Abstract
Purpose:
To report the clinical presentation and outcomes in patients who underwent surgery for proliferative sickle cell retinopathy (PSCR).
Design:
Retrospective, consecutive case series.
Subjects:
All patients who underwent vitreoretinal surgery for complications secondary to PSCR between 1/1/2014 and 12/31/2021 at a university referral center.
Methods:
Retrospective consecutive case series.
Main outcome measures:
Best-corrected visual acuity (BCVA), single operation anatomic success rate.
Results:
The study included 65 eyes of 61 patients. Disease distribution included 24 (44.4%) eyes with SC disease, 14 (25.9%) with SS disease, 13 (24.1%) with sickle cell trait, and 3 (5.6%) with sickle cell-beta thalassemia. Preoperative transfusion was not performed in any study patients. Regional anesthesia with monitored anesthesia care (RA-MAC) was utilized in 58 (89.2%) eyes and general anesthesia in 7 (10.8%). In eyes that underwent surgery for retinal detachment (RD; N = 52) the rate of single operation anatomic success was 72.4% with combined scleral buckling/pars plana vitrectomy (SB/PPV; N = 29) compared to 47.8% with PPV alone (N = 23; P = 0.07). Mean BCVA at the last follow-up examination was 1.27 (20/372) in the SB/PPV group and 1.05 (20/226) in the PPV group (P = 0.48). In all SB cases, an encircling band was utilized and there were no known cases of anterior segment ischemia. All eyes that had surgery for VH (N = 13) underwent PPV with endolaser treatment and mean BCVA improved from 1.67 (20/944) preoperatively to 0.45 (20/56) at last follow-up examination (P < 0.001). Mean preoperative BCVA, type of RD, indication for surgery, single operation success rate, and mean BCVA at last follow-up examination did not differ based on sickle cell disease type (P > 0.05).
Conclusions:
In patients with RD, SB/PPV achieved slightly higher rates of single operation anatomic success compared to PPV alone. Visual acuity outcomes were similar in the 2 groups. The majority of patients received RA-MAC anesthesia and preoperative transfusions were not performed. There were no cases of postoperative anterior segment ischemia. SC disease was the most common disease type in the current study and surgical outcomes did not differ between sickle cell disease types.
Keywords: Proliferative sickle cell retinopathy, retinal detachment, vitreous hemorrhage
Sickle cell retinopathy is characterized by the development of retinal ischemia and corresponding vascular abnormalities.1, 2 Chronic ischemia can eventually lead to proliferative sickle cell retinopathy (PSCR) with neovascularization and vitreoretinal traction.3–6 Although neovascular lesions in PSCR frequently undergo autoinfarction and regression, they may progress to cause complications such as vitreous hemorrhage (VH) and retinal detachment (RD).4, 7
When self-limited, sequelae of PSCR can often be managed conservatively with observation, anti-vascular endothelial growth factor therapy, or panretinal photocoagulation but surgical treatment may be indicated in the setting of non-clearing VH and posterior RD.1 Pars plana vitrectomy (PPV) with or without scleral buckling (SB) is a frequent management approach for RD.3 Data regarding the presentation, management, and outcomes of patients undergoing surgery for PSCR are limited.8, 9 The current study reports the clinical features and outcomes after surgery for PSCR at a university referral center.
Methods
Institutional Review Board/Ethics Committee approval was obtained, and the study conformed to the requirements of the Declaration of Helsinki and the United States Health Insurance Portability and Privacy Act. Patients who underwent vitreoretinal surgery for PSCR at our institution between 1/1/2014 and 12/31/2021 were identified using keyword and International Classification of Diseases search criteria. Exclusion criteria included pregnant women, prisoner status, less than 2 months of follow-up, and surgery for macular hole. The following data were collected: age, sex, ethnicity, laterality, type of sickle cell disease, presence of diabetes mellitus, preoperative best-corrected visual acuity (BCVA), BCVA immediately prior to surgery, reason for surgery, type of surgery, vitrectomy gauge, type of anesthesia, anesthesia-related complications, perfluorocarbon use, tamponade, postoperative BCVA, anatomical success, postoperative anterior segment ischemia, postoperative ocular hypertension, need for additional surgery for RD, and follow-up duration.
