The γ-aminobutyric acid (GABA)ergic system is the primary inhibitory system in the central nervous system, functioning to reduce neuronal excitability. Recently, Liang et al. discovered that this system exists in the atrioventricular node (AVN), contributing to the AVN delay that maintains coordinated, sequential atrioventricular contraction and safeguards against fatal arrhythmias.
The discovery of γ-aminobutyric acid (GABA) in 1950 revolutionized our comprehension of the major inhibitory system in the central nervous system (CNS), catalyzing the development of drugs such as benzodiazepines to mitigate overexcitation in the brain.1 GABA mediates its action at the pre- and post-synaptic terminals, where it binds to either the GABAA receptor (GABAAR), an ionotropic receptor, or GABAB receptor (GABABR), a G-protein coupled receptor. Activation of either receptor induces hyperpolarization of the cell, diminishing overall neuronal excitability.2 Although our understanding of the GABAergic system has deepened significantly over the past half-century, the extent to which this system operates beyond the CNS remains elusive.
The heart contains specialized pacemaker cells in the cardiac electrical conduction system that can spontaneously generate action potentials (APs). The atrioventricular node (AVN) is an integral component that resides at the atrioventricular (AV) junction of the right side of the heart.3 In addition to its primary role of conducting APs from the atria to the ventricles, the AVN supports the heart’s electrical rhythm in the event of sinoatrial node dysfunction. Due to the slow conduction and long refractory period at the AVN, a critical feature of the AVN is its delay, which ensures the coordinated and sequential contraction of the atria and ventricles. This intrinsic property ensures optimal filling of the heart to prevent life-threatening arrhythmias.3
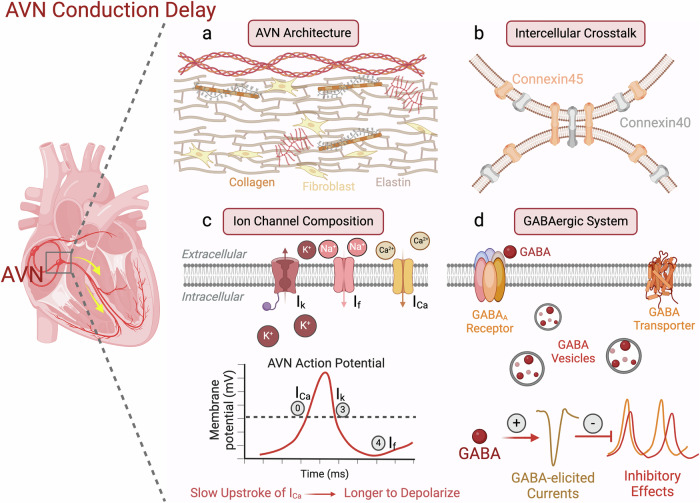
Currently, several factors have been shown to contribute to the AVN delay, including the AVN architecture, ion channel composition, and intercellular communication (Fig. 1).4 In addition to the narrow diameter of the AVN pacemaker cells (AVNPCs), the intricate arrangement of the AVNPCs, fibroblasts, macrophages,5 and extracellular environment creates a convoluted pathway for electrical impulses to traverse, contributing to the slow conduction.4 Furthermore, these pacemaker cells contain sparse amounts of voltage-gated Na+ channels relative to contractile cells. Instead of large and fast Na+ currents, the slow upstroke of the AP in the AVN is primarily mediated by L-type Ca2+ channel current. Additionally, differential expression of gap junction channels (connexin, Cx) within the AVNPCs plays a crucial role in the slow electrical propagation. Cx43 is the primary connexin and mediates fast conductions, whereas Cx40 and Cx45 exhibit smaller conductance.6 The AVN expresses Cx45 and Cx40 but is deficient in Cx43.6 These factors collectively contribute to the AVN delay. However, an important question remains: does the GABAergic system also play a significant role in this process?
Fig. 1. Mechanisms of AVN delay.
The AVN conducts electrical signals from the atria to the ventricles. A critical feature of the AVN is its delay which helps coordinate sequential contraction of the atria and ventricles and prevent life-threatening arrhythmias. Currently known mechanisms of AVN delay include the AVN architecture, intercellular communication, and ion channel composition. a The AVN architecture consists of the extracellular matrix and multiple cell types, creating a convoluted and prolonged pathway for electrical impulses to traverse. b Intercellular communication via low-conductance gap junction channels Cx40 and Cx45, instead of Cx43, contributes to the slow cellular crosstalk. c A pacemaker AP has three distinct phases mediated predominantly by three currents: phases 0 (depolarization; calcium current, ICa), 3 (repolarization; potassium current, Ik) and 4 (spontaneous diastolic depolarization; funny current, If). The upstroke of the AP in the AVN is primarily mediated by the L-type Ca2+ channel current, which leads to the slow depolarization of the AP. d The current study by Liang et al. revealed another important factor which can modulate electrical conduction at the AVN. The authors found that the presence of the GABAergic system (transporters, receptors, and vesicles) within the AVN can exert inhibitory effects on the AVN APs and affect the AVN delay. Figure was created with BioRender.com.
