Abstract
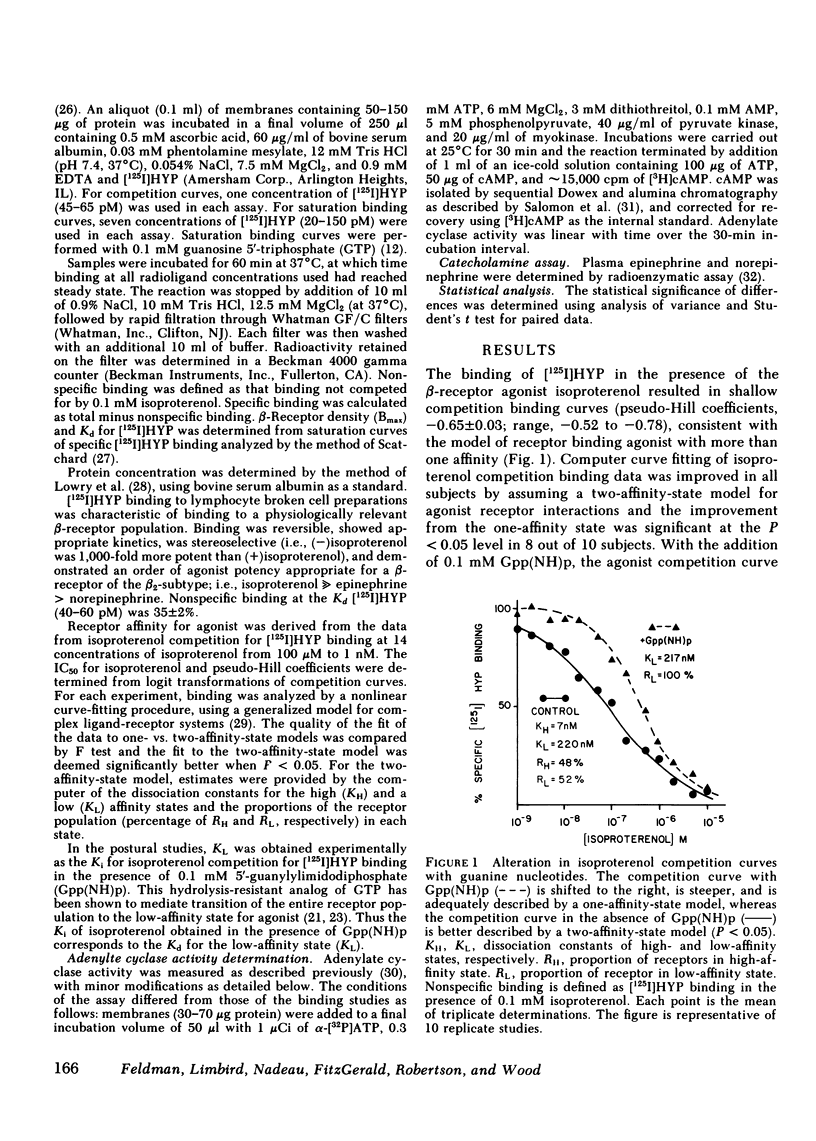
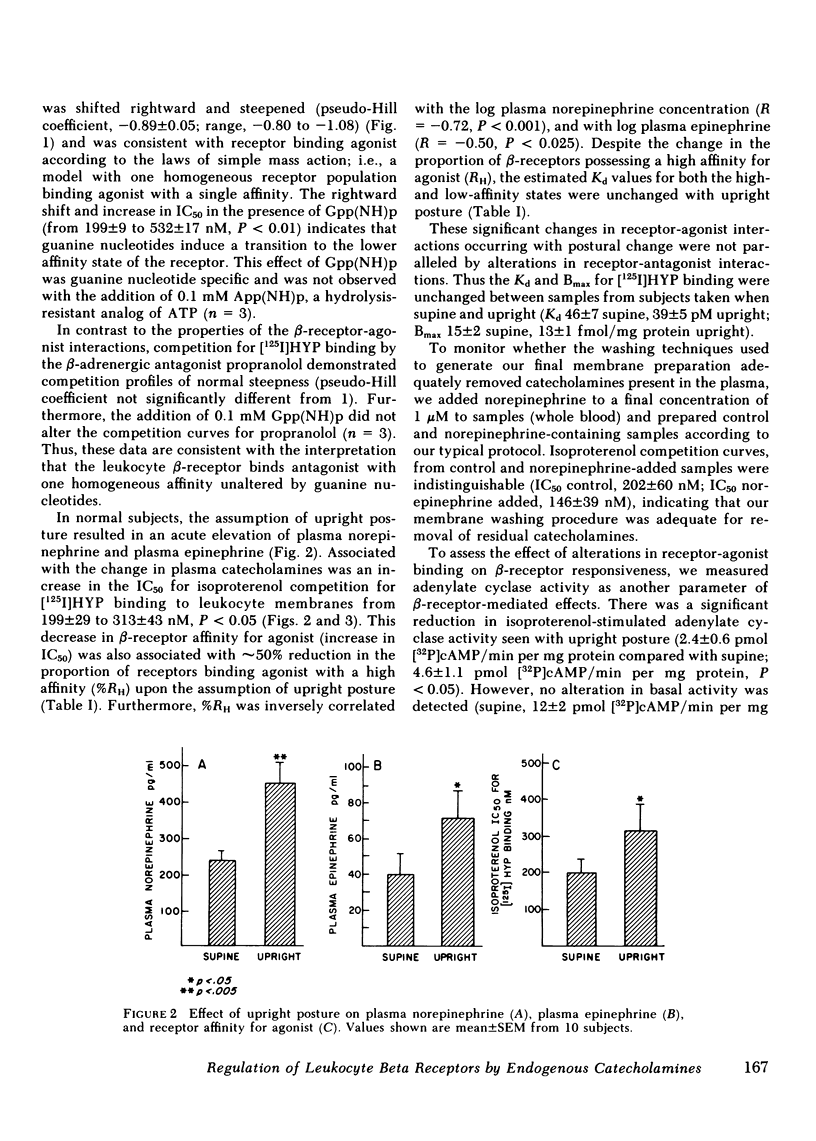
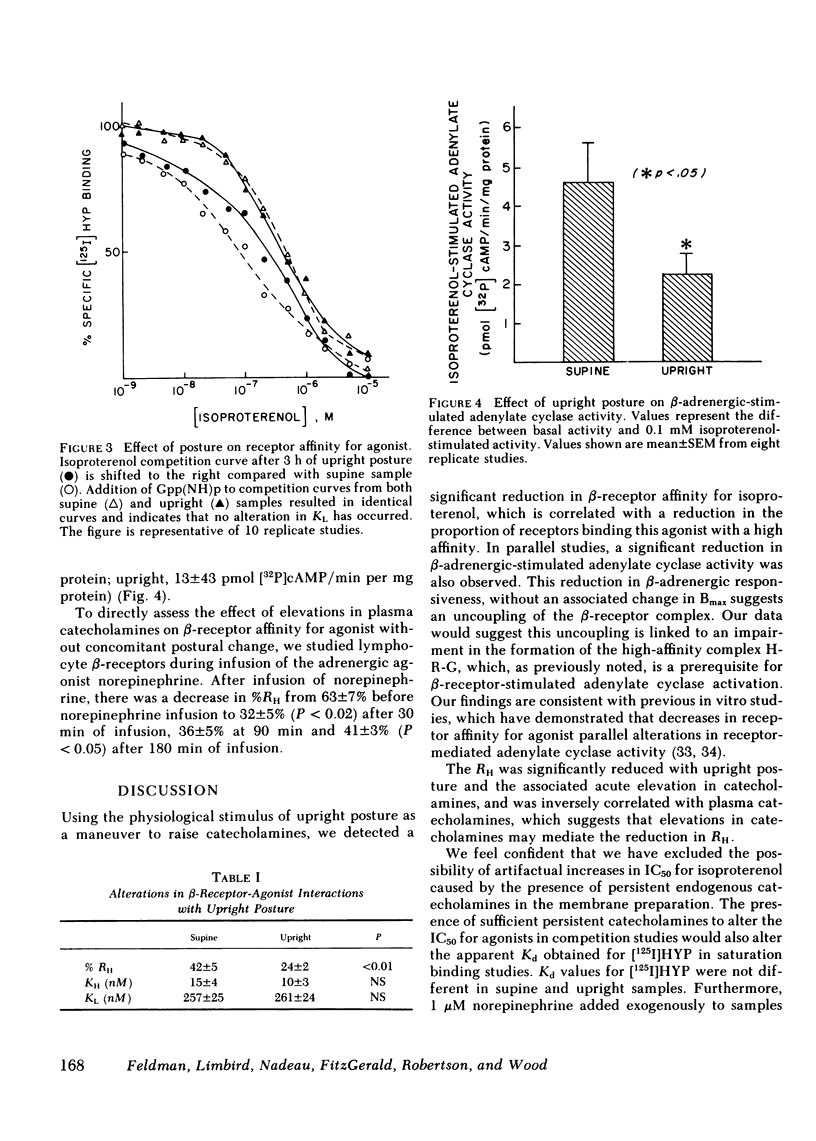
beta-Adrenergic receptors on human mononuclear leukocytes were assessed using [125I]iodohydroxybenzylpindolol binding. Subjects were studied supine and after being ambulatory, a maneuver that increases plasma catecholamines approximately two-fold. beta-Receptor affinity for agonists, measured by the competition of [125I]iodohydroxybenzylpindolol binding by (-)isoproterenol was significantly reduced with ambulation and this reduction was associated with a reduction in the proportion of beta-receptors binding agonist with a high affinity from a mean (+/- SEM) of 42 +/- 5 to 24 +/- 2% (P less than 0.01). In a parallel series, beta-adrenergic-stimulated adenylate cyclase activity was also reduced with postural change from 4.6 +/- 1.1 to 2.4 +/- 0.6 pmol [32P]cAMP/min per mg protein (P less than 0.05) after ambulation. Similar reductions in the proportion of receptors binding agonist with a high affinity were seen after infusion of norepinephrine. We conclude that the maneuver of ambulation reduces leukocyte beta-receptor responsiveness and affinity for agonists, probably by the effect of increased plasma catecholamines mediating an uncoupling of the beta-receptor-adenylate cyclase complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarons R. D., Molinoff P. B. Changes in the density of beta adrenergic receptors in rat lymphocytes, heart and lung after chronic treatment with propranolol. J Pharmacol Exp Ther. 1982 May;221(2):439–443. [PubMed] [Google Scholar]