In patients who underwent regional anesthesia with monitored anesthesia care (RA-MAC), sedation was first achieved through the use of intravenous benzodiazepines, opioids, and/or dexmedetomidine. Peribulbar or retrobulbar 0.75% or 1% ropivacaine with hyaluronidase or with an admixture of equal parts 4% lidocaine and 1% ropivacaine plus hyaluronidase was subsequently administered. Sedation levels were adjusted as necessary throughout the procedure. In those who underwent general anesthesia, sedation was obtained in a similar fashion followed by the administration of general anesthetics. Anesthesia was maintained using a combination of intravenous agents and inhaled anesthetics.
Statistical analysis was performed using IBM SPSS Statistics Version 28 (IBM Corp., Armonk, NY). Descriptive statistics were used to find the mean, standard deviation, range, and proportions for population characteristics and outcomes. Snellen BCVA was converted into logarithm of the minimal angle of resolution for comparison and statistical analyses. Counting fingers, hand motion, light perception, and no light perception visual acuities were given values of 1.9, 2.3, 2.7, and 3.0, respectively. Means were compared with analyses of variance, independent-sample t-tests, and paired sample t-tests. Proportions were compared with chi-square tests. Correlations were measured using linear regression analyses. Covariates were accounted for using analyses of covariance when indicated. All tests employed a significance level of P < 0.05.
Results
The current study included 65 eyes (32 right) of 61 patients (34 males) with a mean age of 41 years. African American patients were the most common subgroup, comprising 64 (98.5%) eyes. There was 1 (1.5%) eye from a Caucasian patient. Disease distribution included 24 (44.4%) eyes with SC disease, 14 (25.9%) with SS disease, 13 (24.1%) with sickle cell trait, and 3 (5.6%) with sickle cell-beta thalassemia. Seven (10.8%) patients had concurrent diabetes mellitus without evidence of diabetic retinopathy. Mean follow-up duration was 42 months (range: 4 months to 7 years).
Indications for surgery included VH in 13 (20.0%) eyes, macula-involving RD in 37 (56.9%), and macula-sparing RD in 15 (23.1%). General anesthesia was administered in 7 (10.8%) cases while 58 (89.2%) received RA-MAC. No preoperative transfusions were performed and no vaso-occlusive events occurred. No patients had removal or repositioning of rectus muscles during surgery.
Pars plana vitrectomy (PPV) was performed in 36 (55.4%) eyes and combined PPV with scleral buckling (SB) was performed in 29 (44.6%) eyes. Vitrectomy gauge included 20-gauge in 2 (3.1%) eyes, 23-gauge in 36 (55.4%) eyes, and 25-gauge in 27 (41.5%) eyes. All eyes with VH (N = 13) underwent PPV without SB. Endolaser was used in all cases. Mean BCVA at last follow-up examination was 1.04 (20/218) in all eyes. Mean preoperative BCVA, type of RD, indication for surgery, anatomic success rate, and mean BCVA at last follow-up examination did not differ based on sickle cell disease type (P > 0.05). There were no differences in anatomic or visual outcomes based on PPV gauge (P > 0.05). No perioperative systemic complications were encountered.
Postoperative ocular hypertension was encountered in 4 of 36 (11.1%) of eyes in the PPV group; 1 case occurred secondary to neovascular glaucoma, 1 due to a hyphema, and 2 cases were idiopathic. Additionally, 1 eye in the PPV group developed neovascularization of the iris and angle and 1 eye demonstrated progressive optic disc cupping in the absence of elevated intraocular pressure. In the SB/PPV group, postoperative ocular hypertension was encountered in 4 of 29 (13.8%) eyes; 3 cases occurred secondary to the presence of silicone oil (SO) in the anterior chamber and 1 case was idiopathic.
