Abstract
The application of exogenous paclobutrazol (PP333) can improve the ability of winter warming to promote flowering in Chaenomeles speciosa, but the underlying mechanism is unclear. In this study, the cultivar ‘Changshouguan’ was sprayed with different concentrations of PP333 during flower bud differentiation, and the changes in the anatomical structures and physiological characteristics of the flower buds during the differentiation process, as well as the growth state of the flower buds and the effect on flowering promotion after winter warming treatment, were comprehensively investigated. The results showed that different concentrations of PP333 could advance the flowering time of ‘Changshouguan’ by 15–24 d under the warming treatment and increase the flowering duration to 17 d compared with those under the warming treatment alone (CK), and 1000 mg/L was the best treatment. Compared with the CK treatment, the PP333 treatment decreased the contents of indole acetic acid (IAA) and gibberellic acid (GAs) and increased the contents of zeatin ribosides (ZRs) and abscisic acid (ABA), thus changing the balance of hormones during flower bud differentiation. The inflection point (low point) of the curve shapes of the ZRs/GAs and ZRs/IAA ratios appeared significantly earlier, which showed a pattern consistent with soluble sugar and protein content and antioxidant activity. Interestingly, the above changes also corresponded to earlier flowering times during the warming process. Taken together, these results indicate that spraying an appropriate concentration of PP333 in the early stage of ‘Changshouguan’ flower bud differentiation promotes the early differentiation of flower buds and early flowering under winter warming treatment by altering their endogenous hormone content and homeostasis and changing their physiological state. The key to maintaining a relatively long flowering period in plants in the PP333 treatment group after flowering promotion was the increased accumulation of sugars and proteins.
Keywords: Paclobutrazol (PP333), Chaenomeles speciosa ‘Changshouguan’, Flowering regulation, Endogenous hormones, Flower bud differentiation
Subject terms: Plant hormones, Plant physiology
Introduction
Chaenomeles speciosa ‘Changshouguan’, also known as ‘Ruby’, is a type of woody ornamental plant in the Rosaceae family1. The trees are graceful with deep red, dense and double-petal flowers, which is ideal for potted plants and landscapes2. Notably, its natural flowering period occurs in spring (March–April), though it can occur as early as winter (between January and February) with proper flowering regulation3. Therefore, ‘Changshouguan’ is considered a premier flower among woody plants for the Chinese New Year4.
Previous studies concerning plant flowering regulation have mainly focused on the control of cultivation conditions and the utilization of exogenous plant growth regulators5,6. It is generally believed that moderate drought and sufficient light can promote flower bud differentiation in woody plants7,8. The impact of temperature is also significant, but different tree species often have different temperature requirements9–12. Since the advent of plant growth regulators, they have been exploited to meet the growing demand for high-quality plants and products13. Among a range of plant growth regulators, gibberellic acid (GA), which inhibits flower bud formation in most deciduous trees but has the opposite effect in conifers, is crucial for the flowering of woody plants14. Accordingly, the application of GA inhibitors, such as paclobutrazol (PP333), also significantly affects the flowering of plants15. Moreover, an adequate supply of tree nutrients, such as carbohydrates, facilitates the transition from vegetative growth to reproductive growth16. At present, the most effective way to advance the flowering period of ‘Changshouguan’ to the Chinese New Year is to increase the temperature in winter (from December to January of the following year). However, this method always consumes a large amount of energy, which is expensive, especially in large-scale production. Moreover, this is also accompanied by a reduction in the quality of the flowers, such as a decrease in flowering duration. How to reduce the production cost and ensure the quality of flowering is an urgent problem facing its industrial applications.
Paclobutrazol is a type of plant growth retardant with high efficiency and low toxicity and can be easily absorbed by the roots, stems, and leaves of plants17. Recently, it has been extensively applied for flowering promotion in horticultural plants, including apple, mango, bayberry, potato, and tomato18,19, and has long efficacy periods and low cost. Spraying PP333 can significantly inhibit the vegetative growth of plants20 and promote the differentiation rate of flower buds, making its application conducive to the formation and proliferation of flower buds18. This is believed to be closely related to PP333-mediated changes in the levels of different endogenous hormones. In essence, PP333 mainly functions in inhibiting GA synthesis. Wilkie et al.14 reported that exogenous GA3 inhibited the flowering of woody plants and promoted the flowering of herbaceous plants, which was contrary to the effect of PP333. GAs reduced the flowering intensity of sweet orange by repressing CiFT gene expression, while the application of PP333 had the opposite effect, increasing the number of flowers by 23% relative to that in the control group21. Moreover, PP333 also has regulatory effects on plant morphology and physiological and biochemical characteristics, thus contributing to the quality of flowering22,23. However, whether it has similar effects on C. speciosa cultivars remains to be investigated. Moreover, most studies have demonstrated only the effects of PP333 treatment alone on plants, while its combination with other flowering regulation techniques, such as warming, as well as the resulting potential gain effects, requires more attention.
Theoretically speaking, PP333 allows plants to complete the transformation from vegetative growth to reproductive growth in advance and reduces nutrient consumption. Considering the relationships between plant flowering and hormone and nutrient levels, it is speculated that PP333 treatment combined with winter warming regulation may promote the flowering of ‘Changshouguan’ and reduce production costs, but the timing of PP333 application is critical. A preliminary experiment was carried out to verify the above hypothesis. The initial results showed that the effect of PP333 treatment was not obvious in the winter warming stage but was significant in the early stage of flower bud differentiation, which led to the development of a method to promote early flowering in ‘Changshouguan’ by spraying PP333 at an appropriate concentration (Patent No. ZL201410364487.1). To further explore the regulatory effect of PP333, potted ‘Changshouguan’ was selected as the experimental material, and the related mechanism was investigated based on the relationship between early flowering and changes in the anatomical structures of flower buds, the levels of different endogenous hormones, and the physiological state influenced by the low-cost regulator PP333. At present, the most effective method for flowering promotion in C. speciosa is winter warming3. There are no reports on the application of exogenous growth regulators in the flower bud differentiation stage of potted ‘Changshouguan’, nor are there reports on the regulation by growth regulators combined with warming treatment. The results of this study will improve the theory and technology of flowering regulation in C. speciosa plants.
Materials and methods
Plant material and growth conditions
In this study, 4-year-old potted ‘Changshouguan’ trees (one tree per pot) that exhibited relatively consistent growth and were suitable for commercial use were selected as the experimental materials. The plants were placed in the nursery of Wangyue Farm, which is located in Nanjing City, Jiangsu Province, China (32°15ʹN, 118°21ʹE). The soil used for the potted plants was typical yellow–brown earth collected from the nursery. The soil texture was clayey (heavy loam), with a total nitrogen content of 0.704 g/kg, available phosphorus content of 1.295 g/kg, available potassium content of 39.7 g/kg, organic matter content of 7.39 g/kg, and pH of 4.5–5.5.
