Abstract
Deficient activity of the guanine nucleotide regulatory protein (G unit), an integral component of the membrane-bound adenylate cyclase complex, has been implicated as the biochemical lesion in many patients with pseudohypoparathyroidism (PHP) type I. In addition to renal resistance to parathyroid hormone in this disorder, there is decreased responsiveness of diverse tissues to hormones that act via 3',5'-cyclic AMP (cAMP). To assess whether a deficiency of G units could account for impaired adenylate cyclase activity, we studied cAMP production in intact cultured fibroblasts and fibroblast plasma membranes from five patients with PHP in response to several activators of adenylate cyclase.
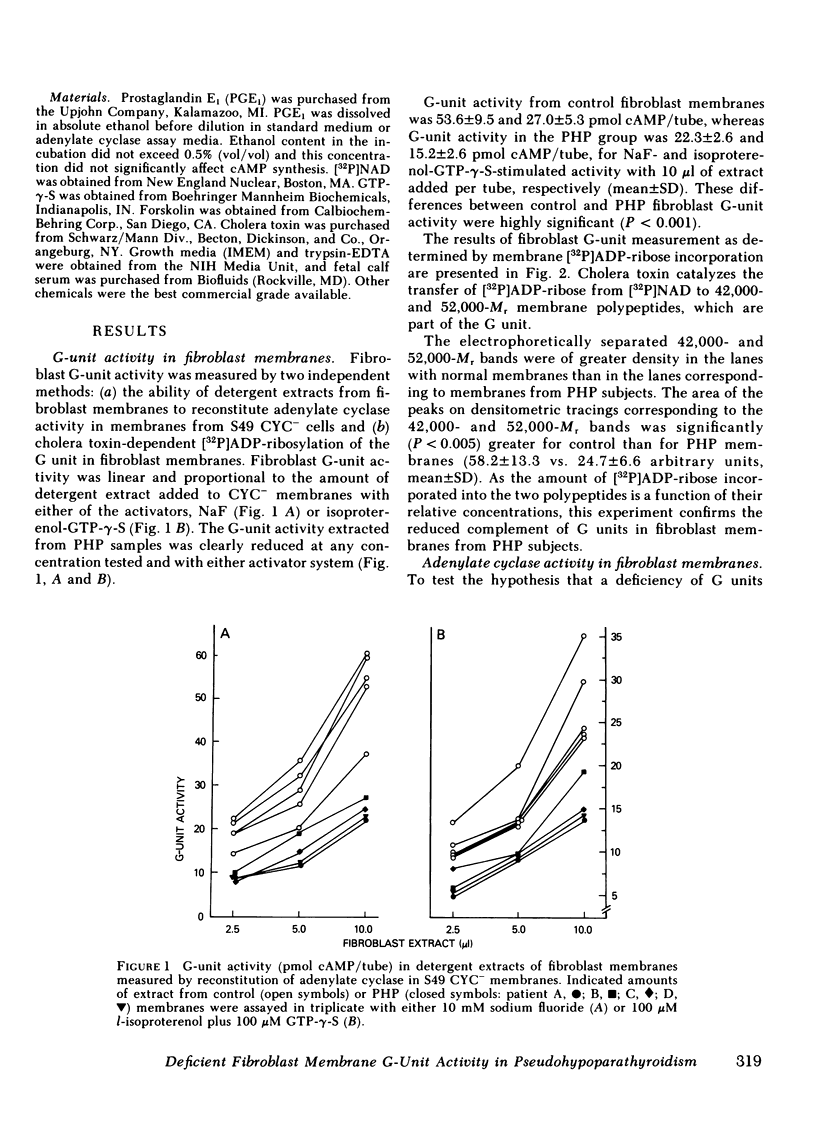
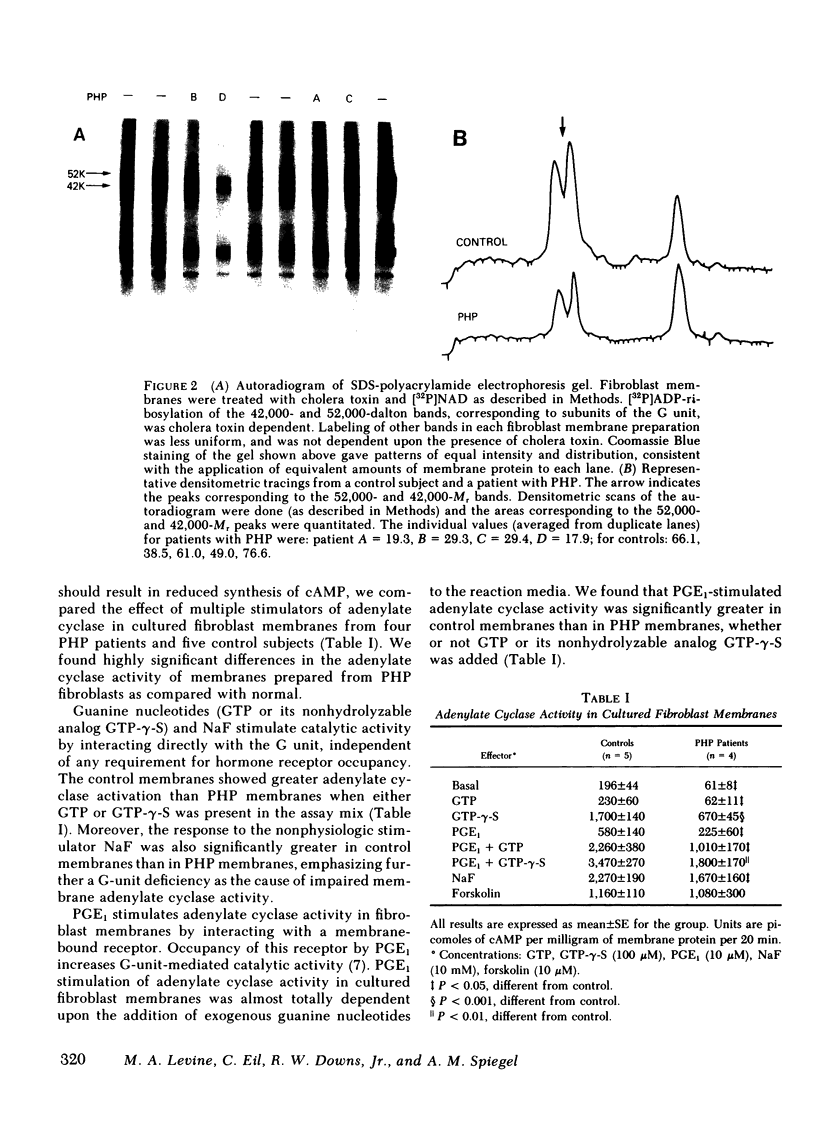
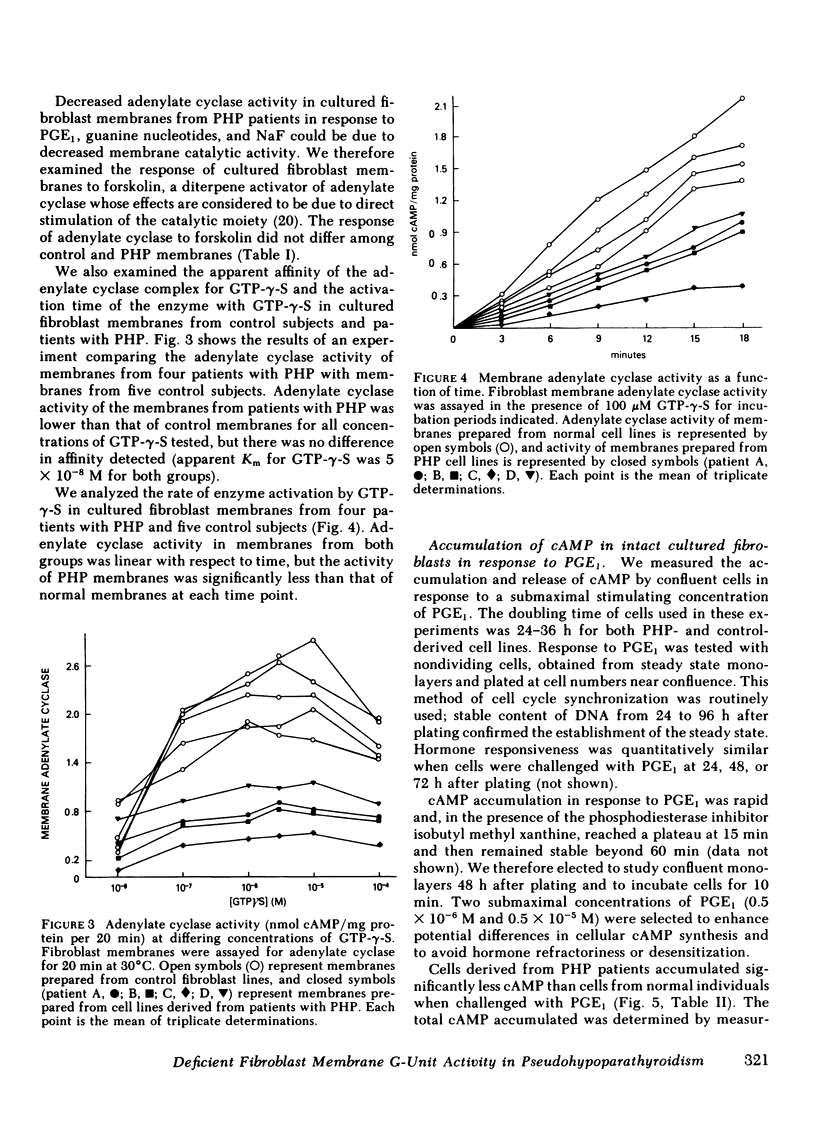
The number of G units in PHP fibroblast membranes, measured by cholera toxin-dependent [32P]ADP ribosylation of G-unit peptides, as well as the G-unit activity, determined by the ability of detergent extracts to reconstitute adenylate cyclase activity in G-unit-deficient S49 CYC- membranes, were found to be markedly reduced compared with control membranes (43 and 40%, respectively), The activation of fibroblast membrane adenylate cyclase by effectors that act directly through the G unit (guanosine triphosphate, guanosine 5'-0-[3-thiotriphosphate] [GTP-γ-S], NaF) was significantly greater in control membranes than in membranes from patients with PHP. Moreover, we found that hormone (prostaglandin E1) stimulated adenylate cyclase activity was also greater in control membranes than in PHP membranes. Neither the apparent affinity of membrane adenylate cyclase for GTP-γ-S (apparent Km =5 X 10-8 M) nor the rate of enzyme activation by GTP-γ-S was significantly different in fibroblast membranes from