Abstract
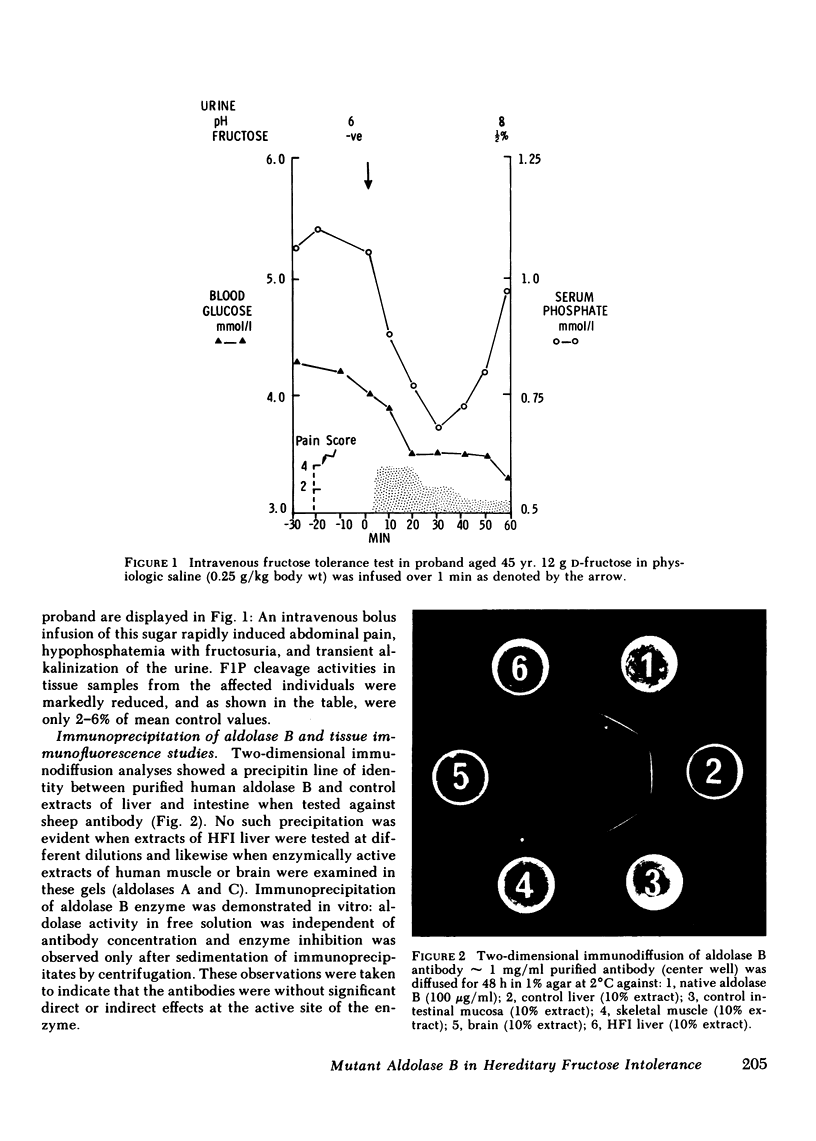
Hereditary fructose intolerance (HFI) is a metabolic disorder caused by enzymic deficiency of aldolase B, a genetically distinct cytosolic isoenzyme expressed exclusively in liver, kidney, and intestine. The molecular basis of this enzyme defect has been investigated in three affected individuals from a nonconsanguineous kindred, in whom fructose-l-phosphate aldolase activities in liver or intestinal biopsy samples were reduced to 2-6% of mean control values.