Experimental design
A total of 50 potted ‘Changshouguan’ that had consistently healthy conditions were selected and randomly divided into 5 groups with 10 pots in each group. Based on the results of preliminary experiments, the concentrations of PP333 used in this study were 1000 mg/L, 1500 mg/L, and 2000 mg/L, and the corresponding treatment groups were represented by A1, A2, and A3, respectively. Moreover, the independent warming treatment group and natural growth group were added, which were represented by CK and CK0, respectively. According to our previously reported patent (No. ZL201410364487.1), the flower bud differentiation of ‘Changshouguan’ started from late May to early June. Therefore, different concentrations of PP333 were sprayed on May 28, June 2, and June 8, and the spray volume per plant was 100 ml. In place of PP333 spraying, an equal amount of deionized water was sprayed instead for the CK and CK0 groups. On December 1, samples from the CK, A1, A2, and A3 groups were transferred to a greenhouse for flowering promotion by warming at 25/16 °C under a light/dark cycle of 12/12 h, while the plants in the CK0 group were placed under natural conditions with a local day/night average temperature of 10/− 3 °C. The temperature-measuring instruments were prepared outdoors and inside the greenhouse to record the temperature dynamics.
Flowering observation of ‘Changshouguan’ under different treatments
To quantify the flowering of ‘Changshouguan’ under different treatments, samples from the CK and A1–A3 groups were transferred to a greenhouse for flowering promotion, and the start dates of different flowering stages, including the initial flowering stage (S1), full flowering stage (S2), initial decline stage (S3), and decay stage (S4), were recorded through daily observation and obtained according to the definitions below. The initial flowering stage refers to the time at which all potted ‘Changshouguan’ plants in each group opened at least their first flower in the greenhouse. The full flowering stage refers to the time at which all potted ‘Changshouguan’ samples in each group were in full bloom (i.e., with a blooming rate of more than 60%). The initial decline stage refers to the time at which at least the first flower of all potted ‘Changshouguan’ plants in each group began to decline, and the decay stage refers to the time at which all the flowers in each group declined. Based on the above observations, the advanced time of flowering and the flowering duration were also calculated. The advanced time of flowering refers to the time at which the plants in the treatment groups (CK, A1, A2, and A3) bloomed earlier than did those in the natural growth group (CK0). The flowering duration is the time from the initial flowering stage to the decay stage.
Determination of flower bud growth in ‘Changshouguan’ under different treatments
From December 1 to January 21 of the following year, the diameter and length of the flower buds of potted ‘Changshouguan’ in the greenhouse were measured with digital Vernier calipers (Mitutoyo, Japan) every 3 days. A total of 18 flower buds from each group were calibrated for dynamic measurements, and the growth curves of the flower buds were plotted.
Determination of flower color in ‘Changshouguan’ under different treatments
Fresh petals were collected from each group at the full flowering stage, and the middle parts of the petals were compared with a color chart from the Royal Horticultural Society (RHSCC). Then, the color of the petals was analyzed using a colorimeter (CR-400 Chroma Meter; Konica Minolta Sensing Americas, Ramsey, NJ). The colorimeter was calibrated with a standard calibration white plate (CR-A43) and configuration luminosity (L*) and two chromaticity coordinates (a* and b*). The L* axis is a grayscale with values ranging from 0 (black) to 100 (white) and is correlated with pigmentation. a* is a red/green axis and correlates with erythema, while positive and negative a* is represented by red and green values, respectively. Similarly, b* is represented by a yellow/blue axis and is correlated with pigmentation and tanning, while positive and negative b* are represented by yellow and blue values, respectively. The analyses were performed in triplicate, and the colorimeter was positioned in a vertical manner in the middle of each petal to ensure equal measurement conditions.
Morphological anatomy of ‘Changshouguan’ flower buds during flower bud differentiation
From June 18 to August 20, the flower buds of the potted ‘Changshouguan’ were collected for morphological observation. The interval between the first two samples was 7 days, and the subsequent interval was 14 days. The flower buds of the A1 and CK groups were obtained according to the sampling time and were immediately stored in 70% formaldehyde–acetic acid–ethanol (FAA) fixative. Then, these flower buds were rapidly descaled under a stereoscopic dissection microscope and photographed.
Determination of the endogenous hormone content in the flower buds of ‘Changshouguan’ under different treatments
From May 28 to July 23, the flower buds of the potted ‘Changshouguan’ were collected to measure the content of endogenous hormones. The interval between the first three samples was 7 days, followed by 14 days. First, the flower buds on the sturdy branches from each group were removed, then their bud scales were removed under a stereoscopic dissection microscope. Afterwards, the samples were sealed in polyethylene plastic bags and stored at – 68 to 70 °C. After all the samples at different stages were collected, the contents of abscisic acid (ABA), indole acetic acid (IAA), zeatin ribosides (ZRs), and GAs were determined by enzyme-linked immunosorbent assay (ELISA) at the Hormone Laboratory of China Agricultural University.
Determination of soluble sugar and soluble protein contents in the flower buds of ‘Changshouguan’ under different treatments
The soluble sugar content was measured via the anthrone–sulfuric acid method24. The descaled flower buds (0.1 g) were mixed with 10 mL distilled water and extracted in boiling water for 30 min (twice). The extract was diluted to 25 mL, and 0.5 mL of the diluent was mixed with 0.5 mL of anthrone ethyl acetate and 5 mL of 98% sulfuric acid, fully shaken, immediately placed in a boiling water bath for 1 min, and then cooled to room temperature naturally. The absorbance of the mixture at 630 nm was measured, and the soluble sugar content was calculated with glucose as a standard. The soluble protein content was measured using the Coomassie Brilliant Blue G-250 method. The descaled flower buds (0.1 g) mixed with 8 mL of distilled water were manually ground into a homogenate, which was subsequently centrifuged at 4000 r/min for 20 min after natural standing for 0.5–1 h. Then, the supernatant was mixed with Coomassie Brilliant Blue G-250, and the absorbance of the mixture was read at 595 nm. The soluble protein content was calculated with bovine serum albumin as a standard.
Determination of antioxidant enzyme activity in ‘Changshouguan’ flower buds under different treatments
The superoxide dismutase (SOD) and peroxidase (POD) activities were measured using the methods reported by Wang et al.25. The descaled flower buds were ground and extracted with precooled phosphate buffer (pH 7.0). After centrifugation, the supernatant was collected as the initial extract of antioxidants. SOD activity was determined by a reaction using the extract mixed with 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 0.75 mM nitro blue tetrazolium, 0.1 mM EDTA, and 0.02 mM riboflavin under illumination at 25 °C for 20 min, and the absorbance of the mixture was read at 560 nm. For POD, the initial extract was mixed with 50 mM phosphate buffer (pH 7.0) and 0.5 mM ascorbic acid, and 0.1 mM H2O2 was added to start the reaction. Then, the POD activity was measured by the rate of guaiacol oxidation at 470 nm.
Statistical analysis
Statistical analysis was conducted using SPSS 17.0 and Microsoft Excel 2003. Multiple comparison analyses were performed using one-way analysis of variance (ANOVA) with Duncan’s test (p < 0.05). Pearson correlation analysis was performed to correlate the degree of different physiological indicators using the online tool ChiPlot (https://www.chiplot.online/). The data obtained are presented as the mean ± standard error (mean ± SE). Microsoft Excel 2003 was used to construct the plots.