control subjects and patients with PHP. In contrast to the notable differences in hormone and G-unit-activated adenylate cyclase shown in fibroblast membranes from PHP patients and control subjects, the intrinsic catalytic activity of membranes, as determined by forskolin-stimulated adenylate cyclase, was not significantly different in the two groups.
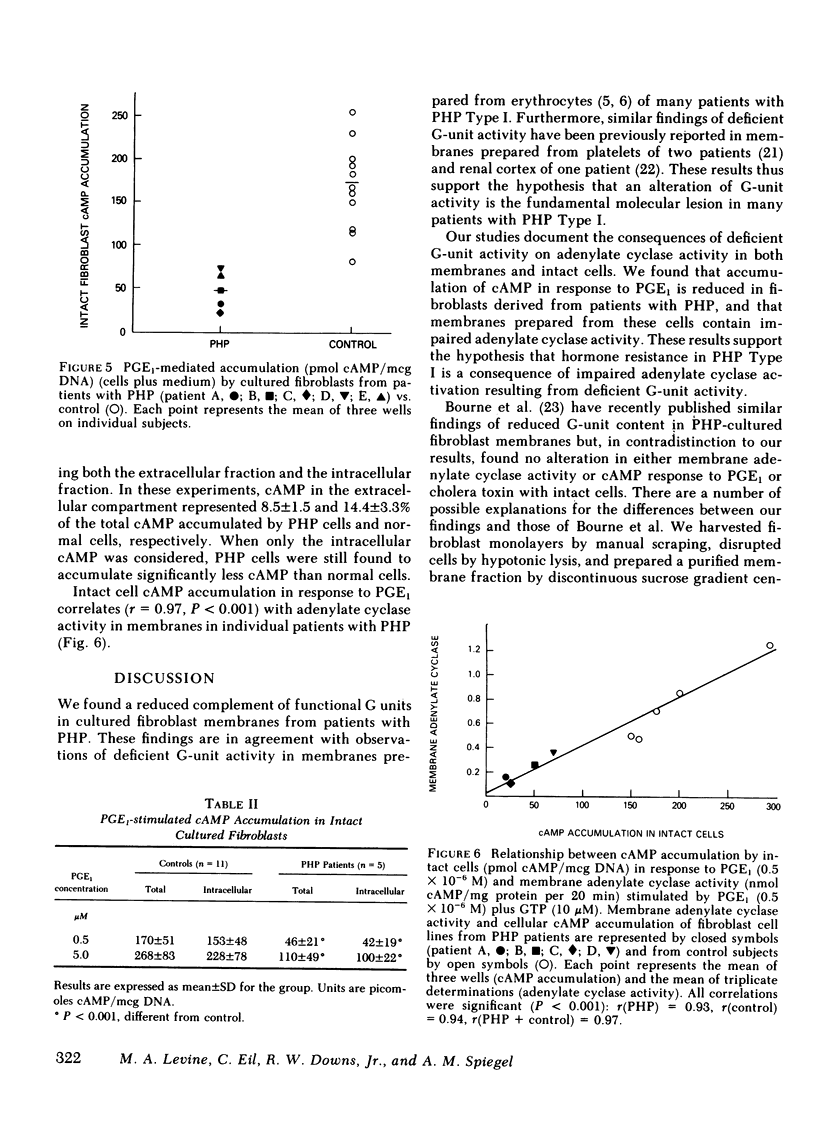
Intact fibroblasts derived from patients with PHP accumulated significantly (P 0.001) less cAMP (46±21 pmol cAMP/mcg DNA, n = 5) than cells from normal individuals (170±51 pmol cAMP/mcg DNA, n = 11) when stimulated with PGE1. PGE1-stimulated accumulation of cAMP by intact fibroblast monolayers correlated closely with PGE1 plus GTP-activated membrane adenylate cyclase activity in both patients and controls (r = 0.97, P < 0.001).
Our data show that, in patients with PHP, (a) fibroblast membranes show a decreased complement of G units, (b) membrane catalytic activity is normal, but adenylate cyclase activity is reduced when stimulated by hormone or by effectors which activate the G unit, (c) the ability of cells to accumulate cAMP in response to hormone stimulation is reduced, and (d) reduced membrane adenylate cyclase activity correlates well with impaired cellular cAMP synthesis. These results, taken together, indicate that a deficiency of G-unit activity can impair synthesis of cAMP by both intact and broken cells, and may explain the resistance of multiple tissues to hormones that act via cAMP observed in PHP.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Kaslow H. R., Brickman A. S., Farfel Z. Fibroblast defect in pseudohypoparathyroidism, type I: reduced activity of receptor-cyclase coupling protein. J Clin Endocrinol Metab. 1981 Sep;53(3):636–640. doi: 10.1210/jcem-53-3-636. [DOI] [PubMed] [Google Scholar]
