Abstract
The correlation functional holds significance in density functional theory as it addresses electron–electron interactions beyond the mean-field approximation, enhancing the accuracy of total energy calculations, electronic excitations, and the prediction of materials properties. There are several expressions to describe this energy, and each of them has a unique set of errors in calculating particular properties of materials. This work offers a new correlation functional by employing the density's dependence on ionization energy. We theoretically derived this functional and combined it with the previously reported ionization energy dependent exchange functional to investigate its effect on the total energy, bond energy, dipole moment, and zero-point energy of 62 molecules. The comparison of this new functional in respect to existing widely used correlation models including QMC, PBE, B3LYP and Chachiyo models shows how well it works in producing accurate results with minimal mean absolute error.
Subject terms: Density functional theory, Electronic properties and materials
Introduction
Density functional theory (DFT) is an effective tool in computational physics that allows for the prediction and analysis of numerous transport and thermal properties of solids1,2. The precision and reliability of DFT computations are greatly influenced by the choice of exchange–correlation functional. Correlation energy is a fundamental concept in quantum mechanics that accounts for the interactions between electrons in a many-electron system. It represents the additional energy required to describe the behavior of electrons beyond what can be explained by the mean-field approximation, such as the Hartree–Fock method3. It basically represents the effects of electron–electron correlation, which arises due to their mutual electrostatic repulsion. This correlation leads to complex quantum effects that cannot be represented by simple mathematical models. Various functionals have been developed over the years, each with its own unique advantages and disadvantages4,5. While some functionals may provide higher overall accuracy, others may be better suitable for specific kinds of systems or features. To accurately correct the positive value of Homo-energy and the orbital energy of anions, for instance, the tuned range-separated DFT approximation has been introduced by satisfying the DFT-Koopmans theory based on basis set alterations and quantum approximations6. The choice of functional depends on the particular problem being solved, the degree of required precision, and the computation time. Fitting the results of accurate Quantum Monte Carlo (QMC) computations of the uniform electron gas (UEG) has yielded multiple equations for the correlation energy in the local density approximation (LDA). These are quite complex expressions, usually denoted by their acronyms, like VWN, VWN5, and so forth. Nevertheless, when applied to molecular computation, they yield remarkably similar results. VWN functional is defined as follows7:
| 1 |
where and the parameters , b, and c are constants that depend on the context and the specific version of the VWN functional being used8. The error may be obtained at a relatively high level for the LDA correlation functional. An improved LDA leads to LSDA0 that is constructed to satisfy specific requirements, but remarkably agrees with favorable results for a homogeneous two-electron density in a restricted curved three-dimensional area. The correlation part of LSDA0 is expressed as follows:
| 2 |
where, is the relative spin polarization defined with the spin densities and , is the Seitz radius, b is constant and the function is 0 for z = ± 1 in order to satisfy the one electron self-correlation constraint and 1 for z = 09,10. The use of Generalized Gradient Approximation (GGA) can increase accuracy. Some of GGA correlations functional are LYP, PBE, and PW91. The PW91 correlation functional has achieved widespread acceptance because it covers short- and long-range electron correlation effects and accomplishes a comparatively low Mean Absolute Error (MAE) for the overall energy for a wide range of molecules11–13. The well-known PBE functional correlation part is given by10,14:
| 3 |
where , is the correlation energy density. The precise mathematical expression for is quite complex and can be found in the original publication by Pedrew, Burke, and Ernzerhof14. This functional's error is better than LDA but still higher than other functionals. The accuracy of the LYP functional combined with Becke's three-parameter exchange functional, or B3LYP, is also better than that of more basic functionals like LDA, especially for molecule systems and transition metals15,16.
Vydrov and Van Voorhis reported that the B97M-V functional was developed for main-group thermochemistry and non-covalent interactions. These families of functions were created by Truhlar and associates, and they exhibit improved accuracy for the total energy, particularly for non-covalent interactions17–19. For both organic and inorganic molecules, it has demonstrated remarkable performance for the total energy with low MAE values20–22. The mathematical formulation of the B97M-V functional is extremely complex and comprises several parameters that are determined through experimentation22.