Plant material statement
The plant materials used in this study are owned by the authors and no permissions are required. The collection of plant material and the research methods involved are in accordance with local guidelines and legislation.
Results
Effect of different treatments on the flowering of ‘Changshouguan’
As shown in Table 1, the flowering time of the CK ‘Changshouguan’ was 87 days earlier than that of the CK0 ‘Changshouguan’. However, the flowering duration after warming treatment 6 days shorter than that of the CK0 treatment. This indicated that increasing temperature promoted early flowering but affected the quality of the flowers. Compared with the CK treatment, the PP333 treatment had a significant effect on the flowering time of ‘Changshouguan’; the average advanced flowering time of all three PP333 treatments was 19 days. Among the groups, the A1 group treated with 1000 mg/L PP333 exhibited the greatest flowering promotion effect, and the advanced flowering time was 24 days. Moreover, the flowering duration after treatment with different PP333 concentrations was more than 30 days, which was 14–17 days longer than that of the CK group. This indicated that an appropriate concentration of PP333 promoted early flowering and prolonged flowering duration during the process of flowering promotion by warming.
Table 1.
Effects of different treatments on the flowering of ‘Changshouguan’.
| Treatment (mg/L) | S1 (M/D) | S2 (M/D) | S3 (M/D) | S4 (M/D) | Advanced time of flowering (d) | Flowering duration (d) |
|---|---|---|---|---|---|---|
| CK0 | 4/1 | 4/10 | 4/20 | 4/26 | 0 | 25 |
| CK | 1/3 | 1/11 | 1/17 | 1/22 | 87 | 19 |
| A1 | 12/10 | 12/22 | 12/31 | 1/12 | 111 | 33 |
| A2 | 12/16 | 12/25 | 12/31 | 1/21 | 105 | 36 |
| A3 | 12/19 | 12/25 | 1/3 | 1/22 | 102 | 34 |
S1: initial flowering stage; S2: full flowering stage; S3: initial decline stage; S4 decay stage. CK0: natural growth group; CK: independent warming treatment group; A1: 1000 mg/L PP333 spraying + warming treatment group; A2: 1500 mg/L PP333 + warming treatment group; A3: 2000 mg/L PP333 + warming treatment group. M/D: month/day.
Effect of PP333 treatment on flower bud growth in ‘Changshouguan’
As shown in Fig. 1, the flower bud diameter and length in both the CK and PP333 treatment groups exhibited S-shaped growth curves during the process of flowering promotion by warming. However, they differed in terms of the occurrence of the inflection point. Treatment with different concentrations of PP333 advanced the dormancy breaking and growth initiation time of the flower buds, which accelerated their growth. The diameter and length in the A1–A3 groups peaked from December 22 to 25, while those in the CK group were greatest around January 12. Moreover, these peak values of diameter and length in the different groups were not significantly different. This indicated that PP333 treatments from May to June promoted the development of flower buds and had no negative effect on floral organs, while the greatest effect occurred in A1, which had relatively large flower buds during flowering.
Figure 1.
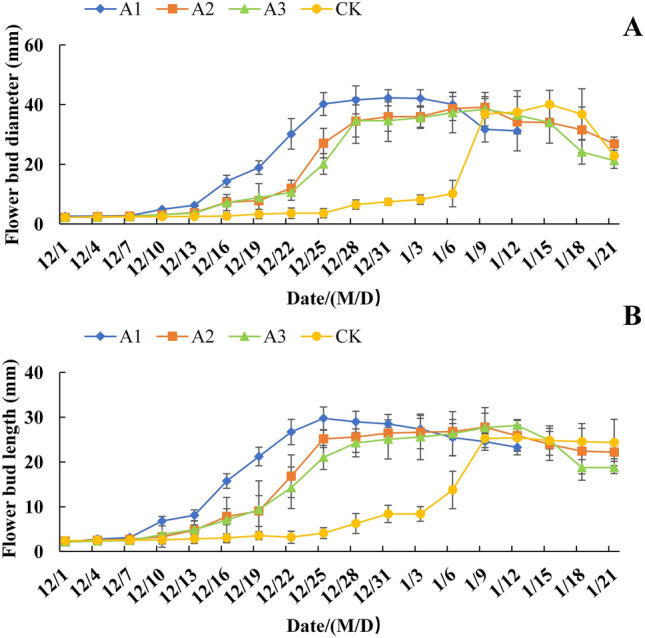
Effect of PP333 treatment on the flowering bud diameter (A) and length (B) of ‘Changshouguan’. Values are presented as mean ± SE (n = 18). CK: independent warming treatment group; A1: 1000 mg/L PP333 spraying + warming treatment group; A2: 1500 mg/L PP333 + warming treatment group; A3: 2000 mg/L PP333 + warming treatment group. M/D: month/day.
Effect of PP333 treatment on flower color in ‘Changshouguan’
As shown in Table 2, the RHSCC in both the CK and A1 groups was 43-C, while that in the A2 and A3 groups was 43-A and 39-B, respectively. However, based on the colorimetric results, the L* values did not significantly differ between the PP333 treatment groups and the CK group, indicating that there was no significant difference in luminosity between the different treatments. The a* values of the different PP333 treatments were significantly lower than that of the CK treatment, which indicated that the green color increased and the red color decreased. The changes in the b* values in the different treatment groups were consistent with those in the a* values, indicating a gradual increase in the blue color, accompanied by a decrease in the yellow color. These combined results further indicated that the application of PP333 did not affect petal luminosity but caused slight fading of petal color.
Table 2.
Measurements of flower color in ‘Changshouguan’ under different treatments.
| Treatment | RHSCC | L* | a* | b* |
|---|---|---|---|---|
| CK | 43-C | 45.9 ± 0.2a | 38.9 ± 0.3a | 29.4 ± 0.4a |
| A1 | 43-C | 43.6 ± 1.1a | 31.3 ± 0.8b | 19.6 ± 1.1b |
| A2 | 43-A | 42.9 ± 2.8a | 31.0 ± 2.0b | 20.3 ± 1.1b |
| A3 | 39-B | 43.6 ± 1.8a | 30.7 ± 1.9b | 18.5 ± 2.3b |
CK: independent warming treatment group; A1: 1000 mg/L PP333 spraying + warming treatment group; A2: 1500 mg/L PP333 + warming treatment group; A3: 2000 mg/L PP333 + warming treatment group. RHSCC: royal horticultural society color chart. L*: luminosity axis; a*: red/green axis; b*: yellow/blue axis. Values are presented as mean ± SE (n = 3). Different letters reflect significant differences among different treatments (p < 0.05).
Observations of flower bud morphological differentiation in ‘Changshouguan’
As shown in Fig. 2, the flower bud primordia of ‘Changshouguan’ in the A1 treatment group began to differentiate, with obvious protrusions observed on June 18, while those in the CK group showed similar phenomena but were less significant than those in the A1 group. By June 25, the protrusions of flower bud primordia were more pronounced in the CK and A1 groups, while protrusions of pistils and stamens were observed in the A1 group. On July 9, petal primordia was clearly developed in the A1 group, indicating that flower bud morphological development in the A1 group had reached the middle stage, and this occurred significantly earlier than in the CK group. The development of flower buds in the A1 group basically ended on July 23 with the gradual formation of sepal primordia, and the flower bud morphology remained unchanged in the later stage, while the development of flower buds in the CK group gradually ended on August 6. Full flower buds could be seen in the photographs after August.
