Abstract
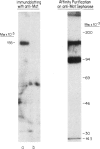
Events that lead to phagocytosis of complement (C3)- or IgG-coated particles after their interaction with specific cell surface receptors are poorly understood. Two mouse monoclonal antibodies (an IgM and an IgG2a) to a human granulocyte-monocyte surface membrane differentiation antigen (Mol) inhibited ingestion by granulocytes both of oil Red O particles opsonized with normal human serum or with IgG and of sheep erythrocytes sensitized with IgG. In addition, they specifically inhibited rosetting between phagocytes and sheep erythrocytes coated with C3bi, a fragment of the complement component C3, generated by cleaving C3b with C3b inactivator and beta IH protein. These monoclonal anti-Mol antibodies did not inhibit IgG Fc, C3b or C3d receptor-mediated binding of erythrocytes coated with the respective proteins. The Fab fragment of the IgG2a monoclonal antibody inhibited noncytotoxic enzyme release from granulocytes when these cells were stimulated with zymosan coated with C3bi. Electrophoretic transfer of polymorphonuclear leukocyte detergent lysates to nitrocellulose, followed by immunofixation with monoclonal antibody, showed that these antibodies were directed to a 155,000-mol wt glycoprotein. This surface membrane structure appears to be involved in Fc and C3 receptor-dependent phagocytosis and closely associated with the C3bi receptor.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Melamed J., Tack B. F., Colten H. R. Characterization of the human complement (c3b) receptor with a fluid phase C3b dimer. J Immunol. 1981 Oct;127(4):1348–1354. [PubMed] [Google Scholar]
- Arnaout M. A., Pitt J., Cohen H. J., Melamed J., Rosen F. S., Colten H. R. Deficiency of a granulocyte-membrane glycoprotein (gp150) in a boy with recurrent bacterial infections. N Engl J Med. 1982 Mar 25;306(12):693–699. doi: 10.1056/NEJM198203253061201. [DOI] [PubMed] [Google Scholar]
- Ault K. A., Springer T. A. Cross-reaction of a rat-anti-mouse phagocyte-specific monoclonal antibody (anti-Mac-1) with human monocytes and natural killer cells. J Immunol. 1981 Jan;126(1):359–364. [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carlo J. R., Ruddy S., Studer E. J., Conrad D. H. Complement receptor binding of C3b-coated cells treated with C3b inactivator, beta 1H globulin and trypsin. J Immunol. 1979 Aug;123(2):523–528. [PubMed] [Google Scholar]
- Crossley L. G. C3b inactivator and beta 1H. Methods Enzymol. 1981;80(Pt 100):112–124. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Stein H. Physicochemical characterization of C3b receptors isolated from human erythrocytes by immunoprecipitation. Immunology. 1980 Dec;41(4):929–936. [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Iida K., Mornaghi R., Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982 May 1;155(5):1427–1438. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melamed J., Arnaout M. A., Colten H. R. Complement (C3b) interaction with the human granulocyte receptor: correlation of binding of fluid-phase radiolabeled ligand with histaminase release. J Immunol. 1982 May;128(5):2313–2318. [PubMed] [Google Scholar]
- NELSON R. A., Jr The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953 Dec 18;118(3077):733–737. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Schreiber R. D., Müller-Eberhard H. J. Interaction of target cell-bound C3bi and C3d with human lymphocyte receptors. Enhancement of antibody-mediated cellular cytotoxicity. J Exp Med. 1981 Jun 1;153(6):1592–1603. doi: 10.1084/jem.153.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Lazarus H., Schlossman S. F. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980 Feb 7;283(5747):583–585. doi: 10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D. Identification of a C3bi-specific membrane complement receptor that is expressed on lymphocytes, monocytes, neutrophils, and erythrocytes. J Exp Med. 1982 Jan 1;155(1):96–110. doi: 10.1084/jem.155.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Hurwitz E. Binding of affinity cross-linked oligomers of IgG to cells bearing Fc receptors. J Immunol. 1977 Apr;118(4):1338–1337. [PubMed] [Google Scholar]
- Springer T. A. Monoclonal antibody analysis of complex biological systems. Combination of cell hybridization and immunoadsorbents in a novel cascade procedure and its application to the macrophage cell surface. J Biol Chem. 1981 Apr 25;256(8):3833–3839. [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Stossel T. P., Alper C. A., Rosen F. S. Serum-dependent phagocytosis of paraffin oil emulsified with bacterial lipopolysaccharide. J Exp Med. 1973 Mar 1;137(3):690–705. doi: 10.1084/jem.137.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Field R. J., Gitlin J. D., Alper C. A., Rosen F. S. The opsonic fragment of the third component of human complement (C3). J Exp Med. 1975 Jun 1;141(6):1329–1347. doi: 10.1084/jem.141.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Schlossman S. F. Analysis of antigenic determinants on human monocytes and macrophages. Blood. 1982 Apr;59(4):775–786. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Van Agthoven A., Schlossman S. F., Terhorst C. Structural analysis of differentiation antigens Mo1 and Mo2 on human monocytes. Hybridoma. 1982;1(3):329–337. doi: 10.1089/hyb.1.1982.1.329. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger R. S., Twarog F. J., Colten H. R. Histaminase release from human granulocytes. J Exp Med. 1976 Oct 1;144(4):1049–1061. doi: 10.1084/jem.144.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]