- Aarons R. D., Nies A. S., Gal J., Hegstrand L. R., Molinoff P. B. Elevation of beta-adrenergic receptor density in human lymphocytes after propranolol administration. J Clin Invest. 1980 May;65(5):949–957. doi: 10.1172/JCI109781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarons R. D., Nies A. S., Gerber J. G., Molinoff P. B. Decreased beta adrenergic receptor density on human lymphocytes after chronic treatment with agonists. J Pharmacol Exp Ther. 1983 Jan;224(1):1–6. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Colucci W. S., Alexander R. W., Williams G. H., Rude R. E., Holman B. L., Konstam M. A., Wynne J., Mudge G. H., Jr, Braunwald E. Decreased lymphocyte beta-adrenergic-receptor density in patients with heart failure and tolerance to the beta-adrenergic agonist pirbuterol. N Engl J Med. 1981 Jul 23;305(4):185–190. doi: 10.1056/NEJM198107233050402. [DOI] [PubMed] [Google Scholar]
- Davies A. O., Lefkowitz R. J. In vitro desensitization of beta adrenergic receptors in human neutrophils. Attenuation by corticosteroids. J Clin Invest. 1983 Mar;71(3):565–571. doi: 10.1172/JCI110801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lean A., Stadel J. M., Lefkowitz R. J. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980 Aug 10;255(15):7108–7117. [PubMed] [Google Scholar]
- DeVellis J., Brooker G. Reversal of catecholamine refractoriness by inhibitors of RNA and protein synthesis. Science. 1974 Dec 27;186(4170):1221–1223. doi: 10.1126/science.186.4170.1221. [DOI] [PubMed] [Google Scholar]
- Fraser J., Nadeau J., Robertson D., Wood A. J. Regulation of human leukocyte beta receptors by endogenous catecholamines: relationship of leukocyte beta receptor density to the cardiac sensitivity to isoproterenol. J Clin Invest. 1981 Jun;67(6):1777–1784. doi: 10.1172/JCI110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galant S. P., Duriseti L., Underwood S., Insel P. A. Decreased beta-adrenergic receptors on polymorphonuclear leukocytes after adrenergic therapy. N Engl J Med. 1978 Oct 26;299(17):933–936. doi: 10.1056/NEJM197810262991707. [DOI] [PubMed] [Google Scholar]