Figure 2.
Morphological observation of flower buds in ‘Changshouguan’ at different differentiation stages under different treatments. CK: independent warming treatment group; A1: 1000 mg/L PP333 spraying + warming treatment group. FP: flower bud primordium; S: stamens; P: pistil; PP: petal primordium; SP: sepal primordium.
Effect of PP333 treatment on endogenous hormones in the flower buds of ‘Changshouguan’
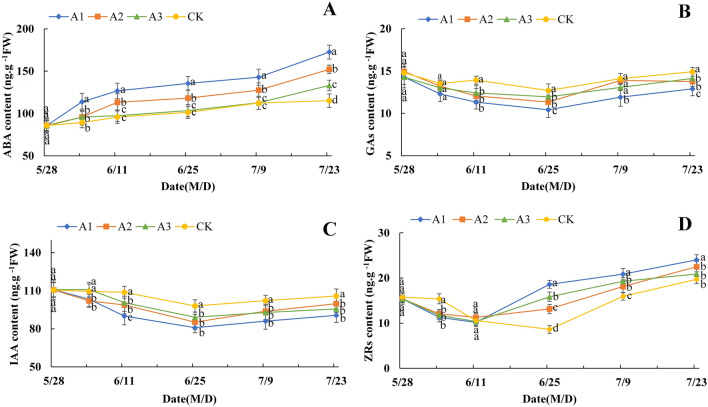
As shown in Fig. 3A, the ABA content of flower buds in both the CK and PP333 treatment groups gradually increased during the experimental period. Compared with those in the CK treatment, the ABA content in the flower buds in the PP333 treatment group significantly increased, especially from May 28–June 11 and July 9–July 23. Moreover, the ABA content in the different PP333 treatment groups was generally greater than that in the CK group, among which the most prominent group was observed in A1, whose content was 49.89% greater than that in the CK group by July 23. The GAs content of flower buds in both the CK and PP333 treatment groups tended to first decrease and then increase, with the lowest values occurring around June 25. Compared with those in the CK treatment group, the GAs content in the flower buds decreased in the PP333 treatment group, with a large decrease occurring from May 28–June 11, while the effect was most obvious in the A1 treatment group. By June 25, the GAs contents in the A1, A2, and A3 groups decreased by 15.67%, 8.36%, and 5.96%, respectively, in comparison with those in the CK group (Fig. 3B). As shown in Fig. 3C, the IAA content of flower buds in both the CK and PP333 treatment groups displayed a trend similar to that of the GA content, with the lowest values occurring around June 25. Compared with that in the CK group, the IAA content in the PP333 treatment group decreased throughout the experiment, and the IAA content in the A1 group was greatest. By June 25, the IAA content in the A1, A2, and A3 groups decreased by 16.90%, 6.14%, and 10.66%, respectively, relative to that in the CK group. The ZRs of flower buds in both the CK and PP333 treatment groups also first decreased and then increased, but their lowest points occurred at different times. From May 28–June 25, the ZRs content in the CK group gradually decreased and reached its lowest value on June 25, while that in the PP333 treatment groups appeared on June 11, 14 days earlier than that in the CK group. Moreover, compared with the CK treatment, the PP333 treatment increased the content of ZRs, while A1 had the most significant effect (Fig. 3D).
Figure 3.
Effect of PP333 treatment on content of ABA (A), GAs (B), IAA (C), and ZRs (D) in ‘Changshouguan’. Values are presented as mean ± SE (n = 3). Different letters reflect significant differences among different treatments on the same date (p < 0.05). CK: independent warming treatment group; A1: 1000 mg/L PP333 spraying + warming treatment group; A2: 1500 mg/L PP333 + warming treatment group; A3: 2000 mg/L PP333 + warming treatment group. M/D: month/day.
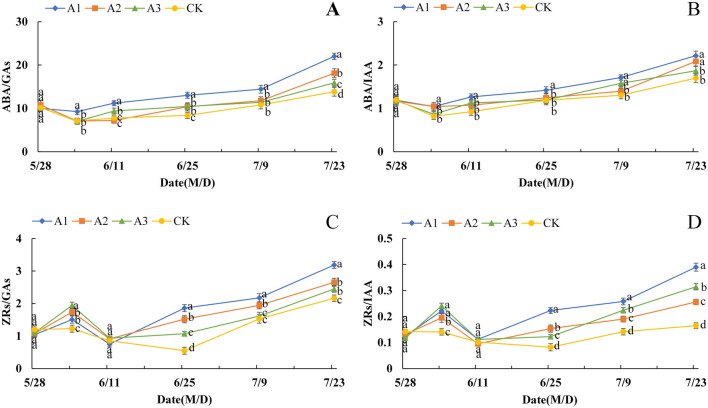
Effect of PP333 treatment on the dynamic balance of endogenous hormones in the flower buds of ‘Changshouguan’
As shown in Fig. 4A, the ABA/GAs ratio of flower buds in both the CK and PP333 treatment groups generally increased gradually but decreased slightly during the initial period between May 28 and June 4. Compared with that of the CK treatment, the ABA/GAs ratio in the PP333 treatment group generally increased, while A1 had the most obvious effect, as the ABA/GAs ratio in the PP333 treatment group was significantly greater than that in the other groups after June 4. The changes in the ABA/IAA ratio of flower buds in the different groups showed a trend of first decreasing and then increasing. On May 28–June 4, the ratio of ABA/IAA decreased to different degrees in the CK and PP333 treatment groups, reached the lowest value on June 4, then increased gradually, and further increased in magnitude on June 25–July 23. Moreover, compared with the CK treatment, the PP333 treatment increased the ABA/IAA ratio, and the most significant effect was observed in A1 (Fig. 4B). The changes in the ZRs/GAs ratio of flower buds in the different treatment groups displayed a general trend of first increasing, then decreasing and then increasing. However, the occurrence time at the lowest point differed between the CK and PP333 treatment groups. For CK, the lowest value was observed on June 25, while that of the PP333 treatment group was on June 11, approximately 14 days earlier than that of the former group. Moreover, compared with that of the CK treatment, the ZRs/GAs ratio increased in the PP333 treatment group at different concentrations, with the most significant effect observed in A1, which was 22.6% and 54.1% greater than that in the CK treatment group at the relatively high (June 4) and low (June 25) points, respectively (Fig. 4C). The overall trend of the ZRs/IAA ratio was similar to that of the ZRs/GAs ratio, which first increased, then decreased and then increased again, with the lowest point occurring at approximately June 25 in the CK treatment and on June 11 in the PP333 treatments, 14 days earlier than in the former. Notably, in comparison with the CK treatment, the PP333 treatment also caused greater ZRs/IAA ratios in the flower buds; A1 had the most obvious effect, 26.3% and 46.7% greater than that of the CK treatment at the relatively high (June 4) and low (June 11) points, respectively (Fig. 4D).
Figure 4.