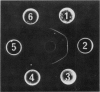
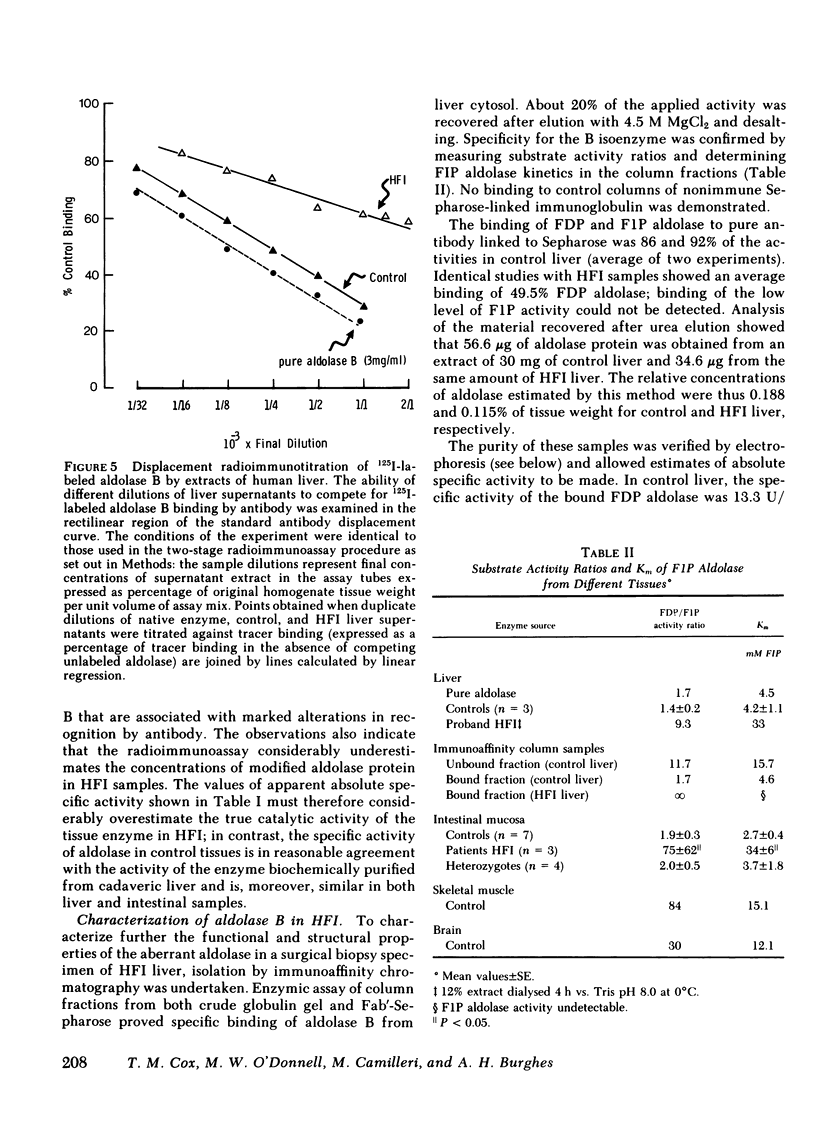
To identify a putative enzyme mutant in tissue extracts, aldolase B was purified from human liver by affinity chromatography and monospecific antibodies were prepared from antiserum raised in sheep. Immunodiffusion gels showed a single precipitin line common to pure enzyme and extracts of normal liver and intestine, but no reaction with extracts of brain, muscle, or HFI liver. However, weak positive staining for aldolase in hepatocyte and enterocyte cytosol was demonstrated by indirect immunofluorescence of HFI tissues. This was abolished by pretreatment with pure enzyme protein. Accordingly, a specific radioimmunoassay (detection limit 7.5 ng) was established to quantify immunoreactive aldolase B in human biopsy specimens. Extracts of tissue from affected patients gave 10-25% immunoreactive enzyme in control samples; immunoreactive aldolase in intestinal extracts from four heterozygotes was reduced (to 55%) when compared with seven samples from normal control subjects (P < 0.05). In extracts of HFI tissues, there was a sevenfold reduction in apparent absolute specific activity (1.02 vs. 8.82 U/mg) of immunoreactive fructose-l-phosphate aldolase B, but the apparent specific activity in heterozygotes (7.71 U/mg) was only slightly impaired. Displacement radioimmunotitration of aldolase B in liver supernatants showed a significant (P < 0.005) decrease in antibody avidity for immunoreactive protein in HFI tissue when compared with the pure enzyme or extract of normal control liver.
