., Goodman D. B. Skeletal muscle calcium metabolism and contractile force in vitamin D-deficient chicks. J Clin Invest. 1979 Nov;64(5):1157–1167. doi: 10.1172/JCI109569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannels D. E., Li J. B., Morgan H. E., Jefferson L. S. Evaluation of hormone effects on protein turnover in isolated perfused organs. Methods Enzymol. 1975;37:238–250. doi: 10.1016/s0076-6879(75)37020-1. [DOI] [PubMed] [Google Scholar]
- Reporter M. 3-methylhistidine metabolism in proteins from cultured mammalian muscle cells. Biochemistry. 1969 Sep;8(9):3489–3496. doi: 10.1021/bi00837a001. [DOI] [PubMed] [Google Scholar]
- Ritz E., Boland R., Kreusser W. Effects of vitamin D and parathyroid hormone on muscle: potential role in uremic myopathy. Am J Clin Nutr. 1980 Jul;33(7):1522–1529. doi: 10.1093/ajcn/33.7.1522. [DOI] [PubMed] [Google Scholar]
- STEENBOCK H., HERTING D. C. Vitamin D and growth. J Nutr. 1955 Dec 10;57(4):449–468. doi: 10.1093/jn/57.4.449. [DOI] [PubMed] [Google Scholar]
- Schott G. D., Wills M. R. Muscle weakness in osteomalacia. Lancet. 1976 Mar 20;1(7960):626–629. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- Shepard R. M., Horst R. L., Hamstra A. J., DeLuca H. F. Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J. 1979 Jul 15;182(1):55–69. doi: 10.1042/bj1820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. G., Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. Dependency of cyclic AMP-induced insulin release on intra- and extracellular calcium in rat islets of Langerhans. J Clin Invest. 1980 Feb;65(2):233–241. doi: 10.1172/JCI109665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströder J. The content of actomyosin in the skeletal muscle of rats with experimentally induced rickets. Helv Paediatr Acta. 1966 Sep;21(4):323–326. [PubMed] [Google Scholar]
- Sugden P. H. The effects of calcium ions, ionophore A23187 and inhibition of energy metabolism on protein degradation in the rat diaphragm and epitrochlearis muscles in vitro. Biochem J. 1980 Sep 15;190(3):593–603. doi: 10.1042/bj1900593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L. C., Buttery P. J. Ntau-Methylhistidine--an index of the true rate of myofibrillar degradation? An appraisal. Life Sci. 1978 Sep 18;23(11):1103–1115. doi: 10.1016/0024-3205(78)90344-2. [DOI] [PubMed] [Google Scholar]
- Wassner S. J., Li J. B. N tau-methylhistidine release: contributions of rat skeletal muscle, GI tract, and skin. Am J Physiol. 1982 Oct;243(4):E293–E297. doi: 10.1152/ajpendo.1982.243.4.E293. [DOI] [PubMed] [Google Scholar]
- Wassner S. J., Orloff S., Holliday M. A. Protein degradation in muscle: response to feeding and fasting in growing rats. Am J Physiol. 1977 Aug;233(2):E119–E123. doi: 10.1152/ajpendo.1977.233.2.E119. [DOI] [PubMed] [Google Scholar]
- Wassner S. J., Schlitzer J. L., Li J. B. A rapid, sensitive method for the determination of 3-methylhistidine levels in urine and plasma using high-pressure liquid chromatography. Anal Biochem. 1980 May 15;104(2):284–289. doi: 10.1016/0003-2697(80)90076-7. [DOI] [PubMed] [Google Scholar]
- Young V. R., Alexis S. D., Baliga B. S., Munro H. N., Muecke W. Metabolism of administered 3-methylhistidine. Lack of muscle transfer ribonucleic acid charging and quantitative excretion as 3-methylhistidine and its N-acetyl derivative. J Biol Chem. 1972 Jun 10;247(11):3592–3600. [PubMed] [Google Scholar]