Effect of PP333 treatment on the ratio of ABA/GAs (A), ABA/IAA (B), ZRs/GAs (C), and ZRs/IAA (D) in ‘Changshouguan’. Values are presented as mean ± SE (n = 3). Different letters reflect significant differences among different treatments on the same date (p < 0.05). CK: independent warming treatment group; A1: 1000 mg/L PP333 spraying + warming treatment group; A2: 1500 mg/L PP333 + warming treatment group; A3: 2000 mg/L PP333 + warming treatment group. M/D: month/day.
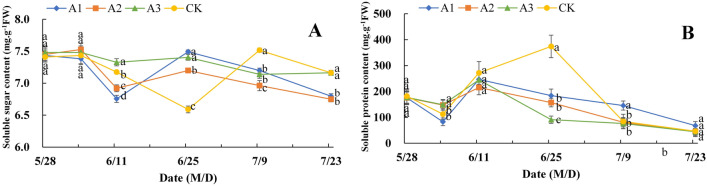
Effect of PP333 treatment on the soluble sugar and soluble protein contents in the flower buds of ‘Changshouguan’
As shown in Fig. 5A, the soluble sugar content in both the CK and PP333 treatment groups tended to first decrease, then increase and finally decrease, with first peak values of approximately 7.4–7.5 mg g−1 FW on June 4. The lowest values in the A1, A2, and A3 groups were observed on June 11, at approximately 6.7–7.3 mg g−1 FW, while that in the CK group was 6.59 mg g−1 FW on June 25. Moreover, the second peak values of soluble sugar content in the A1-A3 groups appeared on June 25, while those in the CK group were observed on July 9, with nonsignificant differences in the values between the CK and A1 groups. After that, the soluble sugar content in the different groups tended to decrease. The soluble protein content in both the CK and PP333 treatment groups also tended to first decrease, then increase and finally decrease, with the lowest values observed on June 4. Notably, the peaks in the A1-A3 groups appeared on June 11, while that in the CK group was observed on June 25. The peak values in the A1–A3 groups decreased by 33.93%, 40.55%, and 33.62%, respectively, compared with those in the CK group. After that, the contents in the different groups gradually decreased and tended to be the same (Fig. 5B).
Figure 5.
Effect of PP333 treatment on soluble sugar (A) and soluble protein content (B) in ‘Changshouguan’. Values are presented as mean ± SE (n = 3). Different letters reflect significant differences among different treatments on the same date (p < 0.05). CK: independent warming treatment group; A1: 1000 mg/L PP333 spraying + warming treatment group; A2: 1500 mg/L PP333 + warming treatment group; A3: 2000 mg/L PP333 + warming treatment group. M/D: month/day.
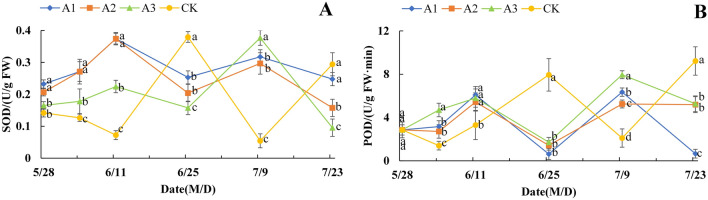
Effect of PP333 treatment on antioxidant enzyme activity in the flower buds of ‘Changshouguan’
As shown in Fig. 6A, the SOD activity in the CK treatment group tended to increase first, then decrease and then increase after a slight decrease at the initial stage. The first peak in CK appeared near 0.377 U/g FW on June 25, and the second peak was approximately 0.294 U/g FW on July 23. In comparison, the SOD activity in the A1–A3 treatments showed a significant “double-peak” pattern. The two peaks in A1–A3 occurred approximately 14 days earlier than those in CK. Interestingly, the first peak in the A1 and A2 groups was 66.67% and 65.73% greater than that in the A3 group, respectively, and the difference was significant, but this was reversed at the second peak. The trend of POD activity in the different treatment groups was similar to that of SOD activity. The first peak of the A1–A3 groups appeared on June 11, and the difference was not significant, while the second peak was consistent with the SOD results. On July 9, the POD activity of A3 increased by 25.37% and 51.58% compared with that of A1 and A2, respectively (Fig. 6B).
Figure 6.
Effect of PP333 treatment on SOD (A) and POD (B) activity in ‘Changshouguan’. Values are presented as mean ± SE (n = 3). Different letters reflect significant differences among different treatments on the same date (p < 0.05). CK: independent warming treatment group; A1: 1000 mg/L PP333 spraying + warming treatment group; A2: 1500 mg/L PP333 + warming treatment group; A3: 2000 mg/L PP333 + warming treatment group. M/D: month/day.
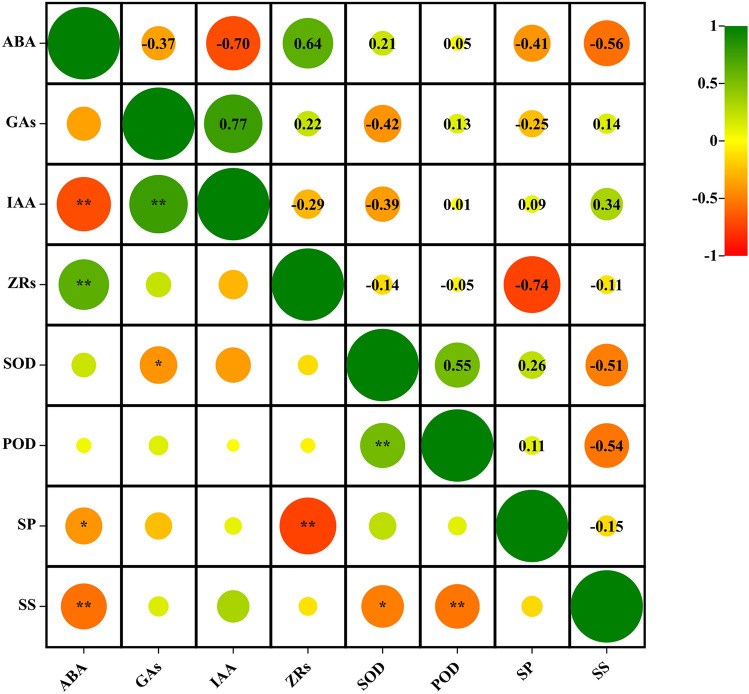
Pearson correlation analysis of physiological indicators in ‘Changshouguan’ flower buds
Pearson correlation analysis was performed to evaluate the relationships among different physiological indicators in the flower buds of ‘Changshouguan’ under the different treatments (Fig. 7). ABA was strongly positively correlated with ZRs (p < 0.01) and strongly negatively correlated with IAA (p < 0.01), while a strong positive correlation was detected between GAs and IAA (p < 0.01). There were also significant negative correlations between ABA and soluble sugars, between the ZRs and soluble proteins, and between POD and soluble sugars (p < 0.01). SOD was negatively correlated with GAs and soluble sugars (p < 0.05), which was consistent with the correlation between ABA and soluble protein (p < 0.05). Moreover, a significant positive correlation was observed between SOD and POD (p < 0.01).
