Abstract
The Japan Diabetes Society (JDS) adopted a sweeping decision to release consensus statements on relevant issues in diabetes management that require updating from time to time and launched a “JDS Committee on Consensus Statement Development.” In March 2020, the committee’s first consensus statement on “Medical Nutrition Therapy and Dietary Counseling for People with Diabetes” was published. In September 2022, a second consensus “algorithm for pharmacotherapy in people with type 2 diabetes” was proposed. In developing an algorithm for diabetes pharmacotherapy in people with type 2 diabetes, the working concept was that priority should be given to selecting such medications as would appropriately address the diabetes pathology in each patient while simultaneously weighing the available evidence for these medications and the prescribing patterns in clinical practice in Japan. These consensus statements are intended to present the committee’s take on diabetes management in Japan, based on the evidence currently available for each of the issues addressed. It is thus hoped that practicing diabetologists will not fail to consult these statements to provide the best available practice in their respective clinical settings. Given that the persistent dual GIP/GLP-1 receptor agonist tirzepatide was approved in April 2023, these consensus statements have been revised1). In this revision, specifically, tirzepatide was added to the end of [likely involving insulin resistance] of “Obese patients” in Step 1: “Select medications to address the diabetes pathology involved” in Fig. 2. While the sentence, “Insulin insufficiency and resistance can be assessed by referring to the various indices listed in the JDS ‘Guide to Diabetes Management.’ was mentioned in the previous edition as well, “While insulin resistance is analogized based on BMI, abdominal obesity, and visceral fat accumulation, an assessment of indicators (e.g., HOMA-IR) is desirable” was added as information in order to more accurately recognize the pathology. Regarding Step 2: “Give due consideration to safety,” “For renal excretion” was added to the “Rule of thumb 2: Avoid glinides in patients with renal impairment.” The order of the medications in “rule of thumb 3: Avoid thiazolidinediones and biguanides in patients with heart failure (in whom they are contraindicated).” to thiazolidinediones then biguanides. In the description of the lowest part of Fig. 2, for each patient failing to achieve his/her HbA1c control goal, “while reverting to step 1” was changed to “while reverting to the opening” and “including reassessment if the patient is indicated for insulin therapy” was added. In the separate table, the column for tirzepatides was added, while the two items, “Characteristic side effects” and “Persistence of effect” were added to the area of interest. The revision also carried additional descriptions of the figure and table such as tirzepatides and “Characteristic side effects” in the statement, and while not mentioned in the proposed algorithm figure, nonalcoholic fatty liver disease (NAFLD) is covered from this revision for patients with comorbidities calling for medical attention. Moreover, detailed information was added to the relative/absolute indication for insulin therapy, the Kumamoto Declaration 2013 for glycemic targets, and glycemic targets for older people with diabetes. Again, in this revision, it is hoped that the algorithm presented here will not only contribute to improved diabetes management in Japan, but will continue to evolve into a better algorithm over time, reflecting new evidence as it becomes available.
Keywords: Type 2 diabetes (T2DM), Pharmacotherapy, Algorithm, Glycemic control (glucose-lowering effects)
Characteristics of Type 2 diabetes in the Japanese and Asian populations
Type 2 diabetes (T2DM) is a metabolic disease in which insulin insufficiency or decreased insulin sensitivity (insulin resistance), combined with relatively decreased insulin action to varying degrees, accounts for such a lack of insulin action, which causes chronic hyperglycemia [2]. Again, multiple genetic factors responsible for insulin insufficiency or insulin resistance and environmental factors (e.g., overeating or lack of physical activity and resultant obesity) combine to lead to such lack of insulin action as to cause T2DM.
A comparison of insulin-secretory capacity and insulin resistance between Westerners and Japanese individuals stratified by glucose tolerance shows that Japanese individuals have less insulin-secretory capacity than Westerners, even though their glucose tolerance is normal and that while Westerners exhibit acutely increased insulin resistance as they move from normal glucose tolerance to diabetes, Japanese individuals tend to exhibit lower insulin-secretory capacity than that usually associated with increased insulin resistance [3, 4]. Again, a study comparing insulin sensitivity and initial insulin response between East Asians, Caucasians, and Blacks showed that these races vary in terms of the balance between their insulin-secretory capacity and insulin resistance, and that East Asians and Blacks are more susceptible to diabetes than Caucasians [5]. The pathology of T2DM in the Japanese population is also shown to be characterized by decreased initial insulin response, regardless of the presence of obesity [6]. On the other hand, a recent study in Hisayama-cho investigated the correlation between pancreatic β cell failure (i.e., low insulinogenic index/HOMA-IR) or insulin resistance and the onset of T2DM and found that while pancreatic β cell failure and insulin resistance are both associated with the risk of T2DM, they are associated with a markedly increased risk of T2DM when they are found together in obese individuals [7].
In addition, histologic studies of the pancreas have shown that, among non-diabetic Westerners, obese individuals have a significantly greater islet mass than non-obese individuals and that, among Westerners with T2DM, both obese and non-obese individuals have an islet mass about 50% lower than that in non-diabetic individuals, and that no increase in pancreatic β-cell mass is noted even in obese Japanese [8, 9]. Studies have also shown that among individuals with T2DM, amyloid deposition is noted in more than 80% of Westerners, but only in 30% of Japanese [10, 11]. Thus, it is suggested that histologic findings on the pancreas differ greatly between different races, suggesting that these differences may contribute to the differences in the pathogenesis of diabetes.
Additionally, advances in the genetic analysis of T2DM have led to the identification of numerous T2DM susceptibility loci, including KCNQ1 [12–14]. A meta-analysis of genome-wide association studies (GWAS) in T2DM has recently shown that many Japanese individuals, but very few Westerners, have the R131Q mutation in the GLP-1 receptor gene (GLP-1R), which is known to be involved in inducing a two-fold increase in insulin secretion. Furthermore, a cross-racial molecular biologic pathway analysis showed that the pathways involved in the onset of maturity-onset diabetes of the young (MODY) are the most strongly associated with T2DM in both races evaluated, and that the pathways involved in the regulation of insulin secretion are significantly associated with T2DM only in Japanese [15].
Taken together, the pathology of T2DM clearly differs between Japanese and Westerners, not only functionally but also histologically and genetically, with decreased insulin-secretory capacity having a greater role in the onset of T2DM in Japanese than in Westerners.
Differences in treatment strategies for Japanese and Western people with T2DM
As detailed above, T2DM can be primarily characterized as having, as an underlying core pathology in most Japanese individuals, insulin resistance and insulin insufficiency, the respective contributions of which vary from individual to individual. In contrast to that, in Westerners, core pathologies can be characterized as having obesity and insulin resistance. Owing to its ability to reduce the risk of microangiopathy, macroangiopathy, and death, as well as its beneficial impact on body weight, low hypoglycemia risk, and low cost [16, 17], metformin has long been recommended as the first-line therapy in Western countries [18, 19]. However, the Standards of Medical Care in Diabetes by the American Diabetes Association (ADA) have been extensively revised in 2022 to address compelling issues in diabetes management, such as diabetic comorbidities (e.g., atherosclerotic cardiovascular disease), patient-related factors in diabetes treatment, and the therapeutic needs of affected individuals [20]. In contrast, the treatment strategy for T2DM in Japan is to allow for the choice of medications from all classes to address the diabetes pathology in each affected individual, while taking into account the extent of their metabolic derangement, their age, the extent of their obesity, the status of their insulin secretion/insulin resistance, the severity of their chronic complications, and the status of their liver/renal function [21]. The rationale for this approach has indeed been provided through the accumulation of relevant evidence, including that from the Kumamoto study [22] and the Japan Diabetes Outcome Interventional Trial 3 (J-DOIT3) [23], which corroborated the importance of multifactorial intervention, including glycemic control, in reducing complications in Japanese people with diabetes.
Initial antidiabetic medication prescribing patterns for people with diabetes Japan
It is not difficult to imagine how significantly such differences in treatment strategies for T2DM might impact the choice of medications or their prescription patterns. In this regard, while there are studies on antidiabetic medication prescribing patterns in Japan [24, 25], they each suffered from a small sample size and lack of data from older people with diabetes, so a nationwide survey is needed to provide a full picture of the prescribing patterns in clinical practice. Thus, the Japan Diabetes Society (JDS) conducted a nationwide survey to clarify prescription patterns in clinical practice as a step in developing an algorithm for diabetes pharmacotherapy [26]. The survey demonstrated that, among the more than 1 million people with T2DM registered with the National Database of Health Insurance Claims and Specific Health Check-ups from the latter half of the fiscal year 2014 to the fiscal year 2017, the most frequently prescribed antidiabetic medications were, unlike those in Western countries [27], dipeptidyl peptidase-4 (DPP-4) inhibitors, followed by biguanides and sodium-glucose cotransporter 2 (SGLT2) inhibitors, with age shown to be the factor most strongly influencing this prescribing pattern. Moreover, the older the patients were, the more likely they were to have been prescribed DPP-4 inhibitors and the markedly less likely they were to have been prescribed biguanides and SGLT2 inhibitors. An analysis of the initial prescription pattern by prefecture also showed that the biguanide and DPP-4 inhibitor prescriptions varied from prefecture to prefecture, while an analysis of the initial prescription pattern by facility (JDS-certified versus non-JDS-certified) showed that no patients receiving initial medication therapy had been initially prescribed biguanides at 38.2% of non-JDS-certified facilities and that the DPP-4 inhibitor prescription pattern varied greatly between JDS-certified and non-JDS-certified facilities (i.e., there were considerable non-JDS-certified facilities where almost 100% of patients had been initially prescribed DPP-4 inhibitors alone). Thus, while survey results suggested that antidiabetic medications were being chosen to address the characteristics of diabetes in each individual patient and that the JDS recommendations on the use of metformin and SGLT2 inhibitors [28, 29] were widely adhered to by primary care physicians, the disparity in DPP-4 inhibitor and biguanide prescribing patterns between regions and facilities. On the other hand, it is pointed out that there is a need to renew awareness of the JDS-proposed principle of medication choice for each patient based not only on the extent of their metabolic derangement, but also on their age, the extent of their obesity, the severity of their chronic complications, the status of their liver/renal function, and the status of their insulin secretion/insulin resistance, Therefore, an algorithm needs to be developed as a tool to promote the appropriate use of antidiabetic medications.