A different correlation functional that was developed by Chachiyo et al.23 yields impressively accurate findings when combined with their exchange functional24–26:
| 4 |
where t () is the gradient parameter, n is the electron density, is the correlation energy density and h is a constant with a value of 0.06672632 Hartree23. Increasing t is intended to decrease the maximum rate of energy through the gradient suppressing factor .
In this paper, we report a new correlation functional that minimizes the mean absolute error (MAE), exceeding existing methods in terms of accuracy. The MAE is a measure of the average difference between computed and experimental values and is an essential measure of a functional's performance. Specifically, we perform a detailed analysis of over 64 different molecules and evaluate their key characteristics, including bond energies, total energy, dipole moments, and zero-point energy. We derive new correlation functional analytically; taking into account the functional's dependency on both the electron density and the ionization energy. By incorporating the ionization energy into the correlation functional, we improve the accuracy. This methodology is a complementary one to our earlier research, wherein we presented a novel exchange functional that is dependent on the ionization energy27. As a result, the ionization energy is included as a significant parameter in both the correlation and exchange functional in our current study, enabling a more comprehensive description of electronic interactions27.
Theoretical method
Equation (4) defines the generic form of the correlation functional23. Based on the second-order Moller–Plesset perturbation (MP2) theory, a straightforward analytical is defined in the local-density approximation as follows28:
| 5 |
where a and b are constants and is the Wigner–Seitz density parameter.
In this work, we used the ionization dependent density as29,30:
| 6 |
where I is the ionization energy and . We take A = 1 into consideration based on formula 29 in reference29. Next, we determine to be:
| 7 |
We obtain the following, using Eqs. (6) and (7):
| 8 |
So:
| 9 |
Remarkably, can be interpreted as an interpolation between the paramagnetic and ferromagnetic instances for spin polarization 31:
| 10 |
where .
Thus, we rewrite Eq. (5) as:
| 11 |
Which is clearly depends on ionization energy8. The Lambert function32 denoted as W, is related to density n. The correlation energy when the density tends to be uniform is represented by in Eq. (5) that is associated with density n28. We obtain the following by substituting Eqs. (9) and (11) in Eq. (4):
| 12 |
This is our new correlation energy in its final form. In order to get the exchange–correlation functional, as previously stated, this relationship was paired with the exchange functional that obtained in our prior study27, namely with:
| 13 |
where, , the parameter and .
We utilized Siam quantum software to do numerical calculations and examine the exchange–correlation functional results33,34. Reference34 was utilized while determining the values of I for atoms and molecules.
Results and discussion
In the first step, we computed the total energy for the elements in the second and third rows of the periodic table using our new exchange–correlation functional. The errors of estimated total energies are compared with the results of the PBE, Chachiyo model, our previous functional27, B3lyp, and QMC in Fig. 1. The exchange functionals in Fig. 1 of "our results in previous work" and the present research are the same, but their correlation functionals differ27.
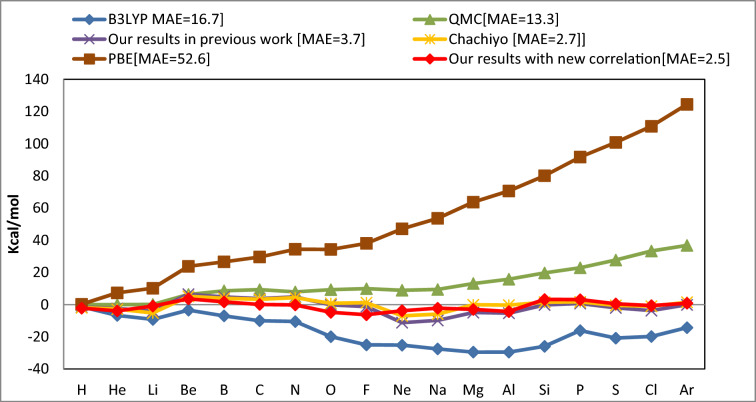
Figure 1.
The total energy mean absolute error (kcal/mol) of various DFT approaches compared to the experimental values for single atoms.
The MAE of new model is 2.5 kcal/mol, as shown in Fig. 1, which is less than the MAE of all QMC, PBE, B3LYP, and Chachiyo models. The MAE of the popular and relevant PBE model is 52.6 kcal/mol, greater than the MAE of all other functionals. The mean absolute error for molecules is plotted in Fig. 2.