Figure 7.
Pearson correlation analysis of different physiological indicators in ‘Changshouguan’. * indicates significant correlations at the p < 0.05 level; ** indicates significant correlations at the p < 0.01 level. ABA, abscisic acid; GAs, gibberellic acid; IAA, indole acetic acid; ZRs, zeatin ribosides; SOD, superoxide dismutase; POD, peroxidase; SP, soluble protein; SS, soluble sugar.
Discussion
In recent years, plant growth regulators have been extensively applied to regulate the growth and development of plants, while spraying regulators at an appropriate concentration can affect blossom formation, reduce plant height, improve the fruit setting rate, and increase crop yield26. In terms of controlling the flowering of ornamental plants, plant growth regulators mainly promote early (or delayed) flowering by breaking (or prolonging) dormancy and promoting (or inhibiting) flower bud growth and opening27, while few studies have been conducted on regulating the stages of flower bud differentiation. In this study, potted ‘Changshouguan’ was treated with different concentrations of PP333 during the early stage of flower bud differentiation, which significantly advanced the dormancy breaking time of flower buds during the promotion of flowering by warming in the winter. Moreover, it promoted the growth of flower buds after dormancy breaking. Notably, the flowering period of the combined warming and PP333 treatment groups occurred 15–24 days earlier than that of the warming treatment group, while the flowering duration of the A1–A3 groups reached 33–36 days, which was significantly greater than that of the CK group, indicating a significant flowering-promoting effect. The disadvantage appears to be a slight decrease in flower color caused by PP333 treatment, which was consistent with the phenomenon in Herbaceous peony reported by Wu et al.28. This could be a result of PP333 acting as a GA inhibitor, reducing GA transport from anther to petal29,30. Given the greatly shortened duration of the warming treatment, as well as the low price and easy application of PP333 by foliar spray, this combined method substantially reduced the cost of flowering promotion and showed strong practical value for production.
Endogenous hormones are closely related to flower formation in plants, and exogenous growth regulators are thought to influence flower bud differentiation by regulating changes in endogenous hormones13,26,31,32. In the present study, the application of PP333 at different concentrations had a significant effect on the contents of endogenous hormones in the flower buds of ‘Changshouguan’, which mainly decreased the contents of IAA and GAs and increased the contents of ZRs and ABA. Studies have reported that a high GAs content significantly inhibits flower bud differentiation in different plants, such as apple, pear, citrus and other tree species33–36. The effect of IAA on flower bud differentiation has not been fully studied. Chen et al.37 suggested that the use of IAA inhibitors can promote the flowering of castor bean. A similar phenomenon was also demonstrated by Zhang et al.38 in a study of Fuji apples. This provides a plausible explanation for the changes in the contents of IAA and GAs in ‘Changshouguan’. Apparently, the regulatory effect of PP333 on flower bud differentiation in ‘Changshouguan’ was mediated by decreasing the GA content, but PP333 did not systematically or quantitatively decrease the GAs content with increasing concentration, indicating that PP333 had a concentration-dependent effect on flower bud differentiation in ‘Changshouguan’, while high concentrations of PP333 might stimulate stress defense in plants to prevent the potential toxicity of PP333. The effect of ABA on flower bud differentiation is controversial. Soler and Cuevas33 reported that high levels of ABA were crucial for flowering promotion, while Baldermann et al.34 further noted that a high ABA content was conducive to the differentiation of flower primordia. Therefore, the high ABA content in ‘Changshouguan’ is important for maintaining the normal physiological and morphological differentiation of flower buds. Many studies have shown that the content of ZRs in flower buds decreases at the physiological differentiation stage, while it increases sharply at the morphological differentiation stage and remains high to further promote the differentiation of flower primordia and the morphological establishment of flower buds39–41. These findings reasonably explain the shapes of the endogenous hormone contents in the flower buds of ‘Changshouguan’ flower buds. Interestingly, the time inflection point (low point) of the ZRs in the flower buds treated with PP333 moved forward significantly, and the most obvious change was observed in Group A1, in which the time of physiological and morphological differentiation was advanced by 14 days, indicating that exogenous PP333 treatment accelerates the formation of flower primordia in ‘Changshouguan’. Taken together, these results suggest that PP333 treatment impacts the levels of different endogenous hormones during the differentiation of flower buds in ‘Changshouguan’ and that these changes promote the induction and formation of flower buds.
The effect of endogenous hormones on flower bud differentiation depends not only on changes in the content of a single hormone but also on the dynamic balance of multiple endogenous hormones42,43. Compared with the CK treatment, the PP333 treatments resulted in significant changes in the ratio of endogenous hormones during the stage of flower bud differentiation in ‘Changshouguan’, which mainly induced increased ratios of ABA/IAA, ABA/GAs, ZRs/GAs and ZRs/IAA. The ratios of ZRs/GAs and ZRs/IAA increased sharply, and they gradually peaked with the progression of flower bud differentiation. Moreover, the PP333 treatments also advanced the inflection point (low point) of the dynamic changes in the ZR content, as well as the ZRs/GAs and ZRs/IAA ratios, by approximately 14 days, and this effect corresponded to the advance in flowering time during the warming process. Therefore, PP333 treatment was speculated to affect the dynamic process of flower bud differentiation in ‘Changshouguan’ by mediating the content and balance of endogenous hormones in flower buds. It has been reported that the ZR content and the dynamics of the ZR/GA and ZR/IAA ratios indirectly reflect the stage of flower primordium formation44,45. The advance in the inflection point promoted the physiological differentiation of flower buds in ‘Changshouguan’, which was regulated by endogenous hormones and accelerated their progress to the stage of morphological differentiation. The most notable feature was that the extreme values of sugar and protein contents as well as antioxidant activity advanced and were closely correlated with each other, which are considered synergistic physiological markers for the initiation of flower bud differentiation46. These physiological changes also corresponded to the observations of flower bud growth in this study.
Warming treatment alone rapidly promoted the growth of flower buds in ‘Changshouguan’, and the flowering time was 87 days earlier than that in the natural growth group, but the flowering duration was significantly shorter, at 6 days shorter than that of the CK0 group. In contrast, on average, the flowering time of the PP333 plus warming treatment group was 19 days earlier than that of the warming group alone, and the flowering duration of the PP333 plus warming treatment group was up to 17 days longer than that of the CK group. The acceleration of flower bud differentiation enables the flower buds of ‘Changshouguan’ to enter dormancy in advance, which is necessary in its life cycle, while complete intrinsic physiological dormancy (endodormancy) is considered to guarantee budding and flowering in the following year9,47. Endodormancy is also considered a process of nutrient accumulation in plants48. Warming treatment alone induced early flowering, but this effect was more dependent on the shortened duration of endodormancy and essential substance accumulation and ultimately caused a shortened flowering duration. In contrast, the soluble sugar content peaked in the PP333 treatment group at the flower bud differentiation stage, especially in the A1 group, which was not significantly different from that in the CK group. Moreover, the consumption of soluble protein in the PP333 treatment groups was lower than that in the CK group, especially in the A1 group. Therefore, a reasonable explanation is that PP333 treatment could benefit the early completion of endodormancy and accelerate the accumulation of sugars and proteins, thus advancing the growth of flower buds, promoting early flowering, and prolonging flowering duration during winter warming.