Working concept of an algorithm for pharmacotherapy in people with T2DM
Given that T2DM differs in pathology between Asians, including Japanese and Westerners, JDS has advanced a different treatment strategy for Japanese people from that for Westerners. The survey results clearly show that the initial diabetes medication prescribing patterns differ greatly between Japan and Western countries [26], suggesting that the JDS-proposed treatment strategy for diabetes has become widespread among diabetologists and general practitioners. It is also likely that the initial diabetes medication prescription patterns reflected the informed use of antidiabetic medications, except imeglimin and tirzepatide, on the part of many physicians, based on their glucose-lowering efficacy and safety profiles that became known after a certain amount of time following their approval. Furthermore, it became clear that the disparity in the prescribing patterns of DPP-4 inhibitors and biguanides between facilities and regions must be resolved to ensure the proper use of these medications. Given that evidence has recently been accumulated, mostly overseas, demonstrating the efficacy of GLP-1 receptor agonists and SGLT2 inhibitors against diabetic comorbidities (i.e., atherosclerotic cardiovascular disease, heart failure (HF), and chronic kidney disease [CKD]), it has been suggested that these additional benefits (i.e., cardio-/renoprotective and mortality-reducing effects) are worth considering in medication selection for people with T2DM. Thus, overall, based on the basic concept (medications can be selected to address the diabetes pathology in Japanese and Asians; the medication selection should reflect the prescribing patterns in clinical practice in Japan; and medications can be selected for their additional benefits in patients with comorbidities that call for medical attention), an algorithm for diabetes pharmacotherapy was developed to allow for such a choice of medications to address each patient’s pathology/condition, with the priority in medication selection determined, with consideration given to current prescribing patterns and other relevant factors (Fig. 1).
Fig. 1.
Working concept of an algorithm for pharmacotherapy in T2DM
Proposed algorithm annotated
Assessing the indications for insulin and determining the HbA1c control target
The overriding premise of diabetes pharmacotherapy is that it must be safe. Thus, medication selection was first assumed to involve assessing whether there were any absolute or relative indications for insulin therapy for each patient (Fig. 2). Among the absolute/relative indications for insulin therapy, the absolute indications include: (1) an insulin-dependent state; (2) hyperglycemic coma (diabetic ketoacidosis, hyperosmolar hyperglycemic state); (3) patients complicated by severe hepatic disorder or renal impairment, severe infection or injury, and moderate-to-severe surgery (such as under general anesthesia); (4) pregnant women with diabetes mellitus (including gestational diabetes, even when favorable glycemic control cannot be achieved by medical nutrition therapy); and (5) glycemic control during parenteral nutrition. Many of these pathologies require hospitalization; therefore, it is desirable to refer patients to diabetologists. Likewise, the relative indications include: (1) significant hyperglycemia (i.e., fasting blood glucose ≥ 250 mg/dL and casual blood glucose ≥ 350 mg/dL) is observed in non-insulin-dependent patients; (2) favorable glycemic control cannot be achieved by oral medication therapy alone; (3) nutritional status is compromised in lean patients; (4) severe hyperglycemia is recognized during steroid treatment; and (5) glucotoxicity is actively eliminated. As individuals aged 65 years or older account for more than half of all people with diabetes in Japan, the HbA1c control goal was determined based on those proposed in the Kumamoto Declaration 2013 and the JDS-proposed “Glycemic targets (HbA1c values) for older people with diabetes” [21, 30]. Based on the evidence in the Kumamoto Declaration 2013 in Japan, the Kumamoto Declaration 2013 proposed that while an HbA1c control goal of less than 7% was determined to prevent complications in people with diabetes and less than 6% for patients in whom a far greater goal is likely to be reached, a less strict control goal of less than 8% was determined for patients in whom strict control was arduous (i.e., patients with a high risk of hypoglycemia). “Glycemic targets for older people with diabetes” recommend that glycemic targets (HbA1c values) for older people with diabetes be determined individually by ADL, cognitive function, the presence or absence of comorbidities, and use of medications associated with a high risk of severe hypoglycemia (insulin, sulfonylureas (SUs), and rapid-acting insulin secretagogues (glinides)). Detailed information on comorbidities, ADL, cognitive function, and medications used represents an important part of safe glycemic control, so consideration should be given to determining glycemic targets (HbA1c values).
Fig. 2.
Proposed algorithm for pharmacotherapy in T2DM
Assessing people with T2DM for the presence of obesity as a relevant measure (step 1)
The insulinogenic index (II) or C-peptide index remains useful as a measure of insulin-secretory capacity, and Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) remains useful as a measure of insulin resistance in people with diabetes. However, the sheer number of patients attending the hospital for T2DM makes it difficult in practical clinical practice to assess all patients using these indicators. Given that one of the important aims of the proposed algorithm is to guide the proper use of antidiabetic medications among non-experts, the presence or absence of obesity was adopted as the most convenient and valid indicator that can reflect the core pathophysiology of diabetes to some extent. Thus, it is recommended that patients be assessed for obesity using the definition of obesity in Japan, body mass index (BMI) 25 kg/m2 or more [31] when selecting medications for T2DM. Given that the extent of obesity (BMI) and insulin resistance are positively correlated [32], insulin resistance is assumed to have a greater contribution to T2DM in highly obese patients, prompting the choice of medications to address the pathology in question. It is important to note that the accumulation of visceral fat is often observed in Japanese and Asians, including Japanese, even if their BMI is much lower than that of obese Westerners; therefore, insulin resistance may be associated with visceral fat accumulation in some of these patients, although they are usually categorized by BMI as non-obese [5, 33, 34]. It is assumed that patients can be accurately assessed for excessive visceral fat accumulation by assessing their BMI and waist circumference at the same time, and it is important to note that excessive visceral fat accumulation may be suspected in men with a waist circumference ≥ 85 cm, as well as in women with a waist circumference of 90 cm or greater [31]. Candidate medications for patients with obesity include non-insulin secretagogues, such as biguanides, SGLT2 inhibitors, and thiazolidinediones, as well as insulin secretagogues, such as GLP-1 receptor agonists with potential for weight-reducing effects, and imeglimin, for which obesity/insulin resistance is a good indication, given its insulin-sensitizing properties. The persistent dual GIP/GLP-1 receptor agonist tirzepatide, approved in April 2023, has been shown to be extremely effective in Japanese patients for glycemic improvement as well as highly potent dose-dependent weight reduction [35, 36], suggesting a good indication in patients with obesity- and insulin resistance-based diabetes for glucose metabolism as well as lipid metabolism, given its weight-reducing properties.
In most non-obese individuals with T2DM in whom insulin insufficiency is assumed to constitute the core pathology, insulin secretagogues should be selected as the mainstay of treatment. Of these, DPP-4 inhibitors remain the most frequently prescribed for people with T2DM in Japan, particularly older patients, probably reflecting the high expectations for their safety even in the older [26]. DPP-4 inhibitors have been shown to exert a far greater glucose-lowering efficacy in Asians than in other races [37, 38], suggesting that non-obese people with T2DM are likely to be a good indication for this medication class in terms of safety and efficacy. While numerous studies have been conducted in the hopes of reducing cardiovascular risk with DPP-4 inhibitors, [39–41]), some of them were reported to be associated with an increased risk of HF, thus calling for their judicious use in patients at a high risk of HF [42]. Of the insulin secretagogues, sulfonylureas (SUs) are also of interest because they are non-glucose-dependent insulin secretagogues and are associated with a high risk of hypoglycemia [43]. On the other hand, glinides and α-glucosidase inhibitors are good medication candidates for patients exhibiting marked postprandial hyperglycemia. Metformin has been shown to exert comparable HbA1c-lowering efficacy in both non-obese and obese Japanese people with T2DM, and thus represents an option for non-obese people with T2DM [44, 45]. Nonobese patients may include older, emaciated patients (BMI < 18.5 kg/m [2]). Therefore, caution should be exercised when using antidiabetic agents with weight-loss properties, i.e., GLP-1 receptor agonists and SGLT2 inhibitors, in lean patients, as they may increase the risk of geriatric syndromes such as sarcopenia and frailty [17]. As mentioned above, in clinical trials of tirzepatide in Japanese people with T2DM, it has been clearly shown to be highly effective in weight reduction [35, 36], while there is a paucity of data in patients with a BMI of less than 23 kg/m2 or older patients; thus, tirzepatide was refrained from being named as a candidate medication in non-obese patients.
