Abstract
Many older patients with COVID-19 likely have co-morbid osteoporosis. We investigated the clinical outcomes of COVID-19 patients with osteoporosis. This was a retrospective cohort study using national claims data from Korea encoded in the common data model. Patients aged ≥ 50 years diagnosed with COVID-19 infection between January 2020 and April 2022 were included and stratified into two groups according to a history of osteoporosis. Clinical outcomes of COVID-19 infection were analyzed using logistic regression analysis after large-scale propensity score stratification. Of the 597,011 patients with COVID-19 included in the study, 105,172 had a history of osteoporosis. In patients with a history of osteoporosis, the odds of mortality decreased (odds ratio [OR] 0.82, P < 0.002), whereas most clinical outcomes of COVID-19 did not exhibit differences compared to those without such a history. Osteoporosis patients with a history of fractures showed increased odds of pneumonia, hospitalization, major adverse cardiac events, venous thromboembolism, and mortality, compared to patients without osteoporosis (ORs 1.34–1.58, P < 0.001 to P = 0.001). Our study suggests that patients with severe osteoporosis who have experienced fractures have an elevated risk of severe complications with COVID-19, while osteoporosis patients without fractures who have sought medical attention have a lower risk of mortality.
Keywords: COVID-19, Fractures, Mortality, Osteoporosis
Subject terms: Viral infection, Metabolic bone disease
Introduction
COVID-19, caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged as a global pandemic in late 2019 and continues to pose a significant threat to public health1. While many individuals experience mild respiratory symptoms, a subset of patients develop severe complications, including acute respiratory distress syndrome (ARDS), multi-organ dysfunction, and death. Advanced age and the presence of comorbidities have been identified as risk factors for severe outcomes in COVID-192. Furthermore, some early case reports and small clinical studies have suggested that COVID-19 has a greater impact on older adults with underlying conditions compared to younger individuals without these conditions3–5. Numerous studies have demonstrated that COVID-19-positive older adults with specific conditions, such as diabetes, cardiovascular disease, and obesity, are at a higher risk of hospitalization and mortality than those without these chronic conditions6–9. This data on the interplay between comorbidities and the risk of adverse events in patients with COVID-19, particularly in older adults, has provided guidance to improve the care of this vulnerable population.
Osteoporosis, a prevalent chronic bone disease characterized by reduced bone density and an increased susceptibility to fractures, affects a significant number of individuals worldwide10. The continued global aging of populations leads to an increase in the incidence of osteoporosis and associated fractures, contributing significantly to medical, social, and economic burdens11. Consequently, there is a high likelihood that many older patients with COVID-19 have comorbid osteoporosis. However, recent studies examining the relationship between osteoporosis and COVID-19 have primarily focused on the impact of the COVID-19 pandemic on musculoskeletal health12–14. A study has reported that infection with SARS-CoV-2, as well as treatment with glucocorticoids, can accelerate bone loss in these patients15. Moreover, medical treatment for osteoporosis was interrupted for many patients during the COVID-19 pandemic, potentially increasing the risk of fractures and related mortality16. However, there has been a scarcity of research exploring the COVID-19-related outcomes in patients with osteoporosis, who may require specialized care to prevent more severe outcomes from the infection17. To fill this research gap, we investigated the clinical outcomes of patients with COVID-19 and osteoporosis using national claims data from Korea.
Methods
Study design and data source
This is a retrospective observational cohort study based on the nationwide claims database provided by the Health Insurance Review and Assessment Service (HIRA) of South Korea. HIRA is a national institution responsible for providing comprehensive health insurance coverage to the entire South Korean population. The database comprises a range of data elements, including demographic information, diagnostic reports, procedures, prescription drugs, medical materials, and healthcare resource utilization. We specifically used the data source that was mapped to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) version 5 by the Big Data Department of the HIRA for COVID-19 research (HIRA COVID-19 OMOP database). This database comprised 9,822,577 patients who used National Health Insurance of South Korea (extracted by stratifying 20% of all users by sex and age) between January 2018 and April 2022. The OMOP-CDM from the distributed research network of the Observational Health Data Science and Informatics (OHDISI) community serves as a standardized and unified data model. It enables integration of heterogenous data sources into a common format (concept IDs)18,19. The Institutional Review Board and Ethics Committee of Inha University Hospital approved the study protocol (IRB No. 2022–07-029), which was conducted in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived by the IRB of Inha University Hospital due to the de-identified nature of the data utilized from the HIRA COVID-19 OMOP database and the retrospective study design.
Study cohorts
The study included patients with COVID-19 aged ≥ 50 years between January 2020 and April 2022 and excluded those who had a previous history of hypercalcemia, hyperparathyroidism, Paget’s disease, osteomalacia, or malignancies. The target cohort was defined as patients who had a documented diagnostic code for osteoporosis or fractures, or who had been prescribed osteoporosis medications such as selective estrogen receptor modulators (SERM), bisphosphonates, denosumab, teriparatide, or romosozumab, as defined by the concept sets listed in Supplemental Table 1 before their COVID-19 infection (the osteoporosis group). The comparator cohort included those who had neither a diagnostic code for osteoporosis nor fractures, as defined by the concept sets in Supplemental Table 1, and who had not been prescribed any osteoporosis medications from the same table before their COVID-19 infection (the non-osteoporosis group). The index date was defined as the date of COVID-19 infection.
Study outcomes
The outcomes were the development of pneumonia, hospitalization, acute respiratory distress syndrome (ARDS), major adverse cardiovascular events (MACE), venous thromboembolism (VTE), and mortality within 30- and 90 days after COVID-19 infection. The concept sets used for the definition of study outcomes are available in Supplemental Table 2.