Conclusion
In summary, the application of PP333 at an appropriate concentration during the period of flower bud differentiation in ‘Changshouguan’ promoted flower bud growth after winter warming, thus promoting early flowering and prolonging flowering duration. The results of the endogenous hormone content analysis showed that PP333 treatment changed the contents and dynamic balance of different hormones during flower bud differentiation, especially at the inflection point of the dynamic changes in ZRs content, as well as in the ZRs/GAs and ZRs/IAA ratios, which occurred approximately 14 days earlier than that in the warming treatment group alone, and this effect corresponded to the advance in flowering time during the warming process. Therefore, PP333 treatment is speculated to affect the dynamic process of flower bud differentiation by mediating the content and homeostasis of endogenous hormones in flower buds, thus accelerating the transformation from physiological differentiation to morphological differentiation, which could be demonstrated by changes in a series of synergistic physiological markers. Moreover, PP333-mediated physiological changes could promote the early completion of endodormancy and accelerate the accumulation of essential substances, which is also crucial for promoting the early flowering and quality of ‘Changshouguan’. Further investigations should focus on PP333-mediated overall changes in plant life history and precise verification based on molecular biology techniques, which will provide a comprehensive theoretical basis for the research and development of low-cost and high-quality flowering regulation technology for the production of ‘Changshouguan’.
Author contributions
W.Z. and Y.X. designed the experiment. S.L., M.S., and W.L. conducted the experiments. All authors contributed to data analysis and review. S.L. and T.W. wrote the manuscript and all authors contributed to the review of the manuscript.
Funding
This research was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data availability
All data are presented in the article, and can be requested from the corresponding author if required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tao Wang, Email: johnwt1007@163.com.
Yinfeng Xie, Email: xxyyff@njfu.edu.cn.
References
- 1.Liang, W. et al. Effects of nitrogen, phosphorus and potassium compound fertilization on the physiological characteristics of Chaenomeles speciosa ‘Changshouguan’ after processing of warming in the post floral stage. J. Nanjing For. Univ.46, 81 (2022). [Google Scholar]
- 2.Watychowicz, K., Janda, K., Jakubczyk, K. & Wolska, J. Chaenomeles–health promoting benefits. Roczniki Państwowego Zakładu Higieny68 (2017). [PubMed]
- 3.Sun, X. et al. Effects of increasing temperature to promote flowering on the diurnal variation of photosynthesis in potted Chaenomeles speciosa (sweet) Nakai “Changshouguan”. Acta Agric. Univ. Jiangxiensis39, 64–71 (2017). [Google Scholar]
- 4.Miao, J. et al. Chemical composition and bioactivities of two common Chaenomeles fruits in China: Chaenomeles speciosa and Chaenomeles sinensis. J. Food Sci.81, H2049–H2058 (2016). 10.1111/1750-3841.13377 [DOI] [PubMed] [Google Scholar]
- 5.Freytes, S. N., Canelo, M. & Cerdán, P. D. Regulation of flowering time: When and where?. Curr. Opin. Plant Biol.63, 102049. 10.1016/j.pbi.2021.102049 (2021). 10.1016/j.pbi.2021.102049 [DOI] [PubMed] [Google Scholar]
- 6.Izawa, T. What is going on with the hormonal control of flowering in plants?. Plant J. Cell Mol. Biol.105, 431–445. 10.1111/tpj.15036 (2021). 10.1111/tpj.15036 [DOI] [PubMed] [Google Scholar]
- 7.Koutinas, N., Pepelyankov, G. & Lichev, V. Flower induction and flower bud development in apple and sweet cherry. Biotechnol. Biotechnol. Equip.24, 1549–1558 (2010). 10.2478/V10133-010-0003-9 [DOI] [Google Scholar]
- 8.Bartolini, S., Viti, R. & Andreini, L. The effect of summer shading on flower bud morphogenesis in apricot (Prunus armeniaca L). Open Life Sci.8, 54–63 (2013). 10.2478/s11535-012-0109-1 [DOI] [Google Scholar]
- 9.Fadón, E., Rodrigo, J. & Herrero, M. Is there a specific stage to rest? Morphological changes in flower primordia in relation to endodormancy in sweet cherry (Prunus avium L.). Trees32, 1583–1594 (2018). 10.1007/s00468-018-1735-7 [DOI] [Google Scholar]
- 10.Sønsteby, A. & Heide, O. M. Temperature effects on growth and floral initiation in sweet cherry (Prunus avium L.). Scientia Horticulturae257, 108762 (2019). 10.1016/j.scienta.2019.108762 [DOI] [Google Scholar]
- 11.O’Hare, T. J. Impact of root and shoot temperature on bud dormancy and floral induction in lychee (Litchi chinensis Sonn.). Scientia Horticulturae99, 21–28 (2004). 10.1016/S0304-4238(03)00083-9 [DOI] [Google Scholar]
- 12.Hidaka, K., Dan, K., Imamura, H. & Takayama, T. Crown-cooling treatment induces earlier flower bud differentiation of strawberry under high air temperatures. Environ. Control Biol.55, 21–27 (2017). 10.2525/ecb.55.21 [DOI] [Google Scholar]
- 13.Gill, K. et al. Physiological perspective of plant growth regulators in flowering, fruit setting and ripening process in citrus. Scientia Horticulturae309, 111628 (2023). 10.1016/j.scienta.2022.111628 [DOI] [Google Scholar]
- 14.Wilkie, J. D., Sedgley, M. & Olesen, T. Regulation of floral initiation in horticultural trees. J. Exp. Bot.59, 3215–3228 (2008). 10.1093/jxb/ern188 [DOI] [PubMed] [Google Scholar]
- 15.Upreti, K. K. et al. Hormonal changes in response to paclobutrazol induced early flowering in mango cv. Totapuri. Scientia Horticulturae150, 414–418 (2013). 10.1016/j.scienta.2012.11.030 [DOI] [Google Scholar]
- 16.Chen, C. & Yu, F. Research progress on flower bud differentiation of trees. Sci. Silvae Sin56, 119–129 (2020). [Google Scholar]
- 17.Soumya, P., Kumar, P. & Pal, M. Paclobutrazol: A novel plant growth regulator and multi-stress ameliorant. Indian J. Plant Physiol.22, 267–278 (2017). 10.1007/s40502-017-0316-x [DOI] [Google Scholar]
- 18.Ashraf, N. & Ashraf, M. Prunus (IntechOpen, 2020). [Google Scholar]
- 19.Lolaei, A., Mobasheri, S., Bemana, R. & Teymori, N. Role of paclobutrazol on vegetative and sexual growth of plants. Int. J. Agric. Crop Sci.5, 958 (2013). [Google Scholar]
- 20.Huo, W. et al. Paclobutrazol and plant-growth promoting bacterial endophyte Pantoea sp. enhance copper tolerance of guinea grass (Panicum maximum) in hydroponic culture. Acta Physiologiae Plantarum34, 139–150 (2012). 10.1007/s11738-011-0812-y [DOI] [Google Scholar]