Giving due consideration to ensuring safety (step 2)
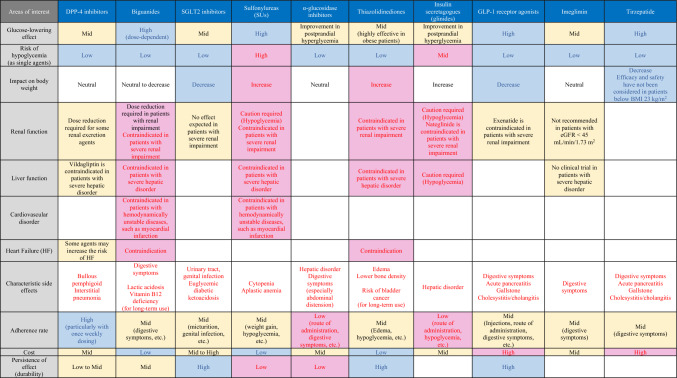
Note that the most desirable attribute required for antidiabetic medications is their ability to “lower blood glucose safely.” Thus, the proposed algorithm has included a summary of their glucose-lowering potency relative to their efficacy, safety, and risk of hypoglycemia, as well as precautions (particularly contraindications) for their use in patients with organ derangement (e.g., renal impairment, hepatic disorder [particularly cirrhosis], cardiovascular disorder, and HF) and newly added characteristic side effects in Table 1, with a running commentary on areas in which caution should be exercised in their use: (1) use in older patients with SUs and glinides, both of which are associated with a high risk of hypoglycemia; (2) safety precautions in medication selection in patients with renal impairment, a highly common comorbidity in people with T2DM; and (3) medications contraindicated in patients with HF.
Table 1.
A summary of the characteristics of antidiabetic medications for safe glycemic control: a comparison of glucose-lowering potency, hypoglycemia risk, contraindications, adherence rates, and medication costs (A ranked listing of medications initially prescribed in Japan by frequency)
Blue letters and color formatting mean favorable effects such as high efficacy, high safety, high weight loss, and high adherence rates, while red letters and formatting mean the opposite for each
According to a network meta-analysis of the HbA1c-lowering efficacy of antidiabetic medications, GLP-1 receptor agonists are the most potent medications in lowering HbA1c, followed by metformin, pioglitazone, and SUs [46]). It was also shown that metformin dose-dependently lowered glucose and exerted highly potent glucose-lowering effects at high doses, and that thiazolidinedione lowered glucose through its insulin-sensitizing effects on adipose tissue and skeletal muscle, indicating that it is more effective in obese patients. Tirzepatide is deemed to be a favorable choice in patients expecting to achieve normoglycemia, given that tirzepatide produces a greater reduction in HbA1c in Japanese people with T2DM than GLP-1 receptor agonist duraglutide, with a high achievement rate of HbA1c less than 5.7% [35, 36].
Safety against hypoglycemia remains the most important requirement for antidiabetic medications. As single agents, antidiabetic medications other than non-glucose-dependent SUs and glinides are generally associated with a low risk of hypoglycemia, whereas SUs are among the agents associated with a high risk of hypoglycemia. Indeed, according to a report from the JDS Committee on a Survey of Severe Hypoglycemia Associated with Diabetes Treatment, patients treated with SUs accounted for approximately 30% of all patients treated with any antidiabetic medication who required emergency transportation for severe hypoglycemia (or about 85% of all patients treated with medications other than insulin therapy) [43]. A finding of particular interest from this survey is that older patients accounted for a large proportion of those transported for severe hypoglycemia, suggesting that caution should be exercised when using SUs in older patients.
The impact of antidiabetic medications on body weight is also particularly relevant to the correction of obesity and prevention of geriatric syndrome. SGLT2 inhibitors have been shown to be associated with a weight reduction of 2 kg compared to a placebo [17], suggesting their suitability for use in obese people with T2DM. GLP-1 receptor agonists are also shown to have weight-reducing effects and are thus deemed suitable for use in obese people with T2DM. The reduction in body weight is reported to be 2 kg on average in patients treated with these medications compared to those treated with a placebo [17]. Of these, semaglutide was evaluated for its efficacy in Japanese people with T2DM in a recent study that demonstrated that the medication was associated with a significant reduction in body weight (ranging from 2 to 3 kg) at high doses [47, 48]). The reduction in body weight by GLP-1 receptor agonists varies from agent to agent; therefore, patients requiring weight loss are likely to be more likely to apply medications that are more effective in reducing weight (i.e., semaglutide). Miglitol, one of α-glucosidase inhibitors, have been shown to be associated with weight reduction in obese Japanese people with T2DM [49]. Conversely, many studies reported a weight gain of approximately 2 kg in patients treated with SUs compared to patients treated with placebo, while pioglitazone was shown to be associated with a weight gain of 1 to 4 kg [50] as well as edema. 5, 10, and 15 mg tirzepatide in Japanese people with T2DM and a BMI of 23 kg/m [2] or more produces a significant reduction in body weight compared to 0.75 mg GLP-1 receptor agonists duraglutide [35], while 5, 10, and 15 mg tirzepatide in SURPASS-2 trials conducted overseas produced a significant reduction in body weight compared to GLP-1 receptor agonists samaglutide 1.0 mg [51]; Thus, tirzepatide is deemed the most effective medication in body weight reduction among existing antidiabetic medications. Its medication is a good indication in highly obese patients with T2DM. However, consideration needs to be given to the indication in non-obese patients (particularly lean patients) at the time of this review, as very few results were shown in Japanese patients with a BMI of less than 23 kg/m2.
Caution should be exercised in the use of multiple antidiabetic medications in people with diabetes complicated by renal impairment. Given that most non-glucose-dependent insulin secretagogues (e.g., SUs and glinides) are renally excreted, their use is thought to be associated with an increased risk of hypoglycemia; therefore, SUs and nateglinide are both contraindicated for use in patients with renal impairment [43], while, as a glinide with biliary excretion, repaglinide is shown to be relatively safer for use in patients with renal impairment than other glinides. Mitiglinide has also been shown to be relatively safer for use in patients with renal impairment, as there is no glucose-lowering efficacy with metabolites. Metformin has been shown to be associated with an increased risk of lactic acidosis in patients with renal dysfunction and is thus contraindicated in patients with eGFR < 30 mL/min/1.73 m2 but is acceptable for use at a daily maximum dose of 750 and 1500 mg in those with eGFR of ≥ 30 mL/min/1.73 m2 and < 45 mL/min/1.73 m2 and of ≥ 45 mL/min/1.73 m2 and < 60 mL/min/1.73 m2, respectively [29]. Pioglitazone is available for use overseas even in renally impaired patients, but is contraindicated for use in severely renally impaired patients in Japan. As their glucose-lowering efficacy is diminished in patients with renal impairment, SGLT2 inhibitors raise concerns over their failure to achieve adequate glucose lowering in severely renally impaired patients.
A severe hepatic disorder is a contraindication for biguanides, SUs, and thiazolidinediones (despite being a relative indication for insulin therapy). Among all patients with cardiovascular disease (CVD), metformin is contraindicated for those with hemodynamically unstable condition or HF but not in Western countries (while still contraindicated in patients with hemodynamically unstable condition or acute HF) based on the reports of reductions in the need for hospitalization due to HF and mortality risk with metformin [52–54]. According to a recent meta-analysis of observational studies investigating the association between metformin use and all-cause mortality in T2DM patients with congestive HF as well as hospitalization for HF, a significant reduction was shown in mortality risk with metformin and hospitalization risk due to HF [55], suggesting significant prognostic findings for the use of metformin in people with T2DM complicated by HF with and without a reduced left ventricular ejection fraction [56, 57]. Accumulation of safety evidence is awaited for the use of metformin in patients with compensated HF in Japan. While thiazolidinediones are deemed a contraindication for patients with stage C or higher symptomatic HF but not those with stage A and B, consideration should be given to their dose adjustment, salt restriction, and concomitant use of diuretics for associated fluid retention.
The characteristic side effects of each antidiabetic medication have been added to Table 1 as a further consideration for safety in the revision of the algorithm. This report outlines several characteristic adverse effects. Among autoimmune bullous diseases, bullous pemphigoid may occur following the initiation of DPP-4 inhibitors, although it occurs less frequently. Note here that bullous pemphigoid is likely to be a factor in septicemia and DIC due to bacterial infection following systemic blisters and erosions [58]. Severe side effects due to biguanides include lactic acidosis, reported mostly in patients complicated by severe renal dysfunction. Whereas, some cases of lactic acidosis were reported in non-older patients with normal renal function, suggesting that caution should be exercised especially in patients with dehydration or who drink heavily. In Japanese patients on the long-term use, thiazolidinediones reduced bone density and increased risk of bone fractures in women [59], and consideration should be given to its use in older women after menopause. Given that long-term use of thiazolidinediones may also be associated with an increased risk of bladder cancer (however, incidence is remarkably low and increased risk is also small [60–62], the use of thiazolidinediones should be avoided in patients with bladder cancer. The risk of bladder cancer should be explained at the initiation of thiazolidinediones, and periodic urinalysis should be conducted. Diseases at increased risk of developing with GLP-1 receptor agonists include gallstones, cholecystitis, cholangitis, and cholestatic jaundice. Consideration should be given to its indication in patients with gallstones or a history of acute biliary tract infection. A meta-analysis of large-scale clinical trials did not show any increased risk of pancreatic cancer and pancreatitis [63]. It is important that patients receiving SGLT2 inhibitors be closely monitored euglycemic diabetic ketoacidosis. SGLT2 inhibitors are expected to increase urinary glucose excretion and lower blood glucose and insulin levels, leading to an increased glucagon/insulin ratio and hepatic glycogenesis/lipolysis in adipose tissue, thus resulting in increased use of lipids for energy metabolism. Therefore, SGLT2 inhibitors are associated with a risk of ketoacidosis due to an acute increase in ketone bodies, even in normoglycemic patients with underlying insulin insufficiency or during sick days. Also of interest are urinary tract and genital infections, among the characteristic side effects of SGLT2 inhibitors, suggesting that consideration should be given to the high risk thereof [64]. Despite the paucity of data from Japan on the use of tirzepatide, its pharmacologic properties are assumed to be associated with the risk of gallstones and acute biliary tract infection, as is the case with GLP-1 receptor agonists. Although the occurrence is low (less than 1%), a summary of side effects such as gallstones, acute biliary tract infection, and acute pancreatitis have been reported from abroad [65] and the risks need to be considered.