- Hancock A. A., DeLean A. L., Lefkowitz R. J. Quantitative resolution of beta-adrenergic receptor subtypes by selective ligand binding: application of a computerized model fitting technique. Mol Pharmacol. 1979 Jul;16(1):1–9. [PubMed] [Google Scholar]
- Harden T. K., Su Y. F., Perkins J. P. Catecholamine-induced desensitization involves an uncoupling of beta-adrenergic receptors and adenylate cyclase. J Cyclic Nucleotide Res. 1979;5(2):99–106. [PubMed] [Google Scholar]
- Johnson G. L., Wolfe B. B., Harden T. K., Molinoff P. B., Perkins J. P. Role of beta-adrenergic receptors in catecholamine-induced desensitization of adenylate cyclase in human astrocytoma cells. J Biol Chem. 1978 Mar 10;253(5):1472–1480. [PubMed] [Google Scholar]
- Kebabian J. W., Zatz M., Romero J. A., Axelrod J. Rapid changes in rat pineal beta-adrenergic receptor: alterations in l-(3H)alprenolol binding and adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3735–3739. doi: 10.1073/pnas.72.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent R. S., De Lean A., Lefkowitz R. J. A quantitative analysis of beta-adrenergic receptor interactions: resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol Pharmacol. 1980 Jan;17(1):14–23. [PubMed] [Google Scholar]
- Krall J. F., Connelly M., Tuck M. L. Acute regulation of beta adrenergic catecholamine sensitivity in human lymphocytes. J Pharmacol Exp Ther. 1980 Sep;214(3):554–560. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Limbird L. E. Activation and attenuation of adenylate cyclase. The role of GTP-binding proteins as macromolecular messengers in receptor--cyclase coupling. Biochem J. 1981 Apr 1;195(1):1–13. doi: 10.1042/bj1950001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., DeLean A., Hickey A. R., Pike L. J., Lefkowitz R. J. Differential effects of GTP on the coupling of beta-adrenergic receptors to adenylate cyclase from frog and turkey