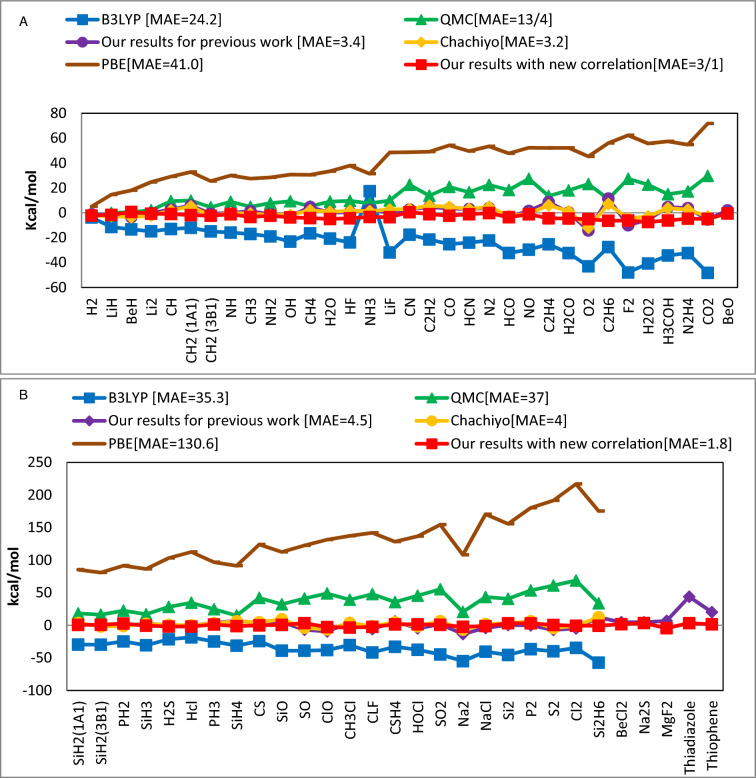
Figure 2.
The total energy mean absolute error (kcal/mol) of various DFT approaches compared to the experimental values for molecules that contain (A) atoms from the first and second rows of the periodic table and (B) atoms from the third row of the periodic table.
Figure 2 makes it evident that the new functional is more accurate for molecules composed of the third rows of the periodic table. This suggests that the incorporation of ionization energy into density results in a more effective correlation functional for these compounds. This outcome is consistent with earlier research35 which demonstrates that the correlation energy improves as the atomic number increases. This indicates that accurate and superior DFT findings are expected for the correlation functional for elements with large atomic numbers. For the molecules under investigation, our new exchange–correlation performs exceptionally well, yielding an absolute mean error of 2.5 kcal/mol—six times more precise than the currently available QMC results36. Taking into account ionization energy can modify the correlation energy by changing the electronic structure of the system and the energies of the occupied and unoccupied orbitals. Therefore, accurate prediction of ionization energies in systems with strong electron correlation (such as transition metals, alloys, or molecules with open-shell configurations) requires taking into account correlation effects beyond basic one-electron models, and vice versa. In Tables 1 and 2, we compare new correlation functional and references34,37 derived total energy values for single atoms and 62 molecules. It's important to note that MAE for the molecules containing the first and second rows is 3.1 kcal/mol whereas for the third row is just 1.8 kcal/mol.
Table 1.
The reference and new functional total energies of single atoms (versus Hartree).
| Atoms | Reference energy37 | New functional estimated energy |
|---|---|---|
| H | −0.5000 | −0.5035 |
| He | −2.9037 | −2.9099 |
| Li | −7.4781 | −7.4795 |
| Be | −14.6674 | −14.6615 |
| B | −24.6539 | −24.6511 |
| C | −37.8450 | −37.8448 |
| N | −54.5892 | −54.5895 |
| O | −75.0673 | −75.0751 |
| F | −99.7339 | −99.7440 |
| Ne | −128.9376 | −128.9437 |
| Na | −162.2546 | −162.2582 |
| Mg | −200.0530 | −200.0579 |
| Al | −242.3460 | −242.3529 |
| Si | −289.3590 | −289.3538 |
| P | −341.2590 | −341.2539 |
| S | −398.1100 | −398.1090 |
| Cl | −460.1480 | −460.1490 |
| Ar | −527.5400 | −527.5385 |
Table 2.