Weighing the additional medication benefits for comorbidities (step 3)
Given that numerous large-scale clinical trials conducted overseas have shown the efficacy of SGLT2 inhibitors and GLP-1 receptor agonists against chronic kidney disease (CKD) (particularly overt nephropathy), CVD, and HF, the proposed algorithm included CVD, HF, and CKD (particularly overt nephropathy) as target diseases for which antidiabetic medications may may provide additional benefits. However, it should be noted that the algorithm draws mainly on the evidence available from overseas due to the paucity of data from Japan on this issue. These comorbidities appear to be a valid indication for the use of SGLT2 inhibitors and GLP-1 receptor agonists, with the caveat that the reduction seen in cardiovascular events in these trials may be accounted for in part by that in HbA1c [66]. Further studies are required to elucidate the mechanisms involved. According to a meta-analysis of recently approved tirzepatide, which assessed the safety of cardiovascular outcomes, tirzepatide did not increase the risk of major cardiovascular disease, all-cause mortality, and cardiovascular death [67]. While tirzepatide binds to GLP-1 and GIP receptors with strong affinity, in vitro studies have shown that the binding affinity of GIP receptors is similar to that of intrinsic GIP, while the binding affinity of the GLP-1 receptor is five-fold lower than that of intrinsic GLP-1. Thus, GIP is deemed to have a different pharmacologic property from GLP-1 receptor agonists in vivo. Therefore, further studies are needed to elucidate whether tirzepatide could reduce cardiorenal events.
Weighing the additional benefit of antidiabetic medications for CVD
Large-scale clinical trials of SGLT2 inhibitors (i.e., EMPA-REG, CANVAS, and DECLARE TIMI [59] have been conducted in people with T2DM and CVD or those with T2DM at a high risk of CVD with major adverse cardiovascular events (MACE) (a composite of cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal ischemic stroke) as the primary endpoint. They demonstrated a significant reduction in MACE using these agents [68–70], with this finding also confirmed by independent meta-analyses of these trials [71, 72]. Likewise, clinical trials of GLP-1 receptor agonists have been conducted (i.e., LEADER, SUSTAIN6, and Harmony), which show significant reductions in MACE [73–75]. The outcome was also confirmed by a meta-analysis [70]. Thus, while it should be noted that there is a paucity of data from trials in Japanese patients and that Japanese people with diabetes are at lower risk of CVD than their Western counterparts, the evidence based on overseas trial is so numerous and of such high quality that SGLT2 inhibitors and GLP-1 receptor agonists were highly recommended in the order listed for their additional benefit against CVD.
Weighing the additional benefit of antidiabetic medications for HF
Assessing and treating HF is of vital importance, given that even asymptomatic people with T2DM are deemed to be in a state called “pre-HF,” i.e., at high risk of HF. In this regard, SGLT2 inhibitors (i.e., such as empagliflozin, canagliflozin, and dapagliflozin) have been shown to be useful in the prevention of HF in clinical trials examining cardiovascular efficacy. [68–70], with a similar finding shown in a subset analysis of Asian subjects [76]. In addition, a study that compared real-world evidence for SGLT2 inhibitors versus DPP-4 inhibitors in global populations, including Japanese and Koreans, also confirmed the usefulness of SGLT2 inhibitors in preventing HF [77]. Furthermore, SGLT2 inhibitors have been shown in a clinical trial of HF people with or without T2DM exhibiting heart failure with reduced ejection fraction (HFrEF) to produce a significant reduction in HF aggravation and cardiovascular death [78, 79], an outcome that was confirmed by a meta-analysis [80]. SGLT2 inhibitors are also shown to produce a significant reduction in HF aggravation and cardiovascular death in people with or without T2DM exhibiting HF with preserved EF (HFpEF) [81, 82]. Given them, SGLT2 inhibitors were included as first-choice medications in people with T2DM and HF.
In contrast, GLP-1 receptor agonists are not consistently shown to be useful in preventing HF in clinical studies evaluating their cardiovascular safety, but are nevertheless shown to be useful as a class in preventing HF in a meta-analysis [83]. However, its usefulness in the Japanese population remains unclear at the time of this review. The FIGHT trial evaluated the effect of liraglutide in patients with acute HF exhibiting decreased left ventricular ejection fraction and demonstrated that, contrary to expectations, treatment with liraglutide increased risk of HF in people especially with T2DM [84], suggesting the need to further investigate the usefulness of GLP-1 receptor agonists for various HF pathology or stage as well as their respective mechanisms of action.
Weighing the additional benefit of antidiabetic medications for CKD (particularly overt nephropathy)
In sub-analyses of data from people with T2DM and CVD or those at a high risk of CVD participating in cardiovascular safety trials, SGLT2 inhibitors (i.e., empagliflozin, canagliflozin, and dapagliflozin) have been shown to be useful in reducing composite renal events [68–70]. Of note, reanalysis of the canagliflozin trial data using a rigorous composite endpoint (doubling of serum creatinine, end-stage renal failure, and renal death) showed a significant reduction in the composite renal endpoint, as well as in renal dysfunction and albuminuria, with the medication [85]. In addition, findings from large-scale clinical trials have evaluated the usefulness of SGLT2 inhibitors (i.e., DAPA-CKD, CREDENCE, and EMPA-kidney) [86–88]. While the DAPA-CKD, CREDENCE, and EMPA-Kidney trials involved different patient populations (CKD people with or without T2DM, of whom those without T2DM accounted for 32.5% [eGFR, 25–75 mL/min/1.73 m2; urinary albumin/creatinine ratio (ACR), 200–5000 mg/g], T2DM people with CKD exhibiting overt albuminuria [eGFR, ≥ 30/ < 90 mL/min/1.73 m2; urinary ACR, 300–5000 mg/g], and EMPA-Kidney patients [non-diabetic individuals 54.0%; eGFR ≥ 20/ < 45 mL/min/1.73 m2 or eGFR ≥ 45/ < 90 mL/min/1.73 m2 and urinary ACR, ≥ 200 mg/g], respectively), all studies showed a significant reduction in the composite renal endpoint. Interestingly, dapagliflozin was examined for its renoprotective effect by the primary cause of CKD in an exploratory analysis of the DAPA-CKD study data, which revealed that despite there being no interaction between its renoprotective effect and any primary cause of CKD, the medication offered renoprotection to people with diabetic nephropathy [89]. Given that most patients in the DAPA-CKD and CREDENCE trials were shown to have overt albuminuria (urinary ACR ≥ 300 mg/g), it appears that the evidence of renoprotection with SGLT2 inhibitors remains nearly limited to people with T2DM having overt nephropathy. In light thereof, this algorithm noted that SGLT2 inhibitors should be considered as medications of first choice in patients with albuminuria (particularly overt nephropathy), regardless of their glucose-lowering effects, while not only SGLT2 inhibitors but GLP-1 receptor agonists should be considered in patients without albuminuria. It should also be noted that the renoprotective effect of SGLT2 inhibitors remains unclear in highly renally impaired patients (eGFR < 20 mL/min/1.73 m2).
Of the GLP-1 receptor agonists currently available, liraglutide has been shown to inhibit the onset of persistent overt albuminuria, thereby reducing the occurrence of a composite renal endpoint in a subset analysis of people with T2DM at a high risk of CVD treated with this medication [90]. Semaglutide has been shown to reduce the composite renal endpoint in people with T2DM at high risk of CVD [74] and duraglutide inhibits the deterioration of eGFR, thereby significantly reducing albuminuria in people with T2DM exhibiting eGFR ≥ 30/ < 60 mL/min/1.73 m2 [91]. GLP-1 receptor agonists have also been reported to reduce the composite cardiovascular endpoint (cardiovascular death, non-fatal MI, and non-fatal cerebral infarction [CI]) in people with T2DM with renal dysfunction (eGFR, < 60 mL/min/1.73 m2) [92], GLP-1 receptor agonists were recommended as medications of second choice in people with T2DM having albuminuria. Hence, in this algorithm, SGLT2 inhibitors and GLP-1 receptor agonists are worth considering in people with T2DM without albuminuria.