- Brooker G., Terasaki W. L., Price M. G. Gammaflow: a completely automated radioimmunoassay system. Science. 1976 Oct 15;194(4262):270–276. doi: 10.1126/science.184530. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Melson G. L., Aurbach G. D. Pseudohypoparathyroidism: defective excretion of 3',5'-AMP in response to parathyroid hormone. J Clin Invest. 1969 Oct;48(10):1832–1844. doi: 10.1172/JCI106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. B., Hill S. C., Ulane M. M. Hormone-stimulated cyclic AMP production by skin fibroblasts cultured from healthy persons and patients with cystic fibrosis. Pediatr Res. 1980 Jul;14(7):863–868. doi: 10.1203/00006450-198007000-00004. [DOI] [PubMed] [Google Scholar]
- Downs R. W., Jr, Levine M. A., Drezner M. K., Burch W. M., Jr, Spiegel A. M. Deficient adenylate cyclase regulatory protein in renal membranes from a patient with pseudohypoparathyroidism. J Clin Invest. 1983 Feb;71(2):231–235. doi: 10.1172/JCI110763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELRICK H., ALBRIGHT F., BARTTER F. C., FORBES A. P., REEVES J. D. Further studies on pseudo-hypoparathyroidism: report of four new cases. Acta Endocrinol (Copenh) 1950;5(3):199–225. doi: 10.1530/acta.0.0050199. [DOI] [PubMed] [Google Scholar]
- Farfel Z., Bourne H. R. Deficient activity of receptor-cyclase coupling protein in platelets of patients with pseudohypoparathyroidism. J Clin Endocrinol Metab. 1980 Nov;51(5):1202–1204. doi: 10.1210/jcem-51-5-1202. [DOI] [PubMed] [Google Scholar]
- Farfel Z., Brickman A. S., Kaslow H. R., Brothers V. M., Bourne H. R. Defect of receptor-cyclase coupling protein in psudohypoparathyroidism. N Engl J Med. 1980 Jul 31;303(5):237–242. doi: 10.1056/NEJM198007313030501. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Haslam R. J., Goldstein S. Adenosine 3': 5'-cyclic monophosphate in young and senescent human fibroblasts during growth and stationary phase in vitro. Effects of prostaglandine E1 and of adrenaline. Biochem J. 1974 Nov;144(2):253–263. doi: 10.1042/bj1440253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. J., Ayad S. R. Trypsin modifies the activity of adenylate cyclase from normal, malignant and hybrid mammalian cells. Biochim Biophys Acta. 1980 Jun 19;630(2):202–209. doi: 10.1016/0304-4165(80)90422-5. [DOI] [PubMed] [Google Scholar]
- Kartner N., Alon N., Swift M., Buchwald M., Riordan J. R. Isolation of plasma membranes from human skin fibroblasts. J Membr Biol. 1977 Sep 14;36(2-3):191–211. doi: 10.1007/BF01868151. [DOI] [PubMed] [Google Scholar]
- Kelly L. A., Butcher R. W. The effects of epinephrine and prostaglandin E-1 on cyclic adenosine 3':5'-monophosphate levels in WI-38 fibroblasts. J Biol Chem. 1974 May 25;249(10):3098–3102. [PubMed] [Google Scholar]
- Kurz J. B., Perkins J. P. Cystic fibrosis fibroblasts respond normally to isoproterenol. Pediatr Res. 1981 Oct;15(10):1328–1333. doi: 10.1203/00006450-198110000-00004. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Downs R. W., Jr, Moses A. M., Breslau N. A., Marx S. J., Lasker R. D., Rizzoli R. E., Aurbach G. D., Spiegel A. M. Resistance to multiple hormones in patients with pseudohypoparathyroidism. Association with deficient activity of guanine nucleotide regulatory protein. Am J Med. 1983 Apr;74(4):545–556. doi: 10.1016/0002-9343(83)91008-2. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Downs R. W., Jr, Singer M., Marx S. J., Aurbach G. D., Spiegel A. M. Deficient activity of guanine nucleotide regulatory protein in erythrocytes from patients with pseudohypoparathyroidism. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1319–1324. doi: 10.1016/0006-291x(80)90563-x. [DOI] [PubMed] [Google Scholar]
