Abstract
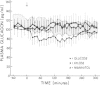
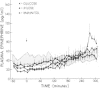
The mechanisms of postprandial glucose counterregulation—those that blunt late decrements in plasma glucose, prevent hypoglycemia, and restore euglycemia—have not been fully defined. To begin to clarify these mechanisms, we measured neuroendocrine and metabolic responses to the ingestion of glucose (75 g), xylose (62.5 g), mannitol (20 g), and water in ten normal human subjects to determine for each response the magnitude, temporal relationships, and specificity for glucose ingestion. Measurements were made at 10-min intervals over 5 h. By multivariate analysis of variance, the plasma glucose (P < 0.0001), insulin (P < 0.0001), glucagon (P < 0.03), epinephrine (P < 0.0004), and growth hormone (P < 0.01) curves, as well as the blood lactate (P < 0.0001), glycerol (P < 0.001), and β-hydroxybutyrate (P < 0.0001) curves following glucose ingestion differed significantly from those following water ingestion. However, the growth hormone curves did not differ after correction for differences at base line. In contrast, the plasma norepinephrine (P < 0.31) and cortisol (P < 0.24) curves were similar after ingestion of all four test solutions, although early and sustained increments in norepinephrine occurred after all four test solutions. Thus, among the potentially important glucose regulatory factors, only transient increments in insulin, transient decrements in glucagon, and late increments in epinephrine are specific for glucose ingestion. They do not follow ingestion of water, xylose, or mannitol.
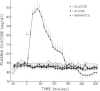
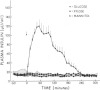
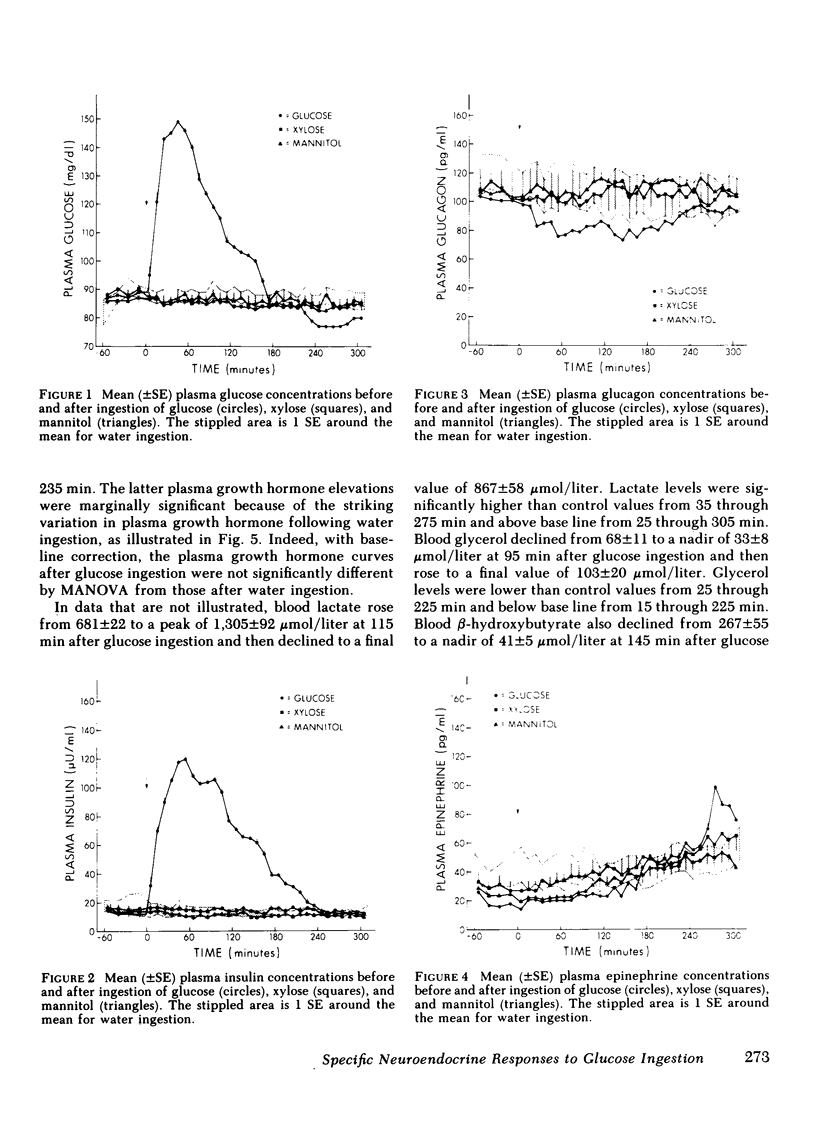
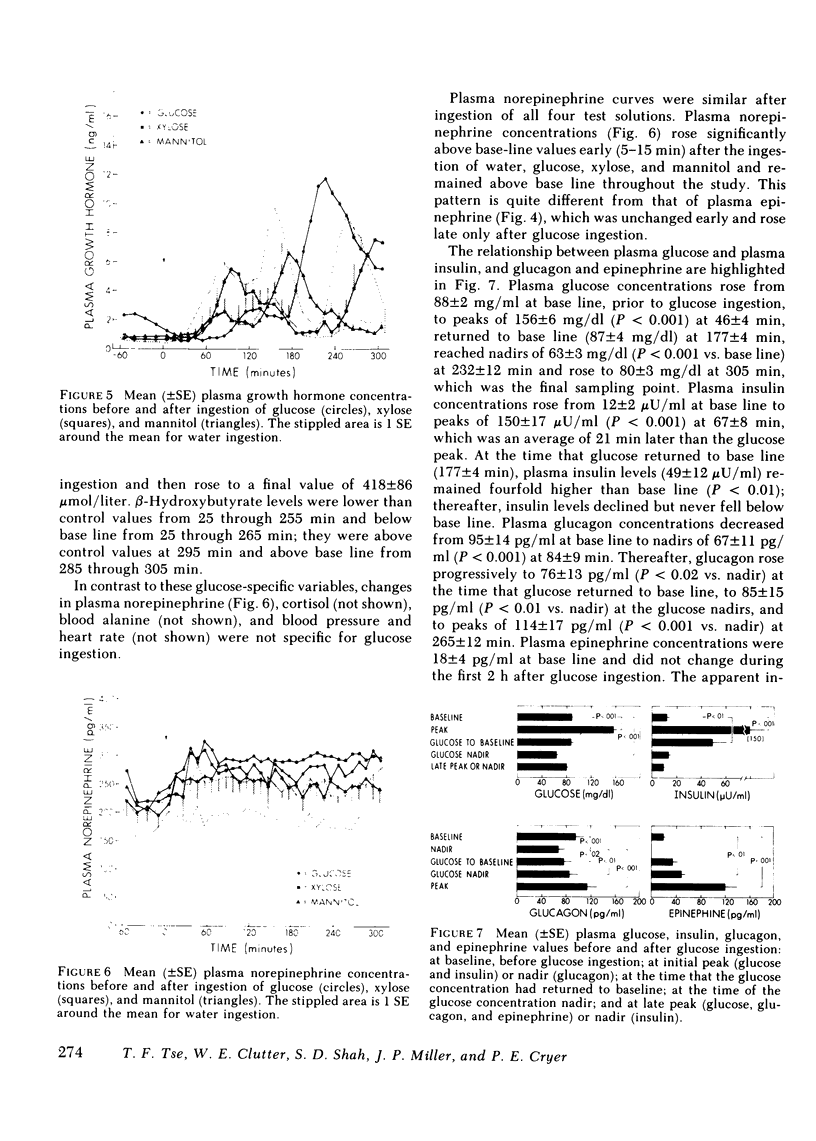
Following glucose ingestion, plasma glucose rose to peak levels of 156±6 mg/dl at 46±4 min, returned to base line at 177±4 min, reached nadirs of 63±3 mg/dl at 232±12 min, and rose to levels comparable to base line at 305 min, which was the final sampling point. Plasma insulin rose to peak levels of 150±17 μU/ml (P < 0.001) at 67±8 min. At the time glucose returned to base line, insulin levels (49±12 μU/ml) remained fourfold higher than base line (P < 0.01); thereafter they declined but never fell below base line. Plasma glucagon decreased from 95±14 pg/ml to nadirs of 67±11 pg/ml (P < 0.001) at 84±9 min and then rose progressively to peak levels of 114±17 pg/ml (P < 0.001 vs. nadirs) at 265±12 min. Plasma epinephrine, which was 18±4 pg/ml at base line, did not change initially and then rose to peak levels of 119±20 pg/ml (P < 0.001) at 271±13 min.