The reference and new functional estimated total energies of molecules (versus Hartree).
| Molecules | Reference energy36,37 | New functional estimated energy | Molecules | Reference energy34,37 | New functional estimated energy | Molecules | Reference energy36,37 | New functional estimated energy |
|---|---|---|---|---|---|---|---|---|
| Li2 | −14.9951 | −14.9962 | CH3Cl | −500.1240 | −500.1299 | SiH2(3B1) | −290.5690 | −290.5678 |
| CH | −38.4788 | −38.4805 | CLF | −559.9820 | −559.9860 | Cl2 | −920.3900 | −920.3909 |
| CH2 (1A1) | −39.1346 | −39.1377 | CSH4 | −438.7120 | −438.7100 | Si2H6 | −582.5670 | −582.5682 |
| CH2 (3B1) | −39.1484 | −39.1522 | HOCl | −535.9800 | −535.9769 | NaCl | −622.5610 | −622.5626 |
| NH | −55.2227 | −55.2246 | SO2 | −548.6590 | −548.6574 | Si2 | −578.8390 | −578.8335 |
| CH3 | −39.8355 | −39.8409 | Na2 | −324.5360 | −324.5397 | P2 | −682.7040 | −682.6992 |
| NH2 | −55.8794 | −55.8832 | LiF | −107.4340 | −107.4401 | S2 | −796.3840 | −796.3832 |
| OH | −75.7371 | −75.7430 | SiH2(1A1) | −290.6020 | −290.6006 | PH2 | −342.5040 | −342.4990 |
| CH4 | −40.5158 | −40.5226 | N2 | −109.5420 | −109.5427 | SiH3 | −291.2210 | −291.2225 |
| H2O | −76.4383 | −76.4467 | HCO | −113.8570 | −113.8626 | CN | −92.7250 | −92.7245 |
| HF | −100.4590 | −100.4663 | NO | −129.9050 | −129.9067 | C2H2 | −77.3355 | −77.3373 |
| H2 | −1.1745 | −1.1781 | C2H4 | −78.5888 | −78.5958 | NH3 | −56.5647 | −56.5702 |
| LiH | −8.0704 | −8.0732 | H2CO | −114.5090 | −114.5164 | CO | −113.3260 | −113.3300 |
| H2S | −399.4030 | −399.4056 | O2 | −150.3270 | −150.3348 | HCN | −93.4311 | −93.4330 |
| HCl | −460.8190 | −460.8220 | C2H6 | −79.8274 | −79.8377 | BeCl2 | −938.9860 | −934.9831 |
| PH3 | −343.1460 | −343.1442 | F2 | −199.5300 | −199.5403 | BeO | −89.8465 | −89.8474 |
| SiH4 | −291.8740 | −291.8765 | H2O2 | −151.5640 | −151.5753 | Na2S | −722.6966 | −722.6910 |
| CS | −436.2290 | −436.2292 | H3COH | −115.7310 | −115.7407 | MgF2 | −399.6619 | −399.6688 |
| SiO | −364.7340 | −364.7325 | N2H4 | −111.8780 | −111.8851 | Thiadiazole | −584.9234 | −584.9179 |
| SO | −473.3780 | −473.3730 | CO2 | −188.6010 | −188.6090 | Thiophene | −552.9535 | −552.9510 |
| ClO | −535.3200 | −535.3246 | BeH | −15.2468 | −15.2456 |
We also compared the total energy errors with our earlier research27, QMC, B3LYP, Chachiyo model, and PBE in Tables 3 and 4.
Table 3.
The comparison of the new functional with other models total energy errors for single atoms.
| Total energy error | Our previous functional27 | New functional | QMC | B3LYP | Chachiyo | PBE |
|---|---|---|---|---|---|---|
| MAE (for all single atoms) | 3.7 | 2.5 | 13.3 | 16.7 | 2.7 | 52.6 |
|
MAE (for first and second row single atoms) |
4.0 | 2.7 | 6.9 | 11.8 | 3.5 | 25.1 |
The unit of every energy is kcal/mol.
Table 4.
The comparison of new functional with other models total energy errors for examined 62 molecules.
| Total energy error | Our previous functional27 | New functional | QMC | B3LYP | Chachiyo | PBE |
|---|---|---|---|---|---|---|
| MAE (for all molecules) | 3.9 | 2.5 | 23.7 | 28.9 | 3.5 | 79.4 |
| MAE (for molecules made up from first and second row atoms) | 3.4 | 3.1 | 13.4 | 24.1 | 3.1 | 41 |
The unit of every energy is kcal/mol.