Eyes with retinal detachment
Macula involvement was present in 37 of 52 (71.2%) eyes undergoing surgery for RD. Mean preoperative BCVA was 1.62 (20/827) in eyes with macula involvement compared to 1.17 (20/298) in eyes without macula involvement (P = 0.10). The RD was combined tractional/rhegmatogenous in 38 (73.1%) eyes, rhegmatogenous alone in 7 (13.5%) eyes, and tractional alone in 7 (13.5%) eyes. Preoperative BCVA did not differ based on type of RD (P = 0.14). Twenty-nine (55.8%) eyes underwent combined SB/PPV while 23 (44.2%) underwent PPV alone. An encircling band was used in all eyes that underwent combined SB/PPV. Twenty-five (86.2%) eyes received a #41 band and 4 (13.8%) received a #240 band. There were no cases of anterior segment ischemia. One eye developed a hyphema and anterior segment inflammation postoperatively which resolved with corticosteroid treatment. Drainage of subretinal fluid was achieved either by a pre-existing break or a drainage retinotomy. External drainage was not performed. Combined SB/PPV was performed in 3 of 7 (42.9%) eyes with rhegmatogenous RDs, 2 of 7 (28.6%) eyes with tractional RDs, and 18 of 38 (47.4%) eyes with combined tractional/rhegmatogenous RDs (P = 0.65). A tamponade agent was used in 48 eyes, including SO in 28 (58.3%), C3F8 in 15 (31.3%), SF6 in 4 (8.3%), and air only in 1 (2.1%). Perfluorocarbon was used in 3 (13.0 %) eyes that underwent PPV alone and 17 (58.6%) eyes that underwent combined SB/PPV (P < 0.001). Silicone oil tamponade was used in 9 (39.1%) eyes that underwent PPV alone and 19 (65.5%) eyes that underwent combined SB/PPV (P = 0.06).
Single operation anatomic success at 6 months was achieved in 32 of 52 (61.5%) eyes. Rates of single operation success did not differ based on perfluorocarbon use (P = 0.86). Eyes that received SO (N = 28) had a single operation success rate of 53.6% while those that received another tamponade (N = 20) had a single operation success rate of 70.8% (P = 0.20). There was a nonstatistically significant higher rate of single operation success in eyes that underwent combined SB/PPV (21 of 29 [72.4%]) compared to PPV alone (11 of 23 [47.8%]; P = 0.07).
Mean postoperative BCVA of all eyes with RDs was 1.19 (20/306) at last follow-up examination and was poorer in eyes with macula-involving RD (1.43 [20/535]) compared to eyes with macula-sparing RD (0.59 [20/77]; P = 0.002). In eyes with macula-involving RD, BCVA at last follow-up examination was 20/40 or better in 5 (13.5%) eyes and 20/200 or worse in 22 (59.5%) eyes. Best-corrected visual acuity at last follow-up examination in eyes with macula-sparing RD was 20/40 or better in 9 (60.0%) eyes and 20/200 or worse in 4 (26.7%) eyes. Best-corrected visual acuity at last follow-up examination did not improve significantly in eyes with macula-involving RD (P = 0.27). There was a trend towards an improvement in BCVA after surgery in eyes with macula-sparing RD (P = 0.06).
There were no differences in mean BCVA at last follow-up exam based on perfluorocarbon use (P = 0.30) or type of RD surgery (P = 0.48; Table 1). Best-corrected visual acuity at last follow-up examination was 20/40 or better in 2 (7.1%) eyes that received SO tamponade and in 12 (50%) eyes that received another tamponade (P < 0.001). Sixteen (57.1%) eyes that received SO tamponade and 10 (41.7%) that received another tamponade had a BCVA of 20/200 or worse (P = 0.27). Analysis of covariance testing with preoperative BCVA as a covariate revealed that mean BCVA at last follow-up examination was worse in eyes that received SO tamponade (1.51 [20/647]) compared to eyes that received another tamponade (0.74 [20/110]; P = 0.006; Table 2).
Table 1.