Statistical analysis
Analysis tools for the OMOP-CDM are built in the interactive analysis platform ATLAS (version 2.7.6) and the OHDSI Methods Library R packages version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). ATLAS is a free, publicly available (https://github.com/OHDSI), web-based tool developed by the OHDSI community. It facilitates the design and execution of analyses on standardized, patient-level observational data in the CDM format. ATLAS is deployed as a web application in conjunction with the OHDSI WebAPI, and it automatically generates the R code needed to execute the study. Propensity score (PS) stratification was performed to reduce potential confounding due to an imbalance in the baseline observed covariates between the target (the osteoporosis group) and the comparator cohorts (the non-osteoporosis group). A large set of covariates was used to estimate the large-scale PSs, including age groups, sex, year and month of cohort entry, and all recorded medical histories within 1 year of index date. After PS stratification, logistic regression analysis was performed to calculate the odds ratio (OR) and 95% confidence interval (CI) of the study outcomes. Subgroup analyses were conducted based on sex and previous history of fractures. Specifically, we compared the study outcomes between the osteoporosis and non-osteoporosis groups among both women and men using logistic regression analyses after PS stratification. Additionally, we assessed the study outcomes between the ‘osteoporosis with fracture’ group (the fracture group) and the ‘non-osteoporosis’ group, as well as between the ‘osteoporosis without fracture’ group (the non-fracture group) and the ‘non-osteoporosis’ group, employing the same statistical methods. All tests were two-tailed, and P < 0.05 was considered statistically significant.
Results
Cohort selection
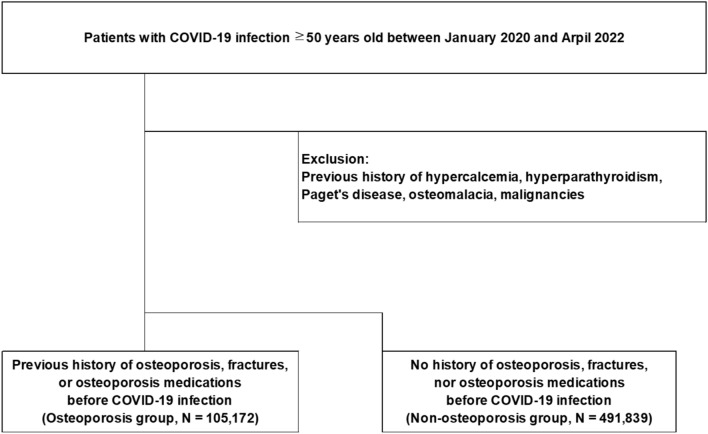
During the period from January 2020 to April 2022, a total of 597,011 study-eligible patients with COVID-19 infection were identified. Among them, there were 105,172 patients in the osteoporosis group and 491,839 in the non-osteoporosis group (Fig. 1).
Figure 1.
Flow chart of cohort selection.
Cohort characteristics
The baseline characteristics of the patients before and after large-scale PS stratification are presented in Table 1. After PS stratification, we found no significant imbalance (absolute standardized mean difference > 1.0) in the baseline variables including age groups, sex, and previous medical histories between the osteoporosis and non-osteoporosis groups. Especially, there was no significant imbalance in the previous histories of respiratory and cardiovascular diseases. The cohort balance before and after PS adjustment is also presented in Supplemental Fig. 1 and preference score distributions for each database are plotted in Supplemental Fig. 2.
Table 1.
Selected baseline characteristics of study participants before and after large-scale PS stratification.
| Characteristic | Before PS stratification | After PS stratification | ||||
|---|---|---|---|---|---|---|
| Osteoporosis group (%) | Non-osteoporosis group (%) | SMD | Osteoporosis group (%) | Non-osteoporosis group (%) | SMD | |
| N = 105,172 | N = 491,839 | N = 105,172 | N = 491,839 | |||
| Age group (years) | ||||||
| 50–54 | 7.7 | 29.1 | − 0.57 | 25.0 | 25.5 | − 0.01 |
| 55–59 | 13.3 | 20.7 | − 0.20 | 19.5 | 19.6 | 0.00 |
| 60–64 | 16.8 | 19.4 | − 0.07 | 18.4 | 19.1 | − 0.02 |
| 65–69 | 17.6 | 12.6 | 0.14 | 12.5 | 13.4 | − 0.03 |
| 70–74 | 14.1 | 6.9 | 0.23 | 8.2 | 7.9 | 0.01 |
| 75–79 | 10.4 | 4.2 | 0.24 | 5.9 | 5.1 | 0.04 |
| 80–84 | 9.6 | 3.3 | 0.26 | 4.9 | 4.3 | 0.03 |
| 85–89 | 6.4 | 2.2 | 0.21 | 3.3 | 2.9 | 0.02 |
| 90–94 | 3.1 | 1.1 | 0.14 | 1.7 | 1.5 | 0.01 |