- 21.Muñoz-Fambuena, N. et al. Gibberellic acid reduces flowering intensity in sweet orange [Citrus sinensis (L.) Osbeck] by repressing CiFT gene expression. J. Plant Growth Regul.31, 529–536 (2012). 10.1007/s00344-012-9263-y [DOI] [Google Scholar]
- 22.Jungklang, J., Saengnil, K. & Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci.24, 1505–1512 (2017). 10.1016/j.sjbs.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker, A., McCall, I. & Whipker, B. E. Growth control of ‘Imperial Dark Blue’ plumbago with ethephon, flurprimidol, and paclobutrazol substrate drenches. HortTechnology26, 493–496 (2016). 10.21273/HORTTECH.26.4.493 [DOI] [Google Scholar]
- 24.Wang, X. et al. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep.9, 8543. 10.1038/s41598-019-44958-x (2019). 10.1038/s41598-019-44958-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, T. et al. Effects of seasonal changes on chlorophyll fluorescence and physiological characteristics in the two Taxus species. Plants12, 2636 (2023). 10.3390/plants12142636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desta, B. & Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric.8, 1–15 (2021). 10.1186/s40538-020-00199-z [DOI] [Google Scholar]
- 27.Ramzan, F. et al. Pre-planting exogenous application of gibberellic acid influences sprouting, vegetative growth, flowering, and subsequent bulb characteristics of ‘Ad-Rem’tulip. Hortic. Environ. Biotechnol.55, 479–488 (2014). 10.1007/s13580-014-0113-7 [DOI] [Google Scholar]
- 28.Wu, Y., Liu, J., Zhao, D. & Tao, J. Effect of paclobutrazol application on plant growth and flower quality in herbaceous peony. Phyton91, 2017–2032 (2022). 10.32604/phyton.2022.020643 [DOI] [Google Scholar]
- 29.Ravid, J. et al. GA as a regulatory link between the showy floral traits color and scent. New Phytol.215, 411–422 (2017). 10.1111/nph.14504 [DOI] [PubMed] [Google Scholar]
- 30.Maheshwari, C. et al. Insight of PBZ mediated drought amelioration in crop plants. Front. Plant Sci.13, 1008993 (2022). 10.3389/fpls.2022.1008993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng, W. et al. SlCAND1, encoding cullin-associated Nedd8-dissociated protein 1, regulates plant height, flowering time, seed germination, and root architecture in tomato. Plant Mol. Biol.102, 537–551 (2020). 10.1007/s11103-020-00963-7 [DOI] [PubMed] [Google Scholar]
- 32.Prat, L., Botti, C. & Fichet, T. Effect of plant growth regulators on floral differentiation and seed production in Jojoba (Simmondsia chinensis (Link) Schneider). Ind. Crops Prod.27, 44–49 (2008). 10.1016/j.indcrop.2007.07.001 [DOI] [Google Scholar]
- 33.Soler, L. & Cuevas, J. Early flower initiation allows ample manipulation of flowering time in cherimoya (Annona cherimola Mill.). Scientia Horticulturae121, 327–332 (2009). 10.1016/j.scienta.2009.02.005 [DOI] [Google Scholar]
- 34.Baldermann, S. et al. Influence of exogenously applied abscisic acid on carotenoid content and water uptake in flowers of the tea plant (Camellia sinensis). J. Sci. Food Agric.93, 1660–1664 (2013). 10.1002/jsfa.5944 [DOI] [PubMed] [Google Scholar]
- 35.Yan, B. et al. The effects of endogenous hormones on the flowering and fruiting of Glycyrrhiza uralensis. Plants8, 519 (2019). 10.3390/plants8110519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lulsdorf, M. M. et al. Endogenous hormone profiles during early seed development of C. arietinum and C. anatolicum. Plant Growth Regul.71, 191–198 (2013). 10.1007/s10725-013-9819-2 [DOI] [Google Scholar]
- 37.Chen, M. & Jiang, L.-J. Effects of exogenous hormones on castor bean’s vegetative growth and flower bud’s differentiation. J. Cent. South Univ. For. Technol.31, 86–90 (2011). [Google Scholar]
- 38.Zhang, S. et al. Effect of exogenous GA3 and its inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘Fuji’ apple (Malus domestica Borkh.). Plant Physiol. Biochem.107, 178–186 (2016). 10.1016/j.plaphy.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Yuhua, W., Chonghui, F., Xiang, S., Guimin, Q. & Jidong, S. Changes in endogenous hormones during the flower bud differentiation of sweet cherry. Acta Agriculturae Boreali-occidentalis Sinica11, 64–67 (2002). [Google Scholar]
- 40.Zhang, C. et al. Photosynthesis and hormone study of male and hermaphroditic Osmanthus fragrans at different flowering stages. J. Nanjing For. Univ.46, 75 (2022). [Google Scholar]
- 41.Gui, R. Effects of plant growth regulators on flowering and changes of contents of endogenous hormones and polyamines during flowering of Dianthus chinensis L. J. Nanjing For. Univ.46, 6 (2003). [Google Scholar]
- 42.Zhang, M., Han, M., Ma, F. & Shu, H. Effect of bending on the dynamic changes of endogenous hormones in shoot terminals of ‘Fuji’ and ‘Gala’ apple trees. Acta physiologiae plantarum37, 1–9 (2015). 10.1007/s11738-015-1813-z [DOI] [Google Scholar]
- 43.Guo, Y., An, L., Yu, H. & Yang, M. Endogenous hormones and biochemical changes during flower development and florescence in the buds and leaves of Lycium ruthenicum Murr. Forests13, 763 (2022). 10.3390/f13050763 [DOI] [Google Scholar]
- 44.Nibau, C., Di Stilio, V. S., Wu, H.-M. & Cheung, A. Y. Arabidopsis and Tobacco SUPERMAN regulate hormone signalling and mediate cell proliferation and differentiation. J. Exp. Bot.62, 949–961 (2011). 10.1093/jxb/erq325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing, L.-B. et al. Transcription profiles reveal sugar and hormone signaling pathways mediating flower induction in apple (Malus domestica Borkh.). Plant Cell Physiol.56, 2052–2068 (2015). 10.1093/pcp/pcv124 [DOI] [PubMed] [Google Scholar]
- 46.Wu, Y. et al. Differential effects of paclobutrazol on the bulblet growth of oriental lily cultured in vitro: Growth behavior, carbohydrate metabolism, and antioxidant capacity. J. Plant Growth Regul.38, 359–372 (2019). 10.1007/s00344-018-9844-5 [DOI] [Google Scholar]
- 47.Yang, Q. et al. Bud endodormancy in deciduous fruit trees: Advances and prospects. Hortic. Res.10.1038/s41438-021-00575-2 (2021). 10.1038/s41438-021-00575-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson, J. V., Gesch, R. W., Jia, Y., Chao, W. S. & Horvath, D. P. Seasonal shifts in dormancy status, carbohydrate metabolism, and related gene expression in crown buds of leafy spurge. Plant Cell Environ.28, 1567–1578 (2005). 10.1111/j.1365-3040.2005.01393.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the article, and can be requested from the corresponding author if required.