Surgical outcomes in eyes that received pars plana vitrectomy alone or pars plana vitrectomy with scleral buckling for retinal detachment secondary to proliferative sickle cell retinopathy
| No. (%) | |||
|---|---|---|---|
| PPV only | Combined SB/PPV | P - value | |
| No. of eyes | 23 (44.2) | 29 (55.8) | |
| Mean preoperative BCVA | 20/576 | 20/659 | 0.79 |
| Mean BCVA at last follow-up exam | 20/226 | 20/372 | 0.48 |
| BCVA ≥ 20/40 | 9 (39.1) | 5 (17.2) | 0.08 |
| BCVA ≤ 20/200 | 10 (43.5) | 16 (55.2) | 0.40 |
| Reattachment at 6 months | 11 (47.8) | 21 (72.4) | 0.07 |
BCVA = best-corrected visual acuity; PPV = pars plana vitrectomy; SB = scleral buckling
Table 2.
Surgical outcomes in eyes that received silicone oil tamponade or another tamponade for retinal detachment secondary to proliferative sickle cell retinopathy
| No. (%) | |||
|---|---|---|---|
| Silicone oil tamponade | Other tamponade | P - value | |
| No. of eyes | 28 (53.8) | 24 (46.2) | |
| Mean preoperative BCVA | 20/704 | 20/530 | 0.57 |
| Mean BCVA at last follow-up exam | 20/647 | 20/110 | 0.006 |
| BCVA ≥ 20/40 | 2 (7.1) | 12 (50.0) | < 0.001 |
| BCVA ≤ 20/200 | 16 (57.1) | 10 (41.7) | 0.27 |
| Reattachment at 6 months | 15 (53.6) | 17 (70.8) | 0.20 |
BCVA = best-corrected visual acuity
Eyes with vitreous hemorrhage
Mean preoperative BCVA in eyes with VH (N = 13) was 1.67 (20/944). Four eyes received a tamponade agent; 1 eye received SF6 for a retinal tear, 2 received air for peripheral vitreoretinal traction, and 1 received air for a submacular hemorrhage. Mean BCVA at last follow-up examination improved to 0.45 (20/56; P < 0.001). Best-corrected visual acuity at last follow-up was 20/40 or better in 9 (69.2%) eyes and 20/200 or worse in 3 (23.1%) eyes. One eye eventually required additional surgery for a combined tractional/rhegmatogenous RD. Mean BCVA at last follow-up exam did not differ in those who received a tamponade agent (0.79 [20/122]) compared to those who did not (0.30 [20/39]; P = 0.39).
Discussion
In the current study, the single operation anatomic success rate in eyes with retinal detachment was 61.5% which is consistent with prior reports of retinal detachment secondary to PSCR (Table 3). While eyes with macula-involving retinal detachment did not demonstrate statistically significant improvements in visual acuity outcomes with surgery, eyes with macula-sparing retinal detachment did show a trend towards an improvement in BCVA after surgery. As expected, eyes with VH alone had a significant improvement in postoperative BCVA, consistent with prior reports.3 Indeed, postoperative visual acuity outcomes after vitreoretinal surgery for PSCR may be variable due to ischemic retinal disease in addition to the potential for cataract formation and glaucoma.
Table 3.