erythrocytes. Application of new methods for the analysis of receptor-effector coupling. Biochim Biophys Acta. 1979 Aug 22;586(2):298–314. doi: 10.1016/0304-4165(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makman M. H. Properties of adenylate cyclase of lymphoid cells. Proc Natl Acad Sci U S A. 1971 May;68(5):885–889. doi: 10.1073/pnas.68.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey J., Tate R., Lefkowitz R. J. Subsensitivity of adenylate cyclase and decreased beta-adrenergic receptor binding after chronic exposure to (minus)-isoproterenol in vitro. J Biol Chem. 1975 Jul 25;250(14):5727–5729. [PubMed] [Google Scholar]
- Newcombe D. S., Ciosek C. P., Jr, Ishikawa Y., Fahey J. V. Human synoviocytes: activation and desensitization by prostaglandins and 1-epinephrine. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3124–3128. doi: 10.1073/pnas.72.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Robertson D., Johnson G. A., Robertson R. M., Nies A. S., Shand D. G., Oates J. A. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation. 1979 Apr;59(4):637–643. doi: 10.1161/01.cir.59.4.637. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Shear M., Insel P. A., Melmon K. L., Coffino P. Agonist-specific refractoriness induced by isoproterenol. Studies with mutant cells. J Biol Chem. 1976 Dec 10;251(23):7572–7576. [PubMed] [Google Scholar]
- Su Y. F., Harden T. K., Perkins J. P. Catecholamine-specific desensitization of adenylate cyclase. Evidence for a multistep process. J Biol Chem. 1980 Aug 10;255(15):7410–7419. [PubMed] [Google Scholar]
- Tohmeh J. F., Cryer P. E. Biphasic adrenergic modulation of beta-adrenergic receptors in man. Agonist-induced early increment and late decrement in beta-adrenergic receptor number. J Clin Invest. 1980 Apr;65(4):836–840. doi: 10.1172/JCI109735. [DOI] [PMC free article] [PubMed] [Google Scholar]