| 95–99 | 0.8 | 0.4 | 0.05 | 0.5 | 0.5 | 0.01 |
| 100–104 | 0.1 | 0.1 | 0.02 | 0.1 | 0.1 | 0.01 |
| Gender: female | 85.3 | 46.5 | 0.90 | 53.7 | 53.1 | 0.01 |
| Medical history: General | ||||||
| Acute respiratory disease | 57.1 | 50.4 | 0.13 | 51.7 | 51.3 | 0.01 |
| Chronic liver disease | 4.9 | 3.5 | 0.07 | 4.4 | 3.7 | 0.04 |
| Chronic obstructive pulmonary disease | 2.1 | 1.3 | 0.06 | 1.6 | 1.4 | 0.01 |
| Dementia | 13.7 | 5.3 | 0.29 | 8.1 | 6.9 | 0.05 |
| Depressive disorder | 15.0 | 7.2 | 0.25 | 9.9 | 8.4 | 0.05 |
| Diabetes mellitus | 25.2 | 18.0 | 0.18 | 21.6 | 19.0 | 0.06 |
| Gastroesophageal reflux disease | 36.7 | 25.0 | 0.25 | 28.3 | 26.6 | 0.04 |
| Gastrointestinal hemorrhage | 2.6 | 1.8 | 0.06 | 2.1 | 1.9 | 0.01 |
| Hyperlipidemia | 55.5 | 38.5 | 0.35 | 44.6 | 41.0 | 0.07 |
| Hypertensive disorder | 50.0 | 38.1 | 0.24 | 43.2 | 39.9 | 0.07 |
| Lesion of liver | 2.3 | 2.2 | 0.00 | 2.6 | 2.2 | 0.03 |
| Obesity | 0.1 | 0.1 | 0.01 | 0.1 | 0.1 | 0.00 |
| Osteoarthritis | 24.8 | 11.9 | 0.34 | 14.5 | 13.5 | 0.03 |
| Pneumonia | 4.0 | 2.3 | 0.10 | 3.1 | 2.6 | 0.03 |
| Psoriasis | 0.7 | 0.8 | 0.00 | 0.8 | 0.8 | 0.01 |
| Renal impairment | 3.3 | 1.8 | 0.09 | 2.8 | 2.1 | 0.04 |
| Rheumatoid arthritis | 2.4 | 0.9 | 0.12 | 1.4 | 1.1 | 0.03 |
| Schizophrenia | 1.2 | 1.3 | − 0.01 | 1.7 | 1.3 | 0.03 |
| Ulcerative colitis | 0.2 | 0.1 | 0.01 | 0.3 | 0.1 | 0.03 |
| Urinary tract infectious disease | 5.6 | 3.5 | 0.10 | 4.7 | 3.8 | 0.04 |
| Viral hepatitis C | 0.4 | 0.2 | 0.03 | 0.3 | 0.3 | 0.01 |
| Visual system disorder | 48.0 | 35.4 | 0.26 | 38.5 | 37.1 | 0.03 |
| Medical history: Cardiovascular disease | ||||||
| Atrial fibrillation | 2.0 | 1.2 | 0.06 | 1.5 | 1.3 | 0.02 |
| Cerebrovascular disease | 8.0 | 4.0 | 0.17 | 5.3 | 4.6 | 0.03 |
| Coronary arteriosclerosis | 1.6 | 1.4 | 0.02 | 1.5 | 1.4 | 0.01 |
| Heart disease | 18.8 | 11.8 | 0.19 | 14.3 | 12.8 | 0.04 |
| Heart failure | 6.9 | 3.7 | 0.14 | 4.5 | 4.1 | 0.02 |
| Ischemic heart disease | 9.8 | 6.4 | 0.12 | 7.7 | 6.8 | 0.03 |
| Peripheral vascular disease | 17.1 | 9.3 | 0.23 | 11.5 | 10.4 | 0.04 |
| Pulmonary embolism | 0.5 | 0.2 | 0.04 | 0.4 | 0.3 | 0.02 |
| Venous thrombosis | 1.0 | 0.5 | 0.05 | 0.7 | 0.6 | 0.02 |
PS propensity score, SMD standardized mean difference.
Clinical outcomes of COVID-19 infection in patients with a history of osteoporosis
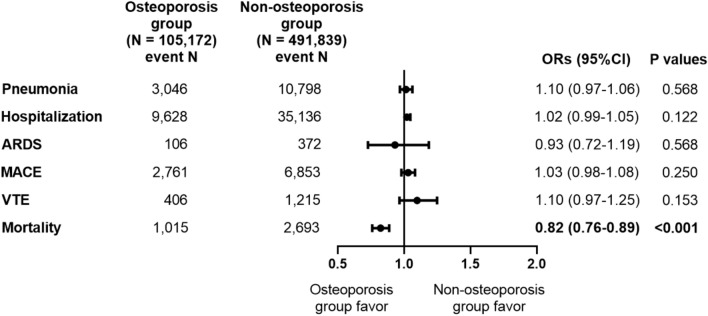
After PS stratification, the odds of clinical outcomes including pneumonia, hospitalization, and ARDS within 30 days after COVID-19 infection were not significantly different between the osteoporosis and non-osteoporosis groups (Fig. 2). Moreover, the odds of cardiovascular outcomes, including MACE and VTE within 30 days after COVID-19 infection were not significantly different in the two groups. However, the odds of mortality within 30 days after COVID-19 infection was significantly lower in the osteoporosis group (OR 0.82, 95% CI 0.76–0.89, P < 0.001). The odds of clinical outcomes including pneumonia, hospitalization, ARDS, MACE, and VTE within 90 days after COVID-19 infection were not significantly different between the osteoporosis and non-osteoporosis groups (Supplemental Fig. 3). However, the odds of mortality within 90 days after COVID-19 infection was significantly lower in the osteoporosis group (OR 0.85, 95% CI 0.79–0.91, P < 0.001).
Figure 2.
Odds ratios for clinical outcomes within 30 days after COVID-19 infection in patients with a previous history of osteoporosis after large-scale PS stratification. ARDS, acute respiratory distress syndrome, MACE, major adverse cardiovascular events, VTE, venous thromboembolism, ORs, odds ratios, CI, confidence interval. Logistic regression analysis was performed after large-scale PS stratification.