Weighing the additional benefit of antidiabetic medications for NAFLD
Although not mentioned in the proposed algorithm, nonalcoholic fatty liver disease (NAFLD) is covered by this revision as a comorbidity that requires medical attention. Given that NAFLD often occurs in people with T2DM, consideration should be given to selecting medications that are expected to be effective in improving NAFLD. According to the Evidence-based Clinical Guidelines for NAFLD /Nonalcoholic Steatohepatitis 2020 (2nd Edition) from the Japanese Society of Gastroenterology, thiazolidinediones are recommended as are SGLT2 inhibitors and GLP-1 receptor agonists also suggested [93]. Evidence in Japanese patients has been provided through some reports, suggesting a correlation between the blood concentration of pioglitazone or its metabolites and improvement of active NAFLD in hepatic tissue [94], and that SGLT2 inhibitors may improve histology of NAFLD [95–97]. Maturation of adipocytes, normalized adipokine, and normalized energy influx [98, 99] are deemed to contribute to NAFLD improvement by pioglitazone. Likewise, SGLT2 inhibitors are expected to reduce the hepatic glycogen content following reduced-insulin concentration in the blood, promote oxidation of fatty acids, and increase lipolysis of adipose tissue [100]. Overseas studies have also reported that of the GLP-1 receptor agonists with significant weight-reducing effects, semaglutide has been shown to be effective in non-alcoholic steatohepatitis (NASH) patients with histologic improvement of NASH without hepatic fibrosis aggravation [101]. The mechanisms include CNS-mediated reduction of food intake and body weight, suppression of hepatic lipid synthesis through decreased expression of fatty acid synthesis genes and inflammatory genes, and suppression of inflammatory cell infiltration. [102]. It has been shown that tirzepatide, a dual GLP-1/GIP receptor agonist, exerts highly potent weight-reducing dose-dependent effects in Japanese people with T2DM [35, 36]. The weight-reducing rate of 7% or higher is likely to be achieved with potential for reduction in liver steatosis, inflammatory cells, and ballooning of adipocytes at high doses. It is hoped that further clinical trials in Japanese people with T2DM will demonstrate the beneficial effects of GLP-1 receptor agonists and GLP-1/GIP receptor agonists on NAFLD.
Patient background to consider (step 4)
In this algorithm, the patient background that should be taken into account is the medication compliance rate and medical cost (Fig. 2, Table 1). Given that medication adherence in people with T2DM has been shown not only to impact their glycemic control but also to be associated with CVD morbidity, mortality, and hospitalization risk, paying attention to maintaining medication adherence represents an extremely important part of diabetes clinical practice. The medical-cost burden should be weighed for each patient, as it includes not only the medication costs but also the associated healthcare costs.
Individuals with chronic diseases, such as diabetes mellitus, must recognize that they need to be on long-term pharmacotherapy while adhering to the dosage and usage of prescribed medications. Indeed, a decline in adherence to these medications not only diminishes their antidiabetic efficacy, but also increases the risk of hypoglycemia through inappropriate intensification of therapy and contributes to polypharmacy. Unfortunately, adherence rates for patients prescribed antidiabetic medications have been reported to be only 68.6% for new prescriptions and 78.1% for all prescriptions [103]. According to a meta-analysis of eight observational studies in people with T2DM, the relative risks for all-cause mortality and hospitalization were shown to be 0.72 (95% confidence interval [CI], 0.62–0.82) and 0.90 (0.87–0.94) among those in the high adherence (80% or higher) group compared to the low adherence (< 80%) group. It is also reported that low adherence is associated with increased CVD risk [104]. Overseas studies have also reported that there is a significant correlation between medication adherence and changes in HbA1c among people with T2DM, that is, a 10% increase in adherence translates into a 0.15% decrease in HbA1c [105, 106]. Similar results have been shown in a questionnaire survey conducted among 1022 people with T2DM in Japan. There was a significant difference in adherence between those receiving and those not receiving their medications as one package [107]. Notably, a systematic review and meta-analysis of medication adherence in people with T2DM has become available [108]. In this review, a comparison of adherence to metformin, SUs, and thiazolidinediones showed that medication adherence was significantly higher with SUs and thiazolidinediones than with metformin; higher with thiazolidinediones than with SUs; and higher with DPP-4 inhibitors than with thiazolidinediones or SUs. In a retrospective study to evaluate the need for intensification of therapy after treatment with biguanides or DPP-4 inhibitors in medication-naïve Japanese people with T2DM, DPP-4 inhibitors are superior to biguanides [109]. While glinides and α-glucosidase inhibitors, which need to be taken before meals, are assumed to be associated with lower adherence rates than SUs, biguanides, or thiazolidinediones [110], this may be remedied by implementing appropriate measures such as ensuring that all other medications are also taken before meals [107]. Thus, based on the evidence summarized above, this algorithm includes a summary of adherence rates for available antidiabetic medications in a separate table. In an increasingly aging society like Japan, it is important to focus on minimizing dosing frequency as well as on maintaining or improving medication adherence through appropriate measures, for example, provision of medications in one package or use of mixture medications.
Medical expenditures are increasing with the aging of the population [111]. A survey on medical expenditure for non-communicable diseases (NCDs) in 2019 by the National Federation of Health Insurance Societies showed that of the 10 NCDs, including cerebrovascular disease, ischemic heart disease, and end-stage kidney disease (dialysis), diabetes mellitus imposes a huge economic burden on affected individuals, accounting for the third largest share in hospitalization costs, the largest share in non-hospitalization costs, and the second largest share next to dialysis in terms of daily health care costs [112]. As high medical expenditures are associated with a decline in adherence to medications [113], it is also likely to be associated with a decline in their efficacy. Thus, consideration needs to be given to the choice of antidiabetic medication(s), as they vary widely in their prices in Japan and expensive choices impose an increased burden on patients. Moreover, the financial burden on patients is not limited by medication costs. Of note here is a survey conducted using the National Database of Health Insurance Claims and Specific Health Check-ups to investigate total medical expenditures incurred by Japanese people with T2DM in the 1 year following their initial antidiabetic prescription (adjusted for age, sex, comorbidities, healthcare institutional attributes, and other relevant factors), which showed that, of all antidiabetic medications, biguanides represented the lowest 1-year expenditure, followed by thiazolidinediones and α-glucosidase inhibitors, while GLP-1 receptor agonists represented the highest [26]. An estimation of the economic burden associated with the use of antidiabetic medications is provided in a separate table in terms of their prices and the associated total medical expenditure. Therefore, to help reduce the economic burden placed on each patient, consideration should be given to using generics, switching to biguanides, or switching from multiple single agents to mixture medications.
While not mentioned in the proposed algorithm, the durability of the effects was added to Table 1 in this revision as a factor that needs to be considered. According to the ADOPT trial, which examined the durability of glycemic improvement effects of three medications (rosiglitazone in the thiazolidinedione class, metformin, and glyburide in the SUs class), greater durability of effects was shown in thiazolidinedione than metformin and SUs [114]. A comparison of pioglitazone and gliclazide also showed that pioglitazone represents a better medication, given the durability of its glycemic improvement effects [115]. The GRADE trial in people with T2DM receiving metformin therapy has recently reported that sitagliptin, glimepiride, liraglutide, or glargine were added to the regimens of those patients receiving metformin to conduct a follow-up survey of the period to reach HbA1c ≥ 7% and found that liraglutide and glargine were superior to other medications in terms of the durability of the effects [116]. The durability of the glucose-lowering effects of thiazolidinediones and SGLT2 inhibitors was shown to be higher than that of SUs, metformin, and DPP-4 inhibitors [117]. It has also been reported that the persistence of HbA1c reduction following the initiation of α-glucosidase inhibitors is approximately 3 years [118]. Thus, based on the reports above, thiazolidinediones, SGLT2 inhibitors, and GLP-1 receptor agonists represent medications with high durability, whereas SUs and α-glucosidase inhibitors represent medications with low durability.
Periodic assessment of treatment efficacy and the need for adjustments in pharmacotherapy
It is proposed in the present algorithm that each medication regimen be reviewed for possible revision every 3 months following its initiation in order to avoid delays when target HbA1c cannot be achieved in addressing patients requiring intensification of therapy. Attention should be focused on promoting medical nutrition therapy tailored to address their diabetes pathology and comorbidities including nephropathy, exercise therapy, and lifestyle modification in each patient. And it is important to revert to the opening of the algorithm, as required, to add further medications, increase their medication doses, or consider alternative medications including the reassessment for insulin therapy.
It should be noted that if left untreated, hyperglycemia increases the subsequent risk of diabetic microangiopathy, macroangiopathy, or death in people with diabetes [119, 120] and that inappropriate glycemic control results, at least in part, from delays in initiation or intensification of therapy (i.e., clinical inertia) [121]. Indeed, it has been reported in a US study that antidiabetic therapy was not appropriately initiated and intensified within 6 months of consultation in 37% and 18% of people with diabetes requiring initiation and intensification of therapy, respectively [122]; moreover, it has also been reported that antidiabetic therapy was not intensified within 6 months of consultation in 44% of people with T2DM having HbA1c 9% or higher [123]. Given the clinical inertia that often occurs in clinical practice, pharmacotherapy needs to be immediately adjusted in patients who fail to achieve their respective HbA1c control goals. While it is recommended by the ADA that any medication regimen be reviewed for efficacy as well as for revision every 3 to 6 months [20], the revision of medication regimen in a 3-month cycle is recommended in Japan. Basically, it is reflecting the usual frequency of hospital visits by people with diabetes in Japan.
Acknowledgements
JDS Committee on Consensus Statement Development: Toshimasa Yamauch is the Chairperson. Ryotaro Bouchi, Tatsuya Kondo, Yasuharu Ohta, Norio Harada, Hideki Kamiya, Ryo Suzuki are the Committee member.