These data indicate that the glucose counterregulatory process late after glucose ingestion is not solely due to the dissipation of insulin and that sympathetic neural norepinephrine, growth hormone, and cortisol do not play critical roles. They are consistent with, but do not establish, physiologic roles for the counterregulatory hormones—glucagon, epinephrine, or both—in that process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke W. L., Santiago J. V., Thomas L., Ben-Galim E., Haymond M. W., Cryer P. E. Adrenergic mechanisms in recovery from hypoglycemia in man: adrenergic blockade. Am J Physiol. 1979 Feb;236(2):E147–E152. doi: 10.1152/ajpendo.1979.236.2.E147. [DOI] [PubMed] [Google Scholar]
- Clutter W. E., Bier D. M., Shah S. D., Cryer P. E. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980 Jul;66(1):94–101. doi: 10.1172/JCI109840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell J. A., Lein A. Diminished insulin response to hyperglycemia in prediabetes and diabetes. Diabetes. 1967 Aug;16(8):560–565. doi: 10.2337/diab.16.8.560. [DOI] [PubMed] [Google Scholar]
- Cryer P. E. Glucose counterregulation in man. Diabetes. 1981 Mar;30(3):261–264. doi: 10.2337/diab.30.3.261. [DOI] [PubMed] [Google Scholar]
- Cryer P. E. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med. 1980 Aug 21;303(8):436–444. doi: 10.1056/NEJM198008213030806. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Santiago J. V., Shah S. Measurement of norepinephrine and epinephrine in small volumes of human plasma by a single isotope derivative method: response to the upright posture. J Clin Endocrinol Metab. 1974 Dec;39(6):1025–1029. doi: 10.1210/jcem-39-6-1025. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Hendler R., Christensen N. Stimulation of counterregulatory hormonal responses in diabetic man by a fall in glucose concentration. Diabetes. 1980 Feb;29(2):125–131. doi: 10.2337/diab.29.2.125. [DOI] [PubMed] [Google Scholar]
- Eigler N., Saccà L., Sherwin R. S. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J Clin Invest. 1979 Jan;63(1):114–123. doi: 10.1172/JCI109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer R. W., Pierce C. E. Plasma cortisol determination: radioimmunoassay and competitive protein binding compared. Clin Chem. 1974 Apr;20(4):411–414. [PubMed] [Google Scholar]
- Fradkin J., Shamoon H., Felig P., Sherwin R. S. Evidence for an important role of changes in rather than absolute concentrations of glucagon in the regulation of glucose production in humans. J Clin Endocrinol Metab. 1980 Apr;50(4):698–703. doi: 10.1210/jcem-50-4-698. [DOI] [PubMed] [Google Scholar]
- Galster A. D., Clutter W. E., Cryer P. E., Collins J. A., Bier D. M. Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l-13C]palmitic acid. J Clin Invest. 1981 Jun;67(6):1729–1738. doi: 10.1172/JCI110211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J., Davis J., Lorenzi M., Rizza R., Bohannon N., Karam J., Lewis S., Kaplan R., Schultz T., Cryer P. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. 1979 Apr;236(4):E380–E385. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- Gray R. S., Scarlett J. A., Griffin J., Olefsky J. M., Kolterman O. G. In vivo deactivation of peripheral, hepatic, and pancreatic insulin action in man. Diabetes. 1982 Oct;31(10):929–936. doi: 10.2337/diab.31.10.929. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburg S., Hendler R., Sherwin R. S. Influence of small increments of epinephrine on glucose tolerance in normal humans. Ann Intern Med. 1980 Oct;93(4):566–568. doi: 10.7326/0003-4819-93-4-566. [DOI] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Peters N., Advani U., Perry G., Rogers J., Brough W. H., Pilkington T. R. Forearm glucose uptake during the oral glucose tolerance test in normal subjects. Diabetes. 1973 Jun;22(6):442–458. doi: 10.2337/diab.22.6.442. [DOI] [PubMed] [Google Scholar]
- Kipnis D. M. Insulin secretion in diabetes mellitus. Ann Intern Med. 1968 Nov;69(5):891–901. doi: 10.7326/0003-4819-69-5-891. [DOI] [PubMed] [Google Scholar]
- Kleinbaum J., Shamoon H. Selective counterregulatory hormone responses after oral glucose in man. J Clin Endocrinol Metab. 1982 Oct;55(4):787–790. doi: 10.1210/jcem-55-4-787. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Leichter S. B., Pagliara A. S., Grieder M. H., Pohl S., Rosai J., Kipnis D. M. Uncontrolled diabetes mellitus and hyperglucagonemia associated with an islet cell carcinoma. Am J Med. 1975 Feb;58(2):285–293. doi: 10.1016/0002-9343(75)90579-3. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Perry J. M., Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979 Jan;48(1):171–175. doi: 10.1210/jcem-48-1-171. [DOI] [PubMed] [Google Scholar]