Clinical outcomes of COVID-19 infection by sex
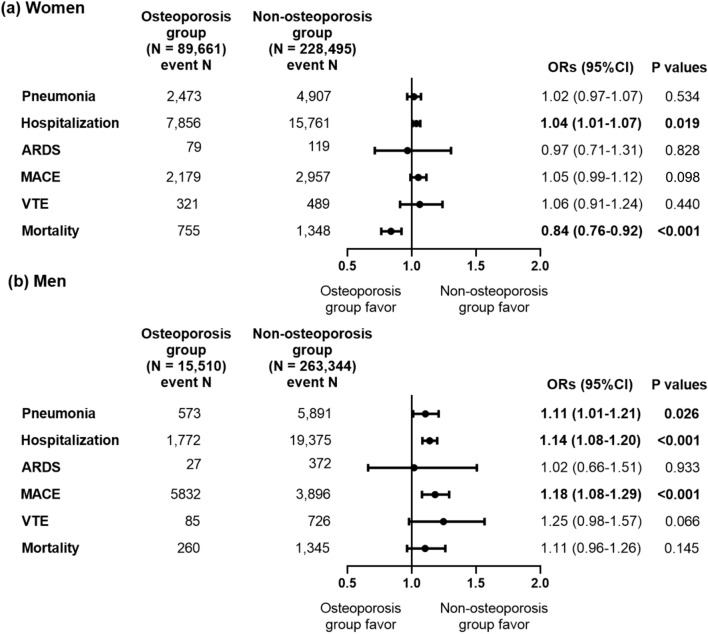
We conducted subgroups analyses according to sex for the comparison of clinical outcomes of COVID-19 infection between the osteoporosis and non-osteoporosis groups. Among women, COVID-19 patients with osteoporosis showed no significant differences in the clinical outcomes within 30 days after COVID-19 infection including pneumonia, ARDS, MACE, and VTE compared to those without osteoporosis (Fig. 3a). Although the odds of hospitalization were higher in female patients with COVID-19 and osteoporosis compared to those without osteoporosis (OR 1.04, 95% CI 1.01–1.07, P = 0.019), their odds of mortality was lower (OR 0.84, 95% CI 0.76–0.92, P < 0.001) within 30 days after COVID-19 infection. The odds of hospitalization were also higher in female patients with COVID-19 and osteoporosis compared to those without osteoporosis (OR 1.04, 95% CI 1.01–1.07, P = 0.015), while the odds of mortality were lower within 90 days of COVID-19 infection (OR 0.84, 95% CI 0.78–0.92, P < 0.001) (Supplemental Fig. 4a). Among men, the odds of clinical outcomes within 30 days after COVID-19 infection including pneumonia (OR 1.11, 95% CI 1.09–1.21, P = 0.026), hospitalization (OR 1.14, 95% CI 1.08–1.20, P < 0.001), and MACE (OR 1.18, 95% CI 1.08–1.29, P < 0.001) were higher in patients with osteoporosis than in those without (Fig. 3b), while the odds of ARDS, VTE, and mortality were not significantly different between the two groups. However, the odds of mortality (OR 1.20, 95% CI 1.06–1.35, P = 0.003) within 90 days of COVID-19 infection were also significantly higher in male patients with osteoporosis than in those without (Supplemental Fig. 4b).
Figure 3.
Odds ratios for clinical outcomes within 30 days after COVID-19 infection in patients with a previous history of osteoporosis according to sex after large-scale PS stratification. (a) Women (b) Men. ARDS, acute respiratory distress syndrome, MACE, major adverse cardiovascular events, VTE, venous thromboembolism, ORs, odds ratios, CI, confidence interval. Logistic regression analysis was performed after large-scale PS stratification.
Clinical outcomes of COVID-19 infection based on previous fracture history
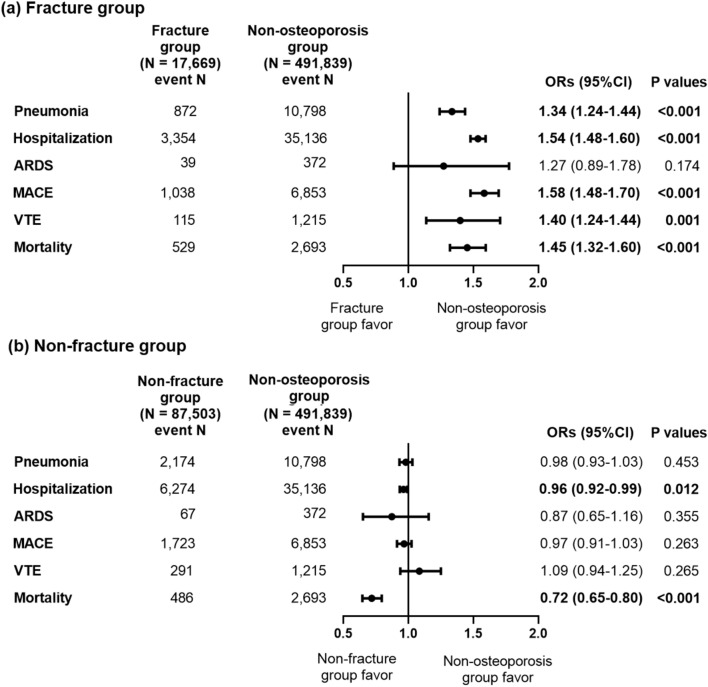
We divided patients in the osteoporosis group into those who had a previous history of fractures (the fracture group; N = 17,669, 16.8%) and those who did not (the non-fracture group; N = 87,503, 83.2%) (Fig. 4). After PS stratification, the odds of clinical outcomes including pneumonia (OR 1.34, 95% CI 1.24–1.44, P < 0.001), hospitalization (OR 1.54, 95% CI 1.48–1.60, P < 0.001), MACE (OR 1.58, 95% CI 1.48–1.70, P < 0.001), VTE (OR 1.40, 95% CI 1.24–1.44, P = 0.001), and mortality (OR 1.45, 95% CI 1.32–1.60, P < 0.001) within 30 days after COVID-19 infection were significantly higher in the fracture group than in the non-osteoporosis group (Fig. 4a). However, in the non-fracture group, the odds of clinical outcomes including pneumonia, ARDS, MACE, and VTE within 30 days after COVID-19 infection were not significantly different from those in the non-osteoporosis group (Fig. 4b). The odds of hospitalization (OR 0.96, 95% CI 0.92–0.99, P = 0.012) and mortality (OR 0.72, 95% CI 0.65–0.80, P < 0.001) within 30 days after COVID-19 infection were significantly lower in the non-fracture than in the non-osteoporosis group. The odds of clinical outcomes including pneumonia (OR 1.33, 95% CI 1.23–1.43, P < 0.001), hospitalization (OR 1.53, 95% CI 1.48–1.59, P < 0.001), MACE (OR 1.55, 95% CI 1.45–1.65, P < 0.001), VTE (OR 1.40, 95% CI 1.14–1.70, P = 0.001), and mortality (OR 1.46, 95% CI 1.34–1.59, P < 0.001) within 90 days after COVID-19 infection were also significantly higher in the fracture group than in the non-osteoporosis group (Supplemental Fig. 5a). However, in the non-fracture group, the odds of clinical outcomes including pneumonia, ARDS, MACE, and VTE within 90 days after COVID-19 infection were not significantly different from those in the non-osteoporosis group, while the odds of mortality (OR 0.76, 95% CI 0.69–0.83, P < 0.001) within 90 days after COVID-19 infection were significantly lower in the non-fracture than in the non-osteoporosis group (Supplemental Fig. 5b).