In a recent article published in Cell Research by Liang et al.,7 the authors investigated the AVN biology from a unique perspective. Given the electrophysiological similarities between neurons and AVNPCs, they sought to determine the existence and function of the GABAergic system within the AVN. They found essential components of an endogenous GABAergic system — metabolic enzymes, GABA receptors, and GABA transporters — at the protein and transcript levels in AVNPCs. Moreover, ultrastructural images of rat and mouse AVNPCs revealed the accumulation of GABA-containing vesicles near the surface membranes. After confirming the existence of the GABAergic system within the AVNPCs, the authors tested the functional consequences in AVN pacemaker excitability by targeting key components of the system using isolated cells, ex vivo rat hearts, and conditional knockout (CKO) mice. Indeed, when isolated AVNPCs were treated with Afloqualone, a GABAAR-specific agonist, spontaneous APs decreased, with apparent prolongation of the AP cycle length. Gabazine, a specific antagonist of GABAAR, attenuated the Afloqualone-induced currents.
Because inhibiting and activating key components of the GABAergic system affected electrical excitability of the AVNPCs, the authors generated a Gabrb2-CKO mouse model to further investigate the GABAergic system. These Gabrb2-CKO mice exhibited a significant depression in Wenckebach periodicity and shortened PR intervals. In response to in vivo programmed electrical stimulation, these Gabrb2-CKO mice demonstrated reduced 2:1 AV conduction duration and AVN effective refractory period, suggesting an increase in AVN conductivity. Ex vivo hearts further confirmed these findings. The authors also clarified the role of Gabrb2 in AVN conduction by applying Afloqualone to rescue this phenotype. After atrial burst stimulation and isoproterenol induction, Gabrb2-CKO mice exhibited faster AVN conduction and a significantly higher incidence of fatal arrhythmias than Cre+/− mice. In the clinic, enhanced AV nodal conduction may lead to fast atrial rates conducted to the ventricles, potentially inducing life-threatening ventricular fibrillation and sudden cardiac death.8 Finally, the authors tested the therapeutic potential of the GABAergic system. In a verapamil-induced AV block model, Gabrb2 or Slc32a1 knockdown using AAV2/9 attenuated the incidence of AV block.
Overall, these findings highlight several important implications in the field of AVN research in health and disease. (1) The existence of an endogenous GABAergic system in the AVN. The GABAergic system has long been considered to primarily act in the CNS. The finding that it has an important role in the AVN not only marks an advancement in our understanding of AVN biology, but will also spur future work on the GABAergic system in non-CNS, excitable tissues. (2) Mechanism of AVN delay. Previous studies found that AVN delay is induced by the AVN architecture, intercellular crosstalk, and ion channel composition. The current study illuminates the critical role of the GABAergic system in contributing to the slow electrical propagation within the AVN. (3) Potential therapy for AVN dysfunction. The AVN ensures that sequential contraction occurs to prevent fatal arrythmias. Enhancing GABAergic activity might be beneficial in conditions where a prolonged AVN delay is needed, whereas inhibiting this system could be advantageous in scenarios requiring improved AVN conduction. In their study, Liang and colleagues helped establish a foundation for future work on the GABAergic system in the AVN.
Competing interests
The authors declare no competing interests.
References
- 1.Koh, W., Kwak, H., Cheong, E. & Lee, C. J. Nat. Rev. Neurosci.24, 523–539 (2023). 10.1038/s41583-023-00724-7 [DOI] [PubMed] [Google Scholar]
- 2.Tang, X., Jaenisch, R. & Sur, M. Nat. Rev. Neurosci.22, 290–307 (2021). 10.1038/s41583-021-00443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurian, T., Ambrosi, C., Hucker, W., Fedorov, V. V. & Efimov, I. R. Pacing Clin. Electrophysiol.33, 754–762 (2010). 10.1111/j.1540-8159.2010.02699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz, S. M. & Lerman, B. B. J. Interv. Card. Electrophysiol.52, 271–279 (2018). 10.1007/s10840-018-0392-5 [DOI] [PubMed] [Google Scholar]
- 5.Hulsmans, M. et al. Cell169, 510–522.e20 (2017). 10.1016/j.cell.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temple, I. P., Inada, S., Dobrzynski, H. & Boyett, M. R. Heart Rhythm10, 297–304 (2013). 10.1016/j.hrthm.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang, D. et al. Cell Res. 10.1038/s41422-024-00980-x (2024).
- 8.Verheul, L. M., Groeneveld, S. A., Tuinenburg, A. E. & Hassink, R. J. J. Electrocardiol.76, 66–70 (2023). 10.1016/j.jelectrocard.2022.11.003 [DOI] [PubMed] [Google Scholar]