Reported surgical outcomes in eyes with retinal detachment associated with proliferative sickle cell retinopathy
| Authors | # Cases | % Macula-involving | Type of surgery | Last best-corrected visual acuity ≥ 20/40 (%) | Single operation success (%) |
|---|---|---|---|---|---|
| Retinal Detachment | |||||
| Freilich (1973)13 | 3 | 68 | 3 SB | 33 | 100 |
| Freilich (1977)19 | 9 | Not reported | 8 SB; 1 scleral shortening | Not reported | Not reported |
| Jampol (1982)10 | 14 | Not reported | 4 SB alone; 10 SB/PPV | 50 | 64 |
| Pulido (1988)11 | 9 | Not reported | 3 PPV alone; 6 SB/PPV | 22 | 56 |
| Downes (2005)20 | 2 | 100 | 1 PPV; 1 not reported | Not reported | 100 |
| Williamson (2009)21 | 6 | Not reported | 6 PPV | 33 | 83 |
| Chen (2014)3 | 8 | 88 | 6 PPV alone; 2 SB/PPV | 0 | 50 |
| Ho (2018)8 | 38 | 76 | PPV | 24 | 71 |
| Okonkwo (2020)22 | 138 | Not reported | 70 PPV alone; 29 SB/PPV | Not reported | 87 |
| Current study | 52 | 71 | 23 PPV; 29 SB/PPV | 27 | 62 |
| Vitreous Hemorrhage without Retinal Detachment | |||||
| Jampol (1982)10 | 5 | N/A | 5 PPV | 40 | N/A |
| Pulido (1988)11 | 2 | N/A | 2 PPV | 0 | N/A |
| Williamson (2009)21 | 8 | N/A | 8 PPV | 88 | N/A |
| Chen (2014)3 | 6 | N/A | 6 PPV | 100 | N/A |
| Ho (2018)8 | 19 | N/A | 19 PPV | Not reported | N/A |
| Okonkwo (2022)23 | 37 | N/A | 37 PPV | Not reported | N/A |
| Current Study | 13 | N/A | 13 PPV | 69 | N/A |
PPV = pars plana vitrectomy; SB = scleral buckling
There were no differences in preoperative or postoperative outcomes between sickle cell disease types in this cohort of eyes undergoing vitreoretinal surgery for PSCR. However, the higher prevalence of SC disease in this sample of patients is evidence for its propensity to cause relatively severe retinopathy compared to other forms of sickle cell disease.
The use of perfluorocarbon and SO tamponade in some cases attests to the complexity of the surgical management of RDs in PSCR. The higher rates compared to prior reports may reflect an increased awareness of this complexity or a trend towards contemporary utilization practices.3 In eyes that received SO (N = 28), visual acuity outcomes were worse when compared to eyes that received another tamponade agent (N = 20). As such, the decision to use silicone oil should consider the potential adverse consequences of this approach. Additionally, the lack of superior outcomes with perfluorocarbon use may attest to its limited utility in managing retinal detachments secondary to PSCR, which are frequently characterized by small breaks that do not require complex flattening maneuvers.
Visual acuity outcomes were similar between eyes treated with PPV alone and those treated with combined SB/PPV. However, there was a trend toward higher rates of single operation anatomic retinal reattachment in eyes that underwent combined SB/PPV. Indeed, the sites of vitreoretinal traction in eyes with PSCR are typically located at areas of peripheral neovascularization. Addressing these areas during PPV alone may be challenging without inadvertently striking the crystalline lens or creating iatrogenic tears.10 As such, scleral buckling may be an effective method to relieve peripheral traction associated with these cases. Although anterior segment ischemia was previously reported with scleral buckling, currently available techniques and adequate intraoperative hydration, oxygenation, intraocular pressure control, avoidance of carbonic anhydrase inhibitor use, and non-removal of rectus muscles have minimized this risk.2, 3, 6, 11–13
Previous authors have demonstrated a trend towards improved functional and anatomic outcomes with 23-gauge PPV compared to 20-gauge PPV in the surgical treatment of PSCR.8 In the current study, the majority of eyes received either 23- or 25-gauge PPV and there were no differences in postoperative outcomes between these two cohorts, consistent with prior reports comparing these 2 PPV gauges in the treatment of other vitreoretinal diseases.14, 15
The majority of patients in the current study underwent regional anesthesia and there were no reported perioperative systemic complications. Indeed, previous studies assessing the utilization of regional versus general anesthesia for ophthalmic surgery have indicated a lack of notable distinctions between the two approaches.16, 17 The benefits of using regional anesthesia with monitored anesthesia care for ophthalmic surgery in these patients include a shorter postoperative recovery period, more effective postoperative pain management, less postoperative nausea and vomiting, and the absence of laryngeal irritation from intubation.16 Furthermore, regional anesthesia offers the advantage of circumventing the hormonal and glycemic stress responses associated with general anesthesia, making it a preferred option for patients with pulmonary and cardiovascular conditions such as those frequently seen in sickle cell disease.18–20
Limitations of the current study include its retrospective nature and the use of outcomes data from multiple vitreoretinal surgeons. There was no defined protocol for management of specific RD types. The surgical approach was determined by the individual surgeon based on the variable pathology and the personal preference of the surgeon. However, due to the variability in clinical presentation and corresponding need for individualized treatment approaches, the feasibility of a prospective, standardized study on this subject is low. An additional limitation of the current study is the variability of disease severity within study groups. While the authors attempted to account for this by using preoperative BCVA as a covariate in statistical analyses, it is possible that additional poorly quantifiable factors may have impacted postoperative outcomes. Finally, while anterior segment ischemia was not observed in the current study, larger series may be more sensitive in detecting this uncommon complication.