Figure 4.
Odds ratios for clinical outcomes within 30 days after COVID-19 infection according to previous history of fractures after large-scale PS stratification. (a) The fracture group (b) The non-fracture group. ARDS, acute respiratory distress syndrome, MACE, major adverse cardiovascular events, VTE, venous thromboembolism, ORs, odds ratios, CI, confidence interval. Logistic regression analysis was performed after large-scale PS stratification.
Discussion
In this study, we found that patients with COVID-19 who had a history of osteoporosis did not exhibit significant differences in clinical outcomes, such as pneumonia, hospitalization, ARDS, MACE, and VTE, compared to those without such a history, but they demonstrated improved mortality outcomes. Conversely, among patients with osteoporosis, male sex and a history of fractures were risk factors for worse COVID-19 outcomes in terms of pneumonia, hospitalization, MACE, VTE, and mortality, compared to patients without osteoporosis.
Although the acute phase of the COVID-19 pandemic appears to be subsiding, the virus has not been eradicated and continues to circulate globally. This ongoing presence necessitates sustained vigilance regarding the impact of the disease, particularly among the global aged population, who are more susceptible to various infectious diseases. Despite the fact that osteoporosis is a very prevalent chronic disease in aged populations, the clinical outcome of COVID-19 in patients with osteoporosis has not been broadly studied yet. Only one previous Korean study reported that the risk of COVID-19 infection was associated with comorbid osteoporosis, whereas the risk of severe COVID-19 infection was not17. In this context, our study results are groundbreaking, offering unique insights into the clinical outcomes of COVID-19 infections in patients with osteoporosis and guiding the management of patients with COVID-19 who have poor bone health.
Our study provides evidence that poor bone health can worsen clinical outcomes in patients with COVID-19. Specifically, patients with osteoporosis who have experienced fractures—a marker of a more severe form of the condition—typically exhibited poorer clinical outcomes, including pneumonia, hospitalization, MACE, VTE, and mortality within both 30 and 90 days of COVID-19 infection. Like our study, previous studies have evaluated the role of vertebral fractures as predictors of COVID-19 outcomes20,21. These studies found that vertebral fractures at the time of COVID-19 infection were associated with respiratory deterioration and mortality. Conversely, studies evaluating the association between low bone mineral density (BMD) at the time of COVID-19 infection and disease severity showed inconsistent results22–24. This suggests that fractures, as indicators of poor bone health, rather than low BMD, are significant factors related to severe COVID-19 infection. The negative effects of fractures on the clinical outcomes of patients with COVID-19 infection can be explained by the characteristics of such patients, including old age, poor general health, and concurrent chronic diseases. However, it has been reported that vertebral fractures themselves are associated with impaired respiratory function and pneumonia25,26 and these have been proven to be linked to mortality, not solely explained by age and concurrent diseases27,28. Moreover, the risk of coronary artery disease and stroke is higher in patients with a history of osteoporotic fractures than in non-osteoporotic patients, or even in those with osteoporosis without fractures29,30. A recent small study reported that patients with hip fractures and concomitant COVID-19 infection had an increased risk of MACE and VTE31. Similarly, the results of our study—after adjusting for various confounding factors, including age and concurrent diseases—still indicated poorer outcomes, such as pneumonia, hospitalization, MACE, VTE, and mortality in patients with COVID-19 who had osteoporotic fractures. This suggests a potential direct impact of poor bone health on the clinical outcomes of COVID-19 infections, underscoring the need for more careful management of COVID-19 patients with severe osteoporosis.
In our study, we observed that males diagnosed with osteoporosis encountered significantly poorer outcomes following their COVID-19 infection when compared to their counterparts without osteoporosis. This pattern contrasted with the outcomes observed within the female subgroup, suggesting a gender-specific differential impact. It is noteworthy that males who eventually seek healthcare services for osteoporosis often present with a more advanced and severe form of the condition, including instances of secondary osteoporosis, in contrast to their female counterparts32. Such severe manifestations of osteoporosis in the male population may have a direct correlation with the worse outcomes of COVID-19 in this group, further emphasizing the potential significant impact of compromised bone health on the overall clinical outcomes of COVID-19 infection. This phenomenon further underscores the critical issue of the significant underdiagnosis of osteoporosis among males despite its considerable prevalence, thereby necessitating increased awareness and proactive screening measures, especially in male patients with COVID-19.