Declarations
Conflict of interest
Ryotaro Bouchi: Honoraria (Sumitomo Pharma; AstraZeneca; Novo Nordisk Pharma), and Research funding (Sumitomo Pharma) Yasuharu Ohta: Research funding (Manpei Suzuki Diabetes Foundation), and Subsidies or Donations (Sumitomo Pharma; Nippon Boehringer Ingelheim; Novo Nordisk Pharma). Daisuke Yabe: Honoraria (Novo Nordisk Pharma; Nippon Boehringer Ingelheim; Eli Lilly Japan; Sanofi; Kyowa Kirin; Sumitomo Pharma), and Research funding (Terumo Corporation; Nippon Boehringer Ingelheim), and Endowed departments by commercial entities (Novo Nordisk Pharma; Taisho Pharmaceutical; ARKRAY). Rimei Nishimura: Honoraria (Sanofi; Medtronic; Nippon Boehringer Ingelheim; KISSEI PHARMACEUTICAL; Eli Lilly Japan; Novo Nordisk Pharma; Astellas Pharma; Abbott Japan; Sumitomo Pharma; AstraZeneca; KOWA; ONO PHARMACEUTICAL; Taisho Pharmaceutical), and Research funding (Mitsubishi Electric), and Subsidies or Donations (Taisho Pharmaceutical; Nippon Boehringer Ingelheim; Abbott Japan; Sumitomo Pharma; Eli Lilly Japan; Arkley). Norio Harada: Research funding (Mitsubishi Tanabe Pharma; Eli Lilly Japan; Nippon Boehringer Ingelheim; ONO PHARMACEUTICAL). Hideki Kamiya: Honoraria (Novo Nordisk Pharma; Sanofi; Sumitomo Pharma; Eli Lilly Japan; Nippon Boehringer Ingelheim; DAIICHI SANKYO; AstraZeneca; ONO PHARMACEUTICAL; KISSEI PHARMACEUTICAL; Mitsubishi Tanabe Pharma; KOWA; Novartis Pharma; MSD; SANWA KAGAKU KENKYUSHO; Otsuka Pharmaceutical), and Research funding (ONO PHARMACEUTICAL; Eli Lilly Japan; KISSEI PHARMACEUTICAL), and Subsidies or Donations (ONO PHARMACEUTICAL; Taisho Pharmaceutical; Sumitomo Pharma; Takeda Pharmaceutical; Mitsubishi Tanabe Pharma; JAPAN TOBACCO; Novo Nordisk Pharma). Ryo Suzuki: Honoraria (Novo Nordisk Pharma; Sanofi; Sumitomo Pharma; Astellas Pharma; KOWA; MSD; Eli Lilly Japan; Mitsubishi Tanabe Pharma; TEIJIN PHARMA), and Research funding (Sumitomo Pharma), and Subsidies or Donations (Nippon Boehringer Ingelheim). Toshimasa Yamauchi: Honoraria (ONO PHARMACEUTICAL; Takeda Pharmaceutical; Sumitomo Pharma; TEIJIN PHARMA; Nippon Boehringer Ingelheim; Novo Nordisk Pharma), and Research funding (KOWA; Minophagen Pharmaceutical; NIPRO CORPORATION; Kyowa Kirin), and Subsidies or Donations (Novo Nordisk Pharma; Mitsubishi Tanabe Pharma; Kyowa Kirin; Takeda Pharmaceutical; ONO PHARMACEUTICAL; Sumitomo Pharma), and Endowed departments by commercial entities (ONO PHARMACEUTICAL; Mitsubishi Tanabe Pharma; Novo Nordisk Pharma; Nippon Boehringer Ingelheim; KOWA; NITTO BOSEKI; Asahi Mutual Life Insurance Company). Dr Daisuke Yabe, Dr Norio Harada, Dr Hideki Kamiya, Dr Toshimasa Yamauchi are Editorial Board members of Journal of Diabetes Investigation and a co-author of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication.
Footnotes
This article is the English version of the “A Consensus Statement from the Japan Diabetes Society: A Proposed Algorithm for Pharmacotherapy in People With Type 2 Diabetes (the Second Edition)” (10.11213/tonyobyo.66.715) released in Japanese on Volume 66 Issue 10, 2023, on the official website of the Japan Diabetes Society, and has been jointly published in Journal of Diabetes Investigation (the official journal of the Asian Association for the Study of Diabetes: https://onlinelibrary.wiley.com/doi/10.1111/jdi.14202) and Diabetology International (the official English journal of JDS).
The authors have received approval from the editors of the Journal of the Japan Diabetes Society, Journal of Diabetes Investigation, and Diabetology International.
JDS Committee on Consensus Statement Development Chairperson (Toshimasa Yamauchi), Ryotaro Bouchi, Tatsuya Kondo, Yasuharu Ohta, Norio Harada, Hideki Kamiya and Ryo Suzuki are the Committee members.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ryotaro Bouchi, Email: rybouchi@hosp.ncgm.go.jp.
JDS Committee on Consensus Statement Development:
Toshimasa Yamauchi, Ryotaro Bouchi, Tatsuya Kondo, Yasuharu Ohta, Norio Harada, Hideki Kamiy, and Toshimasa Yamauchi
References
- 1.Bouchi R, Kondo T, Ohta Y, Goto A, Tanaka D, Satoh H, Yabe D, Nishimura R, Harada N, Kamiya H, Suzuki R, Yamauchi T. The Committee of Establishing Consensus Statements, A Consensus Statement from the Japan Diabetes Society: a proposed algorithm for pharmacotherapy in people with type 2 Diabetes (The Second Edition). J Jpn Diabetes Soc. 2023;66(10):715–33. [Google Scholar]
- 2.Review Committee Survey on the Diagnostic Criteria for Diabetes Mellitus (SRCDCD). A report from the SCCDCD (Globally standardized version). Diabetes. 2012;55:485–504. [Google Scholar]
- 3.Fukushima M, Usami M, Ikeda M, Nakai Y, Taniguchi A, Matsuura T, Suzuki H, Kurose T, Yamada Y, Seino Y. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53:831–5. [DOI] [PubMed] [Google Scholar]
- 4.Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, Groop LC. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49:975–80. [DOI] [PubMed] [Google Scholar]
- 5.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto K, Miyake S, Yano M, Ueki Y, Yamaguchi Y, Akazawa S, Tominaga Y. Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care. 1997;20:1562–8. [DOI] [PubMed] [Google Scholar]
- 7.Yoshinari M, Hirakawa Y, Hata J, Higashioka M, Honda T, Yoshida D, Mukai N, Nakamura U, Kitazono T, Ninomiya T. Comparison of the contributions of impaired beta cell function and insulin resistance to the development of type 2 diabetes in a Japanese community: the Hisayama Study. Diabetologia. 2021;64:1775–84. [DOI] [PubMed] [Google Scholar]
- 8.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. [DOI] [PubMed] [Google Scholar]
- 9.Kou K, Saisho Y, Satoh S, Yamada T, Itoh H. Change in β-cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab. 2013;98:3724–30. [DOI] [PubMed] [Google Scholar]
- 10.Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978;15:417–21. [DOI] [PubMed] [Google Scholar]
- 11.Kamata K, Mizukami H, Inaba W, Tsuboi K, Tateishi Y, Yoshida T, Yagihashi S. Islet amyloid with macrophage migration correlates with augmented β-cell deficits in type 2 diabetic patients. Amyloid. 2014;21:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, Hirota Y, Mori H, Jonsson A, Sato Y, Yamagata K, Hinokio Y, Wang HY, Tanahashi T, Nakamura N, Oka Y, Iwasaki N, Iwamoto Y, Yamada Y, Seino Y, Maegawa H, Kashiwagi A, Takeda J, Maeda E, Shin HD, Cho YM, Park KS, Lee HK, Ng MC, Ma RC, So WY, Chan JC, Lyssenko V, Tuomi T, Nilsson P, Groop L, Kamatani N, Sekine A, Nakamura Y, Yamamoto K, Yoshida T, Tokunaga K, Itakura M, Makino H, Nanjo K, Kadowaki T, Kasuga M. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008;40:1092–7. [DOI] [PubMed] [Google Scholar]
- 13.Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, Ng DP, Holmkvist J, Borch-Johnsen K, Jørgensen T, Sandbaek A, Lauritzen T, Hansen T, Nurbaya S, Tsunoda T, Kubo M, Babazono T, Hirose H, Hayashi M, Iwamoto Y, Kashiwagi A, Kaku K, Kawamori R, Tai ES, Pedersen O, Kamatani N, Kadowaki T, Kikkawa R, Nakamura Y, Maeda S. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet. 2008;40:1098–102. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T, Hara K, Maeda S, Yasuda K, Takahashi A, Horikoshi M, Nakamura M, Fujita H, Grarup N, Cauchi S, Ng DP, Ma RC, Tsunoda T, Kubo M, Watada H, Maegawa H, Okada-Iwabu M, Iwabu M, Shojima N, Shin HD, Andersen G, Witte DR, Jørgensen T, Lauritzen T, Sandbæk A, Hansen T, Ohshige T, Omori S, Saito I, Kaku K, Hirose H, So WY, Beury D, Chan JC, Park KS, Tai ES, Ito C, Tanaka Y, Kashiwagi A, Kawamori R, Kasuga M, Froguel P, Pedersen O, Kamatani N, Nakamura Y, Kadowaki T. A genome-wide association study in the Japanese population identifies susceptibility loci for type 2 diabetes at UBE2E2 and C2CD4A-C2CD4B. Nat Genet. 2010;42:864–8. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Akiyama M, Ishigaki K, Kanai M, Hosoe J, Shojima N, Hozawa A, Kadota A, Kuriki K, Naito M, Tanno K, Ishigaki Y, Hirata M, Matsuda K, Iwata N, Ikeda M, Sawada N, Yamaji T, Iwasaki M, Ikegawa S, Maeda S, Murakami Y, Wakai K, Tsugane S, Sasaki M, Yamamoto M, Okada Y, Kubo M, Kamatani Y, Horikoshi M, Yamauchi T, Kadowaki T. Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat Genet. 2019;51:379–86. [DOI] [PubMed] [Google Scholar]
- 16.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. [DOI] [PubMed] [Google Scholar]
- 17.Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164:740–51. [DOI] [PubMed] [Google Scholar]
- 18.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461–98. [DOI] [PubMed] [Google Scholar]
- 19.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in Type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Professional Practice Committee. 9 Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl. 1):S125–43. [DOI] [PubMed] [Google Scholar]
- 21.Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, Osawa H, Taguchi A, Tanizawa Y, Tobe K, Yoshioka N. Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol Int. 2020;11:165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17. [DOI] [PubMed] [Google Scholar]
- 23.Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, Haraguchi M, Morita A, Ohashi K, Hara K, Morise A, Izumi K, Ishizuka N, Ohashi Y, Noda M, Kadowaki T, J-DOIT 3 Study Group. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT 3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:951–64. [DOI] [PubMed] [Google Scholar]