In conclusion, for patients with RD secondary to PSCR, the use of SB in addition to PPV may improve anatomic success rates and has a low risk of anterior segment ischemia. With adequate hydration, oxygenation, and pain control, RA-MAC was well-tolerated by patients in the current study and avoids the risks of general anesthesia. SC disease was the most common disease type in the current study and surgical outcomes did not differ between sickle cell disease types.
Figure 1. Retinal Detachment Associated with Proliferative Sickle Cell Retinopathy.
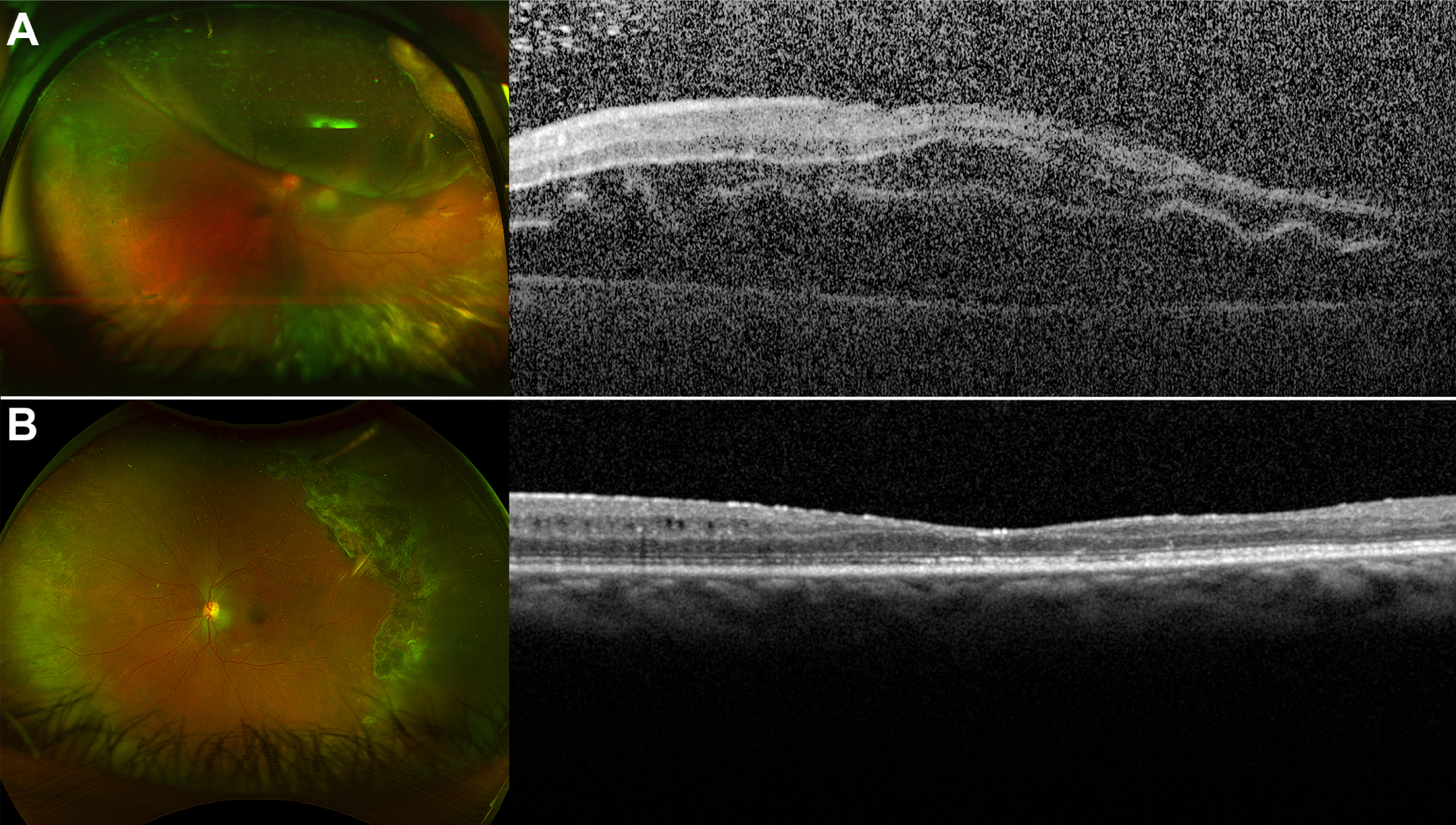
(A) A 50-year-old female with a history of SC disease presented with a macula-involving retinal detachment with areas of peripheral sea fan neovascularization and multiple retinal breaks. Best-corrected preoperative visual acuity was 2.3 (hand motions). She underwent scleral buckling and pars plana vitrectomy with endolaser and silicone oil tamponade. (B) After silicone oil removal 1 year later, the retina was attached and best-corrected visual acuity was 1.3 (20/400).
Figure 2. Vitreous Hemorrhage Secondary to Proliferative Sickle Cell Retinopathy.

(A) A 51-year-old female with a history of sickle cell disease presented with vitreous hemorrhage. Best-corrected preoperative visual acuity was 0.8 (20/126). She underwent pars plana vitrectomy with endolaser. (B) One year later, the vitreous hemorrhage had resolved and best-corrected visual acuity was 0.4 (20/50).