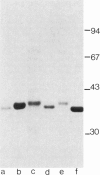
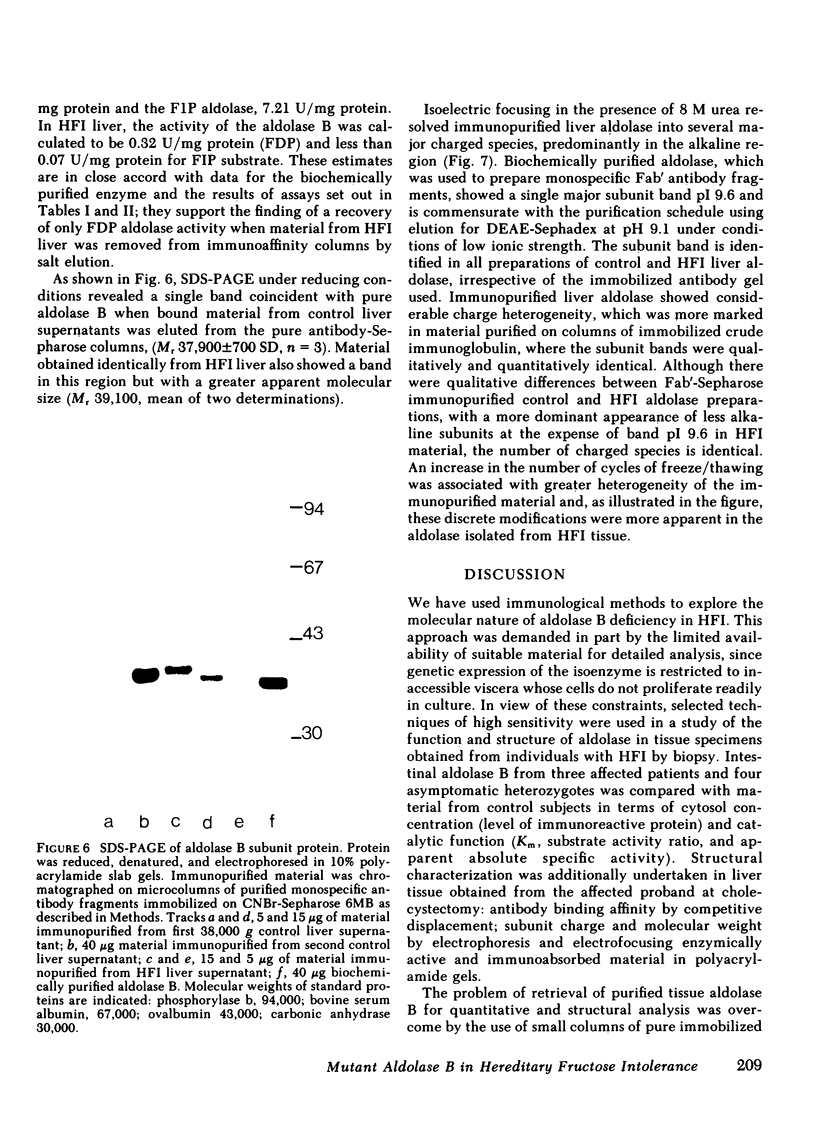
Immunoaffinity chromatography on antialdolase B-Sepharose facilitated isolation and purification of enzyme from liver biopsy specimens. Active aldolase in normal liver, with substrate activity ratios and Michaelis constants identical to biochemically purified human enzyme, could be recovered from antibody columns. Chromatography on monospecific Fab' antialdolase B enabled pure enzyme protein to be retrieved quantitatively from normal control and HFI liver: direct chemical assay showed 1.88 and 1.15 mg aldolase protein/g of tissue, respectively. This confirmed that the catalytic properties of the HFI aldolase were profoundly impaired with specific activities of fructose-l-phosphate cleavage of 7.21 and 0.07 U/mg, respectively. Radioimmunoassay gave estimates of 7.66 and 1.18 U/mg, respectively. Sodium dodecyl sulfate-polyacrylamide electrophoresis indicated that immunopurified aldolase from HFI liver possessed a single subunit size similar to material from control liver extracts: Mr 39,100 vs. 37,900±700 (SD) D, respectively. Electrofocusing under denaturing conditions of aldolase isolated in parallel from control and HFI liver revealed the same complement of subunits and, despite qualitative differences in distribution of bands during degradation, no additional charged species.