- 24.Kohro T, Yamazaki T, Sato H, Harada K, Ohe K, Komuro I, Nagai R. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart. 2013;J54:93–7. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura R, Kato H, Kisanuki K, Oh A, Hiroi S, Onishi Y, Guelfucci F, Shimasaki Y. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9: e025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouchi R, Sugiyama T, Goto A, Imai K, Ihana-Sugiyama N, Ohsugi M, Yamauchi T, Kadowaki T, Ueki K. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Invest. 2022;13:280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999–2018. N Engl J Med. 2021;384:2219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the proper use of SGLT2 inhibitors. Diabetol Int. 2020;11:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Committee on the Proper Use of Biguanides. Recommendations for the Proper Use of Metformin. Recommendations for the Proper Use of Metformin. (Cited Oct 31, 2023, in Japanese) https://www.nittokyo.or.jp/uploads/files/recommendation_metformin_200318.pdf
- 30.Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes, Haneda M, Ito H. Glycemic targets for elderly patients with diabetes. Diabetol Int. 2016;7:331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Japan Society for the Study of Obesity. Clinical practice guidelines for the management of obesity 2016. Ltd: Life Science Publishers Co.; 2016. [Google Scholar]
- 32.Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJ, Dave SS, Qureshi F, Kandula NR. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the Masala and Mesa studies. Daibetes Care. 2014;37:1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015;38:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (surpass J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:623–33. [DOI] [PubMed] [Google Scholar]
- 36.Kadowaki T, Chin R, Ozeki A, Imaoka T, Ogawa Y. Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (surpass J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:634–44. [DOI] [PubMed] [Google Scholar]
- 37.Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. [DOI] [PubMed] [Google Scholar]
- 38.Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: Focus on East Asian perspectives. J Diabetes Invest. 2016;7(Suppl 1):102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F, example Investigators,. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35. [DOI] [PubMed] [Google Scholar]
- 40.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR, TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.26052984 [Google Scholar]
- 41.Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK, Investigators C. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the Carmelina randomized clinical trial. JAMA. 2019;321:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, SAVOR-TIMI53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.23992601 [Google Scholar]
- 43.Namba M, Iwakura T, Nishimura R, Akazawa K, Matsuhisa M, Sato Y, Yamauchi T. Japan Diabetes Society survey committee on severe hypoglycemia associated with antidiabetic drugs. a report from the survey committee on severe hypoglycemia associated with antidiabetic drugs. Diabetes. 2017;60:826–42. [Google Scholar]
- 44.Kaku K, Tajima N, Kawamori R. An observational study of metformin use in type 2 diabetes in clinical practice (MORE study). Diabetes. 2006;49:325–31. [Google Scholar]
- 45.Odawara M, Kawamori R, Tajima N, Iwamoto Y, Kageyama S, Yodo Y, Ueki F, Hotta N. Long-term treatment study of global standard dose metformin in Japanese patients with type 2 diabetes mellitus. Diabetol Int. 2017;8:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, Liakos A, Matthews DR, Bekiari E. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173:278–86. [DOI] [PubMed] [Google Scholar]
- 47.Yamada Y, Katagiri H, Hamamoto Y, Deenadayalan S, Navarria A, Nishijima K, Seino Y, Pioneer 9 investigators. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (pioneer 9): a52-week, phase 2/3 a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020;8:377–91. [DOI] [PubMed] [Google Scholar]
- 48.Yabe D, Nakamura J, Kaneto H, Deenadayalan S, Navarria A, Gislum M, Inagaki N, pioneer 10 Investigators. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (pioneer 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020;8:392–406. [DOI] [PubMed] [Google Scholar]
- 49.Sugihara H, Nagao M, Harada T, Nakajima Y, Tanimura-Inagaki K, Okajima F, Tamura H, Inazawa T, Otonari T, Kawakami M, Oikawa S. Comparison of three α-glucosidase inhibitors for glycemic control and body weight reduction in Japanese patients with obese type 2 diabetes. J Diabetes Invest. 2014;5:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. [DOI] [PubMed] [Google Scholar]
- 51.Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K, SURPASS-2 Investigators. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–15. [DOI] [PubMed] [Google Scholar]
- 52.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–51. [DOI] [PubMed] [Google Scholar]
- 53.Andersson C, Olesen JB, Hansen PR, Weeke P, Norgaard ML, Jørgensen CH, Lange T, Abildstrøm SZ, Schramm TK, Vaag A, Køber L, Torp-Pedersen C, Gislason GH. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia. 2010;53:2546–53. [DOI] [PubMed] [Google Scholar]
- 54.Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6:395–402. [DOI] [PubMed] [Google Scholar]
- 55.Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS, Williams JW Jr. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halabi A, Sen J, Huynh Q, Marwick TH. Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol. 2020;19:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benes J, Kotrc M, Kroupova K, Wohlfahrt P, Kovar J, Franekova J, Hegarova M, Hoskova L, Hoskova E, Pelikanova T, Jarolim P, Kautzner J, Melenovsky V. Metformin treatment is associated with improved outcome in patients with diabetes and advanced heart failure (HFrEF). Sci Rep. 2022;12:13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arai M, Shirakawa J, Konishi H, Sagawa N, Terauchi Y. Bullous pemphigoid and dipeptidyl peptidase 4 inhibitors: a disproportionality analysis based on the Japanese Adverse Drug Event Report Database. Diabetes Care. 2018;41:e130–2. [DOI] [PubMed] [Google Scholar]
- 59.Sasako T, Ueki K, Miyake K, Okazaki Y, Takeuchi Y, Ohashi Y, Noda M, Kadowaki T. Effect of a multifactorial intervention on fracture in patients with type 2 diabetes: subanalysis of the J-DOIT 3 study. J Clin Endocrinol Metab. 2021;106:e2116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuccori M, Filion KB, Yin H, Yu OH, Platt RW, Azoulay L. Pioglitazone use and risk of bladder cancer: population based cohort study. BMJ. 2016;352: i1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levin D, Bell S, Sund R, Hartikainen SA, Tuomilehto J, Pukkala E, Keskimäki I, Badrick E, Renehan AG, Buchan IE, Bowker SL, Minhas-Sandhu JK, Zafari Z, Marra C, Johnson JA, Stricker BH, Uitterlinden AG, Hofman A, Ruiter R, de Keyser CE, MacDonald TM, Wild SH, McKeigue PM, Colhoun HM, Scottish Diabetes Research Network Epidemiology Group; Diabetes and Cancer Research Consortium. Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia. 2015;58:493–504. [DOI] [PubMed] [Google Scholar]
- 62.Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, Ehrlich SF, Mamtani R, Bilker W, Vaughn DJ, Nessel L, Van Den Eeden SK, Ferrara A. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314:265–77. [DOI] [PubMed] [Google Scholar]
- 63.Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, Mannucci E. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab. 2017;19:1233–41. [DOI] [PubMed] [Google Scholar]
- 64.Qiu M, Ding LL, Zhang M, Zhou HR. Safety of four SGLT2 inhibitors in three chronic diseases: a meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc Dis Res. 2021;18:14791641211011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mishra R, Raj R, Elshimy G, Majety P, Edem DR. Adverse events related to tizepatide. J Endocr Soc. 2023;7:bvad16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maiorino MI, Longo M, Scappaticcio L, Bellastella G, Chiodini P, Esposito K, Giugliano D. Improvement of glycemic control and reduction of major cardiovascular events in 18 cardiovascular outcome trials: an updated meta-regression. Cardiovasc Diabetol. 2021;20:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, Zoungas S. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA-REG outcome Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.26378978 [Google Scholar]
- 69.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Canvas Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.28605608 [Google Scholar]
- 70.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, DECLARE-TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.30415602 [Google Scholar]
- 71.Home P. Cardiovascular outcome trials of glucose-lowering medications: an update. Diabetologia. 2019;62:357–69. [DOI] [PubMed] [Google Scholar]
- 72.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9. 10.1016/S0140-6736(18)32590-X. 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 73.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, leader Steering Committee; leader Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.27295427 [Google Scholar]
- 74.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T, SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl JMed. 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 75.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S, Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): A double-blind, randomized placebo-controlled trial. Lancet. 2018;392:1519–29.30291013 [Google Scholar]
- 76.Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, et al. Empagliflozin and cardiovascular outcomes in Asian Patients With Type 2 diabetes and established cardiovascular disease result from EMPA-REG outcome ((R)). Circ. 2017;J81:227–34. [DOI] [PubMed] [Google Scholar]