However, our study's findings require nuanced interpretation due to several factors. While COVID-19 outcomes did not significantly differ between the osteoporosis and non-osteoporosis groups within 30 and 90 days of infection, the osteoporosis group exhibited lower mortality rates, contrary to expectations. Despite this finding appearing contradictory, it may be related to the characteristics of national claims data, indicating that individuals with osteoporosis diagnosis codes may be healthier due to their more frequent use of health services17. However, there is also a possibility that some factors related to osteoporosis themselves could confer a protective effect against mortality in COVID-19 outcomes. While many approaches have been developed to discover new drugs and targets for inhibiting or treating SARS-CoV-2 infection33–35, efforts have also been made to repurpose existing drugs as therapeutic agents to cure or mitigate the impact of COVID-19. In this context, it has been observed that medications for osteoporosis, such as bisphosphonates and SERMs, might improve COVID-19 outcomes. While two studies particularly focusing on the effects in previous bisphosphonate users showed contradictory results, with one demonstrating that previous bisphosphonate use was associated with the severity of COVID-19 infection and the other that it was not, in the study that found a significant association, previous use of bisphosphonates was linked to reduced odds of COVID-19 diagnosis and related hospitalizations36,37. Raloxifene has been found to modulate the replication of SARS-CoV-2 and act as an immunomodulator, decreasing proinflammatory cytokines, and it has also shown evidence of effect on the primary virologic endpoint in the treatment of patients with early mild- to moderate COVID-19 by shortening the time of viral shedding38–40.
Similarly, there is substantial evidence that vitamin D, commonly used as a supplement in patients with osteoporosis and osteopenia, has a beneficial effect on COVID-19 outcomes41. Vitamin D metabolites can modulate immunopathological inflammation and induce the expression of genes encoding antimicrobial peptides42,43; some of these have been shown to bind to the SARS-CoV-2 spike protein and inhibit its binding to the cellular angiotensin-converting enzyme 2 receptor44,45. Accordingly, consistent evidence supports the association between low 25-hydroxy vitamin D levels and poor COVID-19 outcomes46. Data from interventional studies even suggest that vitamin D administration could positively affect outcomes for patients with COVID-19, although these findings need to be corroborated by robust randomized controlled trials47,48. Since patients in the osteoporosis group may have been treated more frequently with medications such as bisphosphonates or SERMs and supplemented with vitamin D, either prescribed or as self-administered supplements, the better mortality of COVID-19 in the osteoporosis group in our study may be attributable to the effect of these drugs and vitamin D on COVID-19.
This study has several strengths. First, this study evaluated very large numbers of patients with COVID-19 (about half a million using national claims data), which allowed us to draw reliable conclusions about clinical outcomes of COVID-19 according to the comorbid osteoporosis status. Second, we used robust methods to account for the confounding factors, including large-scale PS stratification using several covariates. Third, we conducted various subgroup analyses to demonstrate the severity of osteoporosis on the outcome of COVID-19. Fourth, since we conducted this analysis using CDM based on ATLAS tools, this allows independent researchers to replicate our findings rapidly if we provided the codes for this study based on distributed network analyses. However, this study also has limitations. First, since we used national claims data which did not include BMD data, we categorized patients with osteoporosis or fractures based on the diagnostic codes, which may have introduced potential misclassification bias. Second, albeit this categorization may reflect the health service utilization of patients with osteoporosis, we could not statistically adjust these effects between those categorized as having osteoporosis or not. Third, we could not be provided the cause of mortality because of the characteristics of data from HIRA; thus we could not evaluate whether the mortality of patients with COVID-19 in our study was COVID-19 related or not. Lastly, although lower mortality in the osteoporosis group may be attributed to the positive impact of osteoporosis medications on COVID-19, we observed no significant differences in clinical outcomes among the subgroup using these medications (data not shown), as they constituted a small portion (about 7%) of the osteoporosis group. Additionally, we did not assess the effect of vitamin D on COVID-19 since vitamin D supplementation was largely self-administered and not detectable through national claims data.
In conclusion, our study suggests that patients with severe osteoporosis who have experienced fractures have an elevated risk of severe complications with COVID-19. However, patients with osteoporosis who have not experienced fractures and have sought medical attention have demonstrated a lower risk of mortality. This study is among the first to comprehensively investigate the clinical outcomes of COVID-19 infection in patients with osteoporosis, offering significant clinical implications. It suggests that effective management of osteoporosis could lead to improved clinical outcomes in the context of COVID-19, despite the potential for deteriorating bone health itself to exacerbate the severity of COVID-19 complications. Moreover, our findings indicate a need for more meticulous management of COVID-19 patients with a history of severe osteoporosis to mitigate the risk of adverse outcomes.
Supplementary Information
Acknowledgements
This work was supported by the Korean Endocrine Society. Health Insurance Review and Assessment Service Observational Medical Outcome Common Data Model Database for Covid-19 research (HIRA Covid-19 OMOP database) was provided by HIRA in Korea.
Author contributions
S.H.A, S.-H.S, C.Y.J, D.H.Y, Y.K, Y.C, D.H.S, S.H.K, J-I.Y, and S.H reviewed the literature. S.H.A, S.-H.S, C.Y.J, J-I.Y, and S.H conceived and designed the study. S.H.A and D.H.Y acquired the data. S.H.A, S.-H.S and D.H.Y cleaned and analyzed the data. S.H.A draft the initial version of the manuscript. All authors reviewed the initial draft and made critical contribution to the interpretation of the data and approved the manuscript. The corresponding author attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data availability
The data that support the findings of this study are available from the Health Insurance Review and Assessment Service (HIRA) of South Korea but restrictions apply to the availability of these data, which were used under license for the current study from HIRA of South Korea, and so are not publicly available. Data may be available from the authors (Seong Hee Ahn or Dong Han Yu) upon reasonable request and with permission of HIRA of South Korea.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Seong Hee Ahn and Sung-Hyo Seo.