Eyes that underwent scleral buckling with pars plana vitrectomy for proliferative sickle cell retinopathy had slightly better anatomic outcomes compared to eyes that underwent pars plana vitrectomy alone. Anterior segment ischemia was not observed.
Financial Support:
NIH Center Core Grant P30EY014801 (Bethesda, MD), Research to Prevent Blindness-Unrestricted Grant to BPEI (GR004596–1; New York, NY).
Abbreviations:
- BCVA
best-corrected visual acuity
- PPV
pars plan vitrectomy
- PSCR
proliferative sickle cell retinopathy
- RA-MAC
regional anesthesia with monitored anesthesia care
- RD
retinal detachment
- SB
scleral buckling
- SO
silicone oil
- VH
vitreous hemorrhage
Appendix
Proliferative Sickle Cell Retinopathy Study Group
Thomas A. Albini, M.D.,1 Jorge A. Fortun, M.D.,1 Jayanth Sridhar, M.D.,1 Nicolas A. Yannuzzi M.D.,1 Luis J. Haddock M.D.1
1 Department of Ophthalmology, Bascom Palmer Eye Institute, Miami, Florida, USA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
LR – None
SP – None
BW – Consultant for Alcon, Allergan, Alimera, Castel Biosciences, EyePoint, Genentech, Immunocore, Iveric, Regeneron; Stock Options with Lumata Health.