- 77.Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, Tangri N, Goh SY, Thuresson M, Chen H, Surmont F, Hammar N, Fenici P, CVD-REAL Investigators and Study Group. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL2 study. J Am Coll Cardiol. 2018;71:2628–3263. [DOI] [PubMed] [Google Scholar]
- 78.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduction reduction fraction. N Engl J Med. 2019;381:1995–2008.31535829 [Google Scholar]
- 79.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, Emperor - Reduced Trial Investigators. Cardovascular and Outcomes with Empagliflozin Heart Failure. N Engl J Med. 2020;383:1413–24.32865377 [Google Scholar]
- 80.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the emperor - reduced and DAPA - HF trials. Lancet. 2020;396:819–29. [DOI] [PubMed] [Google Scholar]
- 81.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, emperor - Preserved Investigators. Empagliflo failure with heart preservation election fraction. N Engl J Med. 2021;385:1451–61.34449189 [Google Scholar]
- 82.Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, Lamba S, Sharma K, Khan SS, Chandra L, Gordon RA, Ryan JJ, Chaudhry SP, Joseph SM, Chow CH, Kanwar MK, Pursley M, Siraj ES, Lewis GD, Clemson BS, Fong M, Kosiborod MN. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85. [DOI] [PubMed] [Google Scholar]
- 84.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, McNulty SE, Anstrom KJ, Shah MR, Braunwald E, Cappola TP, NHLBI Heart Failure Clinical Research Network. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: results from the canvas Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704. [DOI] [PubMed] [Google Scholar]
- 86.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC, DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436–46.32970396 [Google Scholar]
- 87.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, Credit Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nropephathy. N Engl J Med. 2019;380:2295–306.30990260 [Google Scholar]
- 88.The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, Sammons E, Zhu D, Hill M, Stevens W, Wallendszus K, Brenner S, Cheung AK, Liu ZH, Li J, Hooi LS, Liu W, Kadowaki T, Nangaku M, Levin A, Cherney D, Maggioni AP, Pontremoli R, Deo R, Goto S, Rossello X, Tuttle KR, Steubl PM, Massey D, Eilbracht J, Brueckmann M, Landray MJ, Baigent C, Haynes R. Empaglloifzin patients with chronic kidney disease. N Engl J Med. 2023;388:117–27.36331190 [Google Scholar]
- 89.Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Correa-Rotter R, Rossing P, Toto RD, Sjöström CD, Langkilde AM, Heerspink HJL, DAPA-CKD Trial Committees and Investatorsigators. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:22–31. [DOI] [PubMed] [Google Scholar]
- 90.Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornoe K, Zinman B, Buse JB, Committee LS, Investigators. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–48. [DOI] [PubMed] [Google Scholar]
- 91.Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, Botros FT. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicenter, open-label, randomized trial. Lancet Diabetes Endocrinol. 2018;6:605–17. [DOI] [PubMed] [Google Scholar]
- 92.Mann JFE, Fonseca V, Mosenzon O, Raz I, Goldman B, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. 2018;138:2908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joint committee of the Japanese Society of Gastroenterology and the Japanese Society of Hepatology (2020) NAFLD/Nash Clinical Practice Guidelines 2020 Revision 2nd edition Edited by the Japanese Society of Gastroenterology and the Japanese Society of Hepatology.
- 94.Kawaguchi-Suzuki M, Bril F, Kalavalapalli S. Concentration-dependent response to pioglitazone in nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;46:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akuta N, Kawamura Y, Fujiyama S, Saito S, Muraishi N, Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kumada H. Favorable impact of long-term SGLT2 inhibitor for NAFLD complicated by diabetes mellitus: A5-year follow-up study. Hepatol Commun. 2022;6:2286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi H, Kessoku T, Kawanaka M, Nonaka M, Hyogo H, Fujii H, Nakajima T, Imajo K, Tanaka K, Kubotsu Y, Isoda H, Oeda S, Kurai O, Yoneda M, Ono M, Kitajima Y, Tajiri R, Takamori A, Kawaguchi A, Aishima S, Kage M, Nakajima A, Eguchi Y, Anzai K. Ipragliflozin improves the hepatic outcomes of patients with diabetes with NAFLD. Hepatol Commun. 2022;6:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takeshita Y, Honda M, Harada K, Kita Y, Takata N, Tsujiguchi H, Tanaka T, Goto H, Nakano Y, Iida N, Arai K, Yamashita T, Mizukoshi E, Nakamura H, Kaneko S, Takamura T. Comparison of tofogliflozin and glimepiride effects on nonalcoholic fatty liver disease in participants type with 2 diabetes: a randomized, 48-week, open-label, active-controlled trial. Diabetes Care. 2022;45:2064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. Adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. [DOI] [PubMed] [Google Scholar]
- 99.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating amp -activated protein kinase. Nat Med. 2002;8:1288–95. [DOI] [PubMed] [Google Scholar]
- 100.Obata A, Kubota N, Kubota T, Iwamoto M, Sato H, Sakurai Y, Takamoto I, Katsuyama H, Suzuki Y, Fukazawa M, Ikeda S, Iwayama K, Tokuyama K, Ueki K, Kadowaki T. Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology. 2016;157:1029–42. [DOI] [PubMed] [Google Scholar]
- 101.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA, NN 9931–4296 Investigators. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. [DOI] [PubMed] [Google Scholar]
- 102.Yabut JM, Drucker DJ. Glucagon-like peptide-1 receptor-based therapeutics for metabolic liver disease. Endocr Rev. 2023;44:14–32. [DOI] [PubMed] [Google Scholar]
- 103.Fischer MA, Stedman MR, Lii J, Vogeli C, Shrank WH, Brookhart MA, Weissman JS. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khunti K, Seidu S, Kunutsor S, Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care. 2017;40:1588–96. [DOI] [PubMed] [Google Scholar]
- 105.Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25:1015–21. [DOI] [PubMed] [Google Scholar]
- 106.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27:2800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kurooka N, Eguchi J, Ashida M, Nakajima N, Wada J, Nakajima H. Medication adherence in type 2 diabetes: an investigation into the extent and rate of medication adherence required for favorable glycemic control. Diabetes. 2020;63:609–17. [Google Scholar]
- 108.McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1040–3. [DOI] [PubMed] [Google Scholar]
- 109.Horii T, Iwasawa M, Shimizu J, Atsuda K. Comparing of treatment intensification and clinical outcomes of metformin and dipeptidyl peptidase-4 inhibitors in treatment-naïve patients with type 2 diabetes in Japan. J Diabetes Investig. 2020;11:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boccuzzi SJ, Wogen J, Fox J, Sung JC, Shah AB, Kim J. Utilization of oral hypoglycemic agents in a drug-insured U.S. population. Diabetes Care. 2001;24:1411–5. [DOI] [PubMed] [Google Scholar]
- 111.Estimates of National Medical Care Expenditure, FY 2019, Ministry of Health, Labour and Welfare (cited Oct 31, 2023) https://www.mhlw.go.jp/toukei/saikin/hw/k-iryohi/19/dl/kekka.pdf.
- 112.A survey on medical expenditure for lifestyle-related diseases in 2019 by the Group of Research and Analysis, the Policy Department of the National Federation of Health Insurance Societies (cited Oct 31, 2023) https://www.kenporen.com/toukei_data/pdf/chosa_r03_06_01.pdf
- 113.Kang H, Lobo JM, Kim S, Sohn M-W. Cost-related medication non-adherence among U.S. adults with diabetes. Diabetes Res Clin Practice. 2018;143:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G, Adopt Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. [DOI] [PubMed] [Google Scholar]
- 115.Tan MH, Baksi A, Krahulec B, Kubalski P, Stankiewicz A, Urquhart R, Edwards G, Johns D, Global Study Group. Comparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care. 2005;28:544–50. [DOI] [PubMed] [Google Scholar]
- 116.Grade Study Research Group, Nathan DM, Lachin JM, Balasubramanyam A, Burch HB, Buse JB, Butera NM, Cohen RM, Crandall JP, Kahn SE, Krause-Steinrauf H, Larkin ME, Rasouli N, Tiktin M, Wexler DJ, Younes N. Glycemia reduction in type 2 diabetes - glycemic outcomes. N Engl J Med. 2022;387:1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cherukuri L, Smith MS, Tayek JA. The durability of oral diabetic medications: time to A1c baseline and a review of common oral medications used by the primary care provider. Endocrinol Diabetes Metab J. 2018. 10.31038/EDMJ.2018232. 10.31038/EDMJ.2018232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care. 1999;22:960–4. [DOI] [PubMed] [Google Scholar]
- 119.Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risk of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, Karter AJ. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the diabetes & aging study). Diabetes Care. 2019;42:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS. Clinical inertia. Ann Intern Med. 2001;135:825–34. [DOI] [PubMed] [Google Scholar]
- 122.Gilstrap LG, Mehrotra A, Bai B, Rose S, Blair RA, Chernew ME. National rates of initiation and intensification of antidiabetic therapy among patients with commercial insurance. Diabetes Care. 2018;41:1776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ji X, Kong SX, Milinovich A, Weng W, Bauman J, Ganguly R, Burguera B, Kattan MW, Zimmerman RS. Clinical inertia in type 2 diabetes management: evidence from a large, real-world data set. Diabetes Care. 2018;41:e113–4. [DOI] [PubMed] [Google Scholar]