Fructose phosphate aldolase deficiency in hereditary fructose intolerance is attended by the synthesis of an immunoreactive, but functionally and structurally modified enzyme variant that results from a restricted genetic mutation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- CHAMBERS R. A., PRATT R. T. Idiosyncrasy to fructose. Lancet. 1956 Aug 18;271(6938):340–340. doi: 10.1016/s0140-6736(56)92196-1. [DOI] [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSBY W. H., KUGLER H. W. Intraluminal biopsy of the small intestine; the intestinal biopsy capsule. Am J Dig Dis. 1957 May;2(5):236–241. doi: 10.1007/BF02231100. [DOI] [PubMed] [Google Scholar]
- Cox T. M., Camilleri M., O'Donnell M. W., Chadwick V. S. Pseudodominant transmission of fructose intolerance in an adult and three offspring: Heterozygote detection by intestinal biopsy. N Engl J Med. 1982 Aug 26;307(9):537–540. doi: 10.1056/NEJM198208263070906. [DOI] [PubMed] [Google Scholar]
- FROESCH E. R., PRADER A., LABHART A., STUBER H. W., WOLF H. P. Die hereditäre Fructoseintoleranz, eine bisher nicht bekannte kongenitale Stoffwechselstörung. Schweiz Med Wochenschr. 1957 Sep 14;87(37):1168–1171. [PubMed] [Google Scholar]
- Gitzelmann R., Steinmann B., Bally C., Lebherz H. G. Antibody activation of mutant human fructosediphosphate aldolase B in liver extracts of patients with hereditary fructose intolerance. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1270–1277. doi: 10.1016/0006-291x(74)90451-3. [DOI] [PubMed] [Google Scholar]
- Grégori C., Besmond C., Kahn A., Dreyfus J. C. Characterization of messenger RNA for aldolase B in adult and fetal human liver. Biochem Biophys Res Commun. 1982 Jan 29;104(2):369–375. doi: 10.1016/0006-291x(82)90646-5. [DOI] [PubMed] [Google Scholar]
- Gürtler B., Bally C., Leuthardt F. Reindarstellung und Eigenschaften der menschlichen Leberaldolase. 19. Uber Aldolasen. Hoppe Seylers Z Physiol Chem. 1971 Oct;352(10):1455–1462. [PubMed] [Google Scholar]
- Gürtler B., Leuthardt F. Uber die Heterogenität der Aldolasen. Helv Chim Acta. 1970;53(3):654–658. doi: 10.1002/hlca.19700530326. [DOI] [PubMed] [Google Scholar]
- HERS H. G., JOASSIN G. [Anomaly of hepatic aldolase in intolerance to fructose]. Enzymol Biol Clin (Basel) 1961;1:4–14. [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Kido H., Vita A., Horecker B. L. A one-step procedure for the isolation of fructose 1,6-bisphosphatase and fructose 1,6-bisphosphate aldolase from rabbit liver. Anal Biochem. 1980 Aug;106(2):450–454. doi: 10.1016/0003-2697(80)90547-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lachmann P. J. The purification of specific antibody as F(ab')2 by the pepsin digestion of antigen-antibody precipitates, and its application to immunoglobulin and complement antigens. Immunochemistry. 1971 Jan;8(1):81–88. doi: 10.1016/0019-2791(71)90423-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemonnier F., Gregori C., Schapira F. Possible role of thiol groups in the abnormal kinetics of aldolase in hereditary fructose intolerance. Biochem Biophys Res Commun. 1974 Nov 6;61(1):306–312. doi: 10.1016/0006-291x(74)90567-1. [DOI] [PubMed] [Google Scholar]
- MacGregor J. S., Singh V. N., Davoust S., Melloni E., Pontremoli S., Horecker B. L. Evidence for formation of a rabbit liver aldolase--rabbit liver fructose-1,6-bisphosphatase complex. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3889–3892. doi: 10.1073/pnas.77.7.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann Y., Schapira F., Dreyfus J. C. A structurally modified liver aldolase in fructose intolerance: immunological and kinetic evidence. Biochem Biophys Res Commun. 1968 Jun 28;31(6):884–889. doi: 10.1016/0006-291x(68)90534-2. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Penhoet E. E., Rutter W. J. Catalytic and immunochemical properties of homomeric and heteromeric combinations of aldolase subunits. J Biol Chem. 1971 Jan 25;246(2):318–323. [PubMed] [Google Scholar]
- Schapira F., Hatzfeld A., Gregori C. Structural mutation of aldolase B in hereditary fructose intolerance: electrofocusing results. Monogr Hum Genet. 1978;9:2–6. doi: 10.1159/000401600. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E. D. Differentiation of epithelial cells in human jejunum: localization and quantification of aminopeptidase, alkaline phosphatase and aldolase isozymes in tissue sections. Histochemistry. 1976 Aug 12;48(2):101–109. doi: 10.1007/BF00494548. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Baugher B. W., Landa L., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Purification and characterization of mutant forms of the enzyme. J Biol Chem. 1981 Oct 25;256(20):10306–10312. [PubMed] [Google Scholar]