Contributor Information
Jun-Il Yoo, Email: furim@hanmail.net.
Seongbin Hong, Email: sbhongmd@inha.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68356-0.
References
- 1.The species Severe acute respiratory syndrome-related coronavirus. classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol.5, 536–544. 10.1038/s41564-020-0695-z (2020). 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tisminetzky, M. et al. Age, multiple chronic conditions, and COVID-19: A literature review. J. Gerontol. A Biol. Sci. Med. Sci.77, 872–878. 10.1093/gerona/glaa320 (2022). 10.1093/gerona/glaa320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arentz, M. et al. Characteristics and outcomes of 21 Critically Ill patients with COVID-19 in Washington State. Jama323, 1612–1614. 10.1001/jama.2020.4326 (2020). 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli, G. et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med.180, 1345–1355. 10.1001/jamainternmed.2020.3539 (2020). 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Analysis on 54 Mortality Cases of Coronavirus Disease. in the Republic of Korea from January 19 to March 10, 2020. J. Korean Med. Sci.35(e132), 2020. 10.3346/jkms.2020.35.e132 (2019). 10.3346/jkms.2020.35.e132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkins, J. L. et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci.75, 2224–2230. 10.1093/gerona/glaa183 (2020). 10.1093/gerona/glaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan, W. J. et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J.10.1183/13993003.00547-2020 (2020). 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature584, 430–436. 10.1038/s41586-020-2521-4 (2020). 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, J. et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin. Microbiol. Infect.26, 767–772. 10.1016/j.cmi.2020.04.012 (2020). 10.1016/j.cmi.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement17, 1–45 (2000). [PubMed]
- 11.Wright, N. C. et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res.29, 2520–2526. 10.1002/jbmr.2269 (2014). 10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awosanya, O. D., Dadwal, U. C., Imel, E. A., Yu, Q. & Kacena, M. A. The impacts of COVID-19 on musculoskeletal health. Curr. Osteoporos. Rep.20, 213–225. 10.1007/s11914-022-00734-x (2022). 10.1007/s11914-022-00734-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang, J. COVID-19 pandemic and osteoporosis in elderly patients. Aging Dis.13, 960–969. 10.14336/ad.2021.1201 (2022). 10.14336/ad.2021.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu, C. L. et al. COVID-19 and bone health. Eur. Rev. Med. Pharmacol. Sci.27, 3191–3200. 10.26355/eurrev_202304_31953 (2023). 10.26355/eurrev_202304_31953 [DOI] [PubMed] [Google Scholar]
- 15.Creecy, A. et al. COVID-19 and bone loss: A review of risk factors, mechanisms, and future directions. Curr. Osteoporos. Rep.22, 122–134. 10.1007/s11914-023-00842-2 (2024). 10.1007/s11914-023-00842-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu, E. W., Tsourdi, E., Clarke, B. L., Bauer, D. C. & Drake, M. T. Osteoporosis management in the era of COVID-19. J. Bone Miner. Res.35, 1009–1013. 10.1002/jbmr.4049 (2020). 10.1002/jbmr.4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji, W. et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: A nationwide case-control study. J. Korean Med. Sci.35, e237. 10.3346/jkms.2020.35.e237 (2020). 10.3346/jkms.2020.35.e237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hripcsak, G. et al. Observational health data sciences and informatics (OHDSI): Opportunities for observational researchers. Stud. Health Technol. Inform.216, 574–578 (2015). [PMC free article] [PubMed] [Google Scholar]
- 19.Voss, E. A. et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J. Am. Med. Inform. Assoc.22, 553–564. 10.1093/jamia/ocu023 (2015). 10.1093/jamia/ocu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.di Filippo, L. et al. Radiological thoracic vertebral fractures are highly prevalent in COVID-19 and predict disease outcomes. J. Clin. Endocrinol. Metab.106, e602–e614. 10.1210/clinem/dgaa738 (2021). 10.1210/clinem/dgaa738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.di Filippo, L. et al. Vertebral fractures at hospitalization predict impaired respiratory function during follow-up of COVID-19 survivors. Endocrine77, 392–400. 10.1007/s12020-022-03096-7 (2022). 10.1007/s12020-022-03096-7 [DOI] [PubMed] [Google Scholar]
- 22.Kottlors, J. et al. Early extrapulmonary prognostic features in chest computed tomography in COVID-19 pneumonia: Bone mineral density is a relevant predictor for the clinical outcome: A multicenter feasibility study. Bone144, 115790. 10.1016/j.bone.2020.115790 (2021). 10.1016/j.bone.2020.115790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tahtabasi, M. et al. The prognostic value of vertebral bone density on chest CT in hospitalized COVID-19 patients. J. Clin. Densitom.24, 506–515. 10.1016/j.jocd.2021.07.007 (2021). 10.1016/j.jocd.2021.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakhsh, N., Banjar, M. & Baig, M. Correlation of bone density measured on CT chest with the severity of COVID-19 infection: A retrospective study. PLoS One18, e0286395. 10.1371/journal.pone.0286395 (2023). 10.1371/journal.pone.0286395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe, R., Shiraki, M., Saito, M., Okazaki, R. & Inoue, D. Restrictive pulmonary dysfunction is associated with vertebral fractures and bone loss in elderly postmenopausal women. Osteoporos. Int.29, 625–633. 10.1007/s00198-017-4337-0 (2018). 10.1007/s00198-017-4337-0 [DOI] [PubMed] [Google Scholar]
- 26.Kim, B. et al. Risk of pneumonia after vertebral compression fracture in women with low bone density: A population-based study. Spine43, E830-e835. 10.1097/brs.0000000000002536 (2018). 10.1097/brs.0000000000002536 [DOI] [PubMed] [Google Scholar]