WS – None
AB – Consultant for Alcon, Allergan, ProQR, REGENXBIO, DORC, Zeiss, Oculus
JT – None
SG – None
HP – None
HF – None
TA – None
JF – Consultant for Alcon, DORC
JS – Consultant for Alcon, Allergan, Apellis, DORC, Eyepoint Pharmaceutical, Genentech, Ocuterra, Regeneron, Samsara
NY – Consultant for EyePoint Pharmaceutical, Genentech, Regeneron, Alimera Sciences
LH – Advisory Board for Alimera, Regeneron
References
- 1.Mishra K, Bajaj R, Scott AW. Variable practice patterns for management of sickle cell retinopathy. Ophthalmol Retina 2021; 5(7):715–717. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg MF. Classification and pathogenesis of proliferative sickle retinopathy. Am J Ophthalmol 1971; 71(3):649–665. [DOI] [PubMed] [Google Scholar]
- 3.Chen RW, Flynn HW Jr., Lee WH, et al. Vitreoretinal management and surgical outcomes in proliferative sickle retinopathy: a case series. Am J Ophthalmol 2014; 157(4):870–875 e871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdalla Elsayed MEA, Mura M, Al Dhibi H, et al. Sickle cell retinopathy. A focused review. Graefes Arch Clin Exp Ophthalmol 2019; 257(7):1353–1364. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg MF. Natural history of untreated proliferative sickle retinopathy. Arch Ophthalmol 1971; 85(4):428–437. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg MF. Sickled erythrocytes, hyphema, and secondary glaucoma: I. The diagnosis and treatment of sickled erythrocytes in human hyphemas. Ophthalmic Surg 1979; 10(4):17–31. [PubMed] [Google Scholar]
- 7.Welch RB, Goldberg MF. Sickle-cell hemoglobin and its relation to fundus abnormality. Arch Ophthalmol 1966; 75(3):353–362. [DOI] [PubMed] [Google Scholar]
- 8.Ho J, Grabowska A, Ugarte M, Muqit MM. A comparison of 23-gauge and 20-gauge vitrectomy for proliferative sickle cell retinopathy - clinical outcomes and surgical management. Eye (Lond) 2018; 32(9):1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nithianandan H, Sridhar J. Surgical and medical perioperative management of sickle cell retinopathy: a literature review. Int Ophthalmol Clin 2020; 60(4):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jampol LM, Green JL Jr., Goldberg MF, Peyman GA. An update on vitrectomy surgery and retinal detachment repair in sickle cell disease. Arch Ophthalmol 1982; 100(4):591–593. [DOI] [PubMed] [Google Scholar]
- 11.Pulido JS, Flynn HW Jr., Clarkson JG, Blankenship GW. Pars plana vitrectomy in the management of complications of proliferative sickle retinopathy. Arch Ophthalmol 1988; 106(11):1553–1557. [DOI] [PubMed] [Google Scholar]
- 12.Ryan SJ, Goldberg MF. Anterior segment ischemia following scleral buckling in sickle cell hemoglobinopathy. Am J Ophthalmol 1971; 72(1):35–50. [DOI] [PubMed] [Google Scholar]
- 13.Freilich DB, Seelenfreund MH. Hyperbaric oxygen, retinal detachment, and sickle cell anemia. Arch Ophthalmol 1973; 90(2):90–93. [DOI] [PubMed] [Google Scholar]
- 14.Sedova A, Steiner I, Matzenberger RP, et al. Comparison of safety and effectiveness between 23-gauge and 25-gauge vitrectomy surgery in common vitreoretinal diseases. PLoS One 2021; 16(3):e0248164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthrie G, Magill H, Steel DH. 23-gauge versus 25-gauge vitrectomy for proliferative diabetic retinopathy: a comparison of surgical outcomes. Ophthalmologica 2015; 233(2):104–111. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Hudson JL, Fan KC, et al. Evolving use of regional versus general anesthesia for the surgical repair of open globe injuries. Am J Ophthalmol 2023; 251:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell DN, Lim M, Muir MK, et al. A prospective randomised study of local versus general anaesthesia for cataract surgery. Anaesthesia 1993; 48(5):422–428. [DOI] [PubMed] [Google Scholar]
- 18.Barker JP, Vafidis GC, Robinson PN, Hall GM. Plasma catecholamine response to cataract surgery: a comparison between general and local anaesthesia. Anaesthesia 1991; 46(8):642–645. [DOI] [PubMed] [Google Scholar]
- 19.Barker JP, Vafidis GC, Robinson PN, et al. The metabolic and hormonal response to cataract surgery. A comparison between retrobulbar and peribulbar blockade. Anaesthesia 1993; 48(6):488–491. [DOI] [PubMed] [Google Scholar]
- 20.Sachdev V, Rosing DR, Thein SL. Cardiovascular complications of sickle cell disease. Trends Cardiovasc Med 2021; 31(3):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]