- MacGorman L. R., Rizza R. A., Gerich J. E. Physiological concentrations of growth hormone exert insulin-like and insulin antagonistic effects on both hepatic and extrahepatic tissues in man. J Clin Endocrinol Metab. 1981 Sep;53(3):556–559. doi: 10.1210/jcem-53-3-556. [DOI] [PubMed] [Google Scholar]
- Parker M. L., Pildes R. S., Chao K. L., Cornblath M., Kipnis D. M. Juvenile diabetes mellitus, a deficiency in insulin. Diabetes. 1968 Jan;17(1):27–32. doi: 10.2337/diab.17.1.27. [DOI] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Watson J. A. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 1967 Aug;121(2):404–414. doi: 10.1016/0003-9861(67)90094-x. [DOI] [PubMed] [Google Scholar]
- Popp D. A., Shah S. D., Cryer P. E. Role of epinephrine-mediated beta-adrenergic mechanisms in hypoglycemic glucose counterregulation and posthypoglycemic hyperglycemia in insulin-dependent diabetes mellitus. J Clin Invest. 1982 Feb;69(2):315–326. doi: 10.1172/JCI110455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSON S. A. Secretion of human growth hormone: physiologic and experimental modification. Metabolism. 1963 Jul;12:577–579. [PubMed] [Google Scholar]
- Radziuk J., McDonald T. J., Rubenstein D., Dupre J. Initial splanchnic extraction of ingested glucose in normal man. Metabolism. 1978 Jun;27(6):657–669. doi: 10.1016/0026-0495(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Shen S. W., Silvers A., Farquhar J. W. Is there a delay in the plasma insulin response of patients with chemical diabetes mellitus? Diabetes. 1971 Jun;20(6):416–423. doi: 10.2337/diab.20.6.416. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Saccà L., Cryer P. E., Sherwin R. S. Blood glucose regulates the effects of insulin and counterregulatory hormones on glucose production in vivo. Diabetes. 1979 Jun;28(6):533–536. doi: 10.2337/diab.28.6.533. [DOI] [PubMed] [Google Scholar]
- Saccà L., Sherwin R., Hendler R., Felig P. Influence of continuous physiologic hyperinsulinemia on glucose kinetics and counterregulatory hormones in normal and diabetic humans. J Clin Invest. 1979 May;63(5):849–857. doi: 10.1172/JCI109384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santeusanio F., Bolli G., Massi-Benedetti M., De Feo P., Angeletti G., Compagnucci P., Calabrese G., Brunetti P. Counterregulatory hormones during moderate, insulin-induced, blood glucose decrements in man. J Clin Endocrinol Metab. 1981 Mar;52(3):477–482. doi: 10.1210/jcem-52-3-477. [DOI] [PubMed] [Google Scholar]
- Santiago J. V., Clarke W. L., Shah S. D., Cryer P. E. Epinephrine, norepinephrine, glucagon, and growth hormone release in association with physiological decrements in the plasma glucose concentration in normal and diabetic man. J Clin Endocrinol Metab. 1980 Oct;51(4):877–883. doi: 10.1210/jcem-51-4-877. [DOI] [PubMed] [Google Scholar]
- Seltzer H. S., Allen E. W., Herron A. L., Jr, Brennan M. T. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967 Mar;46(3):323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoon H., Hendler R., Sherwin R. S. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab. 1981 Jun;52(6):1235–1241. doi: 10.1210/jcem-52-6-1235. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Kramer K. J., Tobin J. D., Insel P. A., Liljenquist J. E., Berman M., Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974 May;53(5):1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Liljenquist J. E., Williams P. E., Lacy W. W., Cherrington A. D. Glucose disposal during insulinopenia in somatostatin-treated dogs. The roles of glucose and glucagon. J Clin Invest. 1978 Aug;62(2):487–491. doi: 10.1172/JCI109150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Glucagon physiology and pathophysiology. N Engl J Med. 1971 Aug 19;285(8):443–449. doi: 10.1056/NEJM197108192850806. [DOI] [PubMed] [Google Scholar]
- Welle S., Lilavivat U., Campbell R. G. Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism. 1981 Oct;30(10):953–958. doi: 10.1016/0026-0495(81)90092-5. [DOI] [PubMed] [Google Scholar]
- Welle S., Lilavivathana U., Campbell R. G. Increased plasma norepinephrine concentrations and metabolic rates following glucose ingestion in man. Metabolism. 1980 Sep;29(9):806–809. doi: 10.1016/0026-0495(80)90118-3. [DOI] [PubMed] [Google Scholar]
- Young J. B., Rowe J. W., Pallotta J. A., Sparrow D., Landsberg L. Enhanced plasma norepinephrine response to upright posture and oral glucose administration in elderly human subjects. Metabolism. 1980 Jun;29(6):532–539. doi: 10.1016/0026-0495(80)90078-5. [DOI] [PubMed] [Google Scholar]