- 27.Jalava, T. et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J. Bone Miner. Res.18, 1254–1260. 10.1359/jbmr.2003.18.7.1254 (2003). 10.1359/jbmr.2003.18.7.1254 [DOI] [PubMed] [Google Scholar]
- 28.Kado, D. M. et al. Vertebral fractures and mortality in older women: A prospective study: Study of Osteoporotic Fractures Research Group. Arch. Intern. Med.159, 1215–1220. 10.1001/archinte.159.11.1215 (1999). 10.1001/archinte.159.11.1215 [DOI] [PubMed] [Google Scholar]
- 29.Laroche, M. et al. Osteoporosis and ischemic cardiovascular disease. Joint Bone Spine84, 427–432. 10.1016/j.jbspin.2016.09.022 (2017). 10.1016/j.jbspin.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 30.Pineda-Moncusí, M. et al. Estimating the incidence and key risk factors of cardiovascular disease in patients at high risk of imminent fracture using routinely collected real-world data from the UK. J. Bone Miner. Res.37, 1986–1996. 10.1002/jbmr.4648 (2022). 10.1002/jbmr.4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koutalos, A. A., Ntalouka, M. P., Angelis, F. A., Hantes, M. & Arnaoutoglou, E. Venous thromboembolism and major adverse cardiovascular events in patients with hip fractures suffering from SARS-CoV-2 infection: A systematic review. Hip Int.33, 1122–1132. 10.1177/11207000221132489 (2023). 10.1177/11207000221132489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinonapoli, G. et al. Osteoporosis in men: A review of an underestimated bone condition. Int. J. Mol. Sci.10.3390/ijms22042105 (2021). 10.3390/ijms22042105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, M. et al. Deciphering the binding mechanism of inhibitors of the SARS-CoV-2 main protease through multiple replica accelerated molecular dynamics simulations and free energy landscapes. Phys. Chem. Chem. Phys.24, 22129–22143. 10.1039/d2cp03446h (2022). 10.1039/d2cp03446h [DOI] [PubMed] [Google Scholar]
- 34.Sun, J. et al. Molecular insights and optimization strategies for the competitive binding of engineered ACE2 proteins: A multiple replica molecular dynamics study. Phys. Chem. Chem. Phys.25, 28479–28496. 10.1039/d3cp03392a (2023). 10.1039/d3cp03392a [DOI] [PubMed] [Google Scholar]
- 35.Suwen, Hu. et al. Races of small molecule clinical trials for the treatment of COVID-19: An up-to-date comprehensive review. Drug Dev Res.83, 16–54. 10.1002/ddr.21895 (2022). 10.1002/ddr.21895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, R. H. et al. Association of prior treatment with nitrogen-containing bisphosphonates on outcomes of COVID-19 positive patients. Osteoporos. Int.35, 181–187. 10.1007/s00198-023-06912-6 (2024). 10.1007/s00198-023-06912-6 [DOI] [PubMed] [Google Scholar]
- 37.Thompson, J. et al. Association between bisphosphonate use and COVID-19 related outcomes. Elife.10.7554/eLife.79548 (2023). 10.7554/eLife.79548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iaconis, D. et al. Characterization of raloxifene as a potential pharmacological agent against SARS-CoV-2 and its variants. Cell Death Dis.13, 498. 10.1038/s41419-022-04961-z (2022). 10.1038/s41419-022-04961-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicastri, E. et al. A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19. EClinicalMedicine48, 101450. 10.1016/j.eclinm.2022.101450 (2022). 10.1016/j.eclinm.2022.101450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allegretti, M. et al. Repurposing the estrogen receptor modulator raloxifene to treat SARS-CoV-2 infection. Cell Death Differ29, 156–166. 10.1038/s41418-021-00844-6 (2022). 10.1038/s41418-021-00844-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilezikian, J. P. et al. Consensus and controversial aspects of vitamin D and COVID-19. J. Clin. Endocrinol. Metab.108, 1034–1042. 10.1210/clinem/dgac719 (2023). 10.1210/clinem/dgac719 [DOI] [PubMed] [Google Scholar]
- 42.Greiller, C. L. & Martineau, A. R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients7, 4240–4270. 10.3390/nu7064240 (2015). 10.3390/nu7064240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ismailova, A., Dimeloe, S., Hewison, M. & White, J. H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus5, e10405. 10.1002/jbm4.10405 (2021). 10.1002/jbm4.10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keutmann, M. et al. The ratio of serum LL-37 levels to blood leucocyte count correlates with COVID-19 severity. Sci. Rep.12, 9447. 10.1038/s41598-022-13260-8 (2022). 10.1038/s41598-022-13260-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kudryashova, E. et al. Inhibition of SARS-CoV-2 infection by human defensin HNP1 and retrocyclin RC-101. J. Mol. Biol.434, 167225. 10.1016/j.jmb.2021.167225 (2022). 10.1016/j.jmb.2021.167225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panagiotou, G. et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin. Endocrinol. (Oxf)93, 508–511. 10.1111/cen.14276 (2020). 10.1111/cen.14276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Niet, S. et al. Positive effects of vitamin D supplementation in patients hospitalized for COVID-19: A randomized, double-blind, placebo-controlled trial. Nutrients.10.3390/nu14153048 (2022). 10.3390/nu14153048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dilokpattanamongkol, P. et al. Impact of vitamin D supplementation on the clinical outcomes of COVID-19 pneumonia patients: A single-center randomized controlled trial. BMC Comp. Med. Ther.24, 97. 10.1186/s12906-024-04393-6 (2024). 10.1186/s12906-024-04393-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Health Insurance Review and Assessment Service (HIRA) of South Korea but restrictions apply to the availability of these data, which were used under license for the current study from HIRA of South Korea, and so are not publicly available. Data may be available from the authors (Seong Hee Ahn or Dong Han Yu) upon reasonable request and with permission of HIRA of South Korea.