- Manganiello V. C., Breslow J. Effects of prostaglandin E1 and isoproterenol on cyclic AMP content of human fibroblasts modified by time and cell density in subculture. Biochim Biophys Acta. 1974 Oct 8;362(3):509–520. doi: 10.1016/0304-4165(74)90146-9. [DOI] [PubMed] [Google Scholar]
- McSwigan J. D., Hanson D. R., Lubiniecki A., Heston L. L., Sheppard J. R. Down syndrome fibroblasts are hyperresponsive to beta-adrenergic stimulation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7670–7673. doi: 10.1073/pnas.78.12.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Hughes R. J., Brickman A. S., Farfel Z., Bourne H. R., Insel P. A. Platelets of pseudohypoparathyroid patients: evidence that distinct receptor-cyclase coupling proteins mediate stimulation and inhibition of adenylate cyclase. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4193–4197. doi: 10.1073/pnas.79.13.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T. B., Lad P. M., Preston M. S., Rodbell M. Characteristics of the guanine nucleotide regulatory component of adenylate cyclase in human erythrocyte membranes. Biochim Biophys Acta. 1980 Apr 17;629(1):143–155. doi: 10.1016/0304-4165(80)90273-1. [DOI] [PubMed] [Google Scholar]
- Nusynowitz M. L., Frame B., Kolb F. O. The spectrum of the hypoparathyroid states: A classification based on physiologic principles. Medicine (Baltimore) 1976 Mar;55(2):105–119. doi: 10.1097/00005792-197603000-00001. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Levine M. A., Aurbach G. D., Downs R. W., Jr, Marx S. J., Lasker R. D., Moses A. M., Breslau N. A. Deficiency of hormone receptor-adenylate cyclase coupling protein: basis for hormone resistance in pseudohypoparathyroidism. Am J Physiol. 1982 Jul;243(1):E37–E42. doi: 10.1152/ajpendo.1982.243.1.E37. [DOI] [PubMed] [Google Scholar]