Comprehending bond energies enables scientists to forecast and interpret a range of chemical phenomena, including molecular interactions, reaction mechanism, and the stability of materials. Additionally, it can be ascertained through DFT computations, so we computed the MAE of bond energy generated by new functional and compared it with the results of QMC and our earlier research for 62 molecules in Fig. 3. Molecule bond energy is computed by us using24:
| 14 |
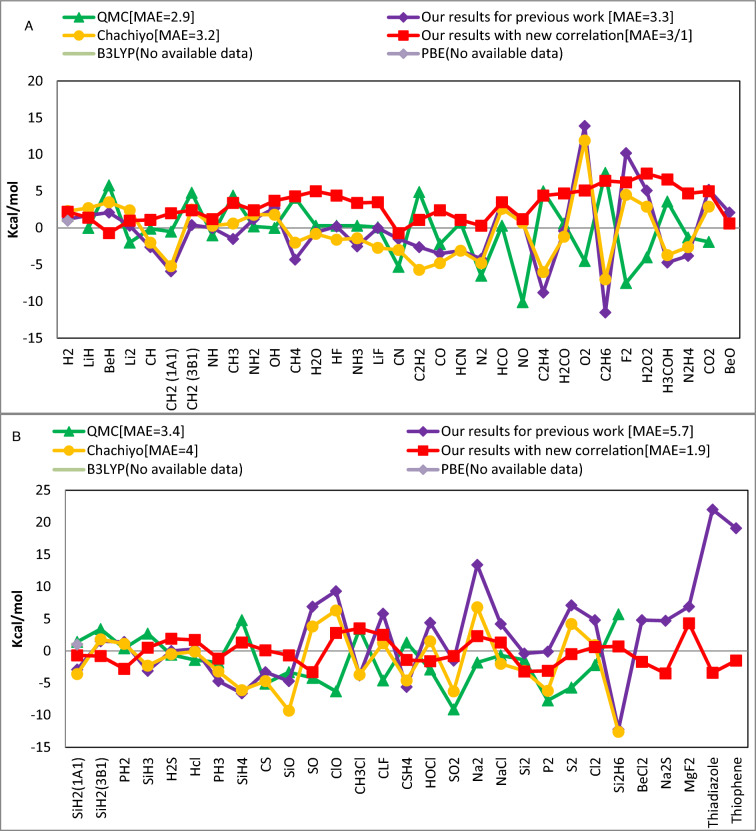
where the total energy of atoms and molecules are described by E(A) and E(M). Figure 3 illustrates that, for third row molecules, our new functional performs better than QMC's results36 , while QMC's results for first and second row molecules are marginally better than our functional for bond energy. Overall, for all 62 molecules, as Table 5 demonstrates, our functional performs better than the QMC and Chachiyo models.
Figure 3.
The bond energies mean absolute error (kcal/mol) obtained by various models compared to the experimental values for (A) molecules containing atoms of the first and second rows of the periodic table and (B) molecules containing atoms of the third row of the periodic table.
Table 5.
The comparison of the bond energy errors for new functional and other models.
| Bond energy error | Our previous functional | New functional | QMC | Chachiyo |
|---|---|---|---|---|
| MAE (for all molecules) | 4.4 | 2.5 | 3.1 | 3.5 |
| MAE (for molecules made up from first and second row atoms) | 3.3 | 3.1 | 2.9 | 3.1 |
The unit of every energy is kcal/mol.
The dipole moment is another quantity that can be evaluated using DFT and offers important details regarding the distribution of charge inside a system. The dipole moment is theoretically equivalent to , which is directly related to density. Predicting and interpreting a wide range of chemical and physical properties, including molecule polarity, solvation effects, molecular interactions and reactivity, and the electric field response of materials, requires an understanding of dipole moments. Figure 4 shows the precision of our functional for dipole moment calculation in comparison to the B3LYP and Chachiyo models. Table 6 summarizes the dipole moment MAE.
Figure 4.
The dipole moment mean absolute error (Debye) of various DFT approaches compared to the experimental values for molecules.
Table 6.
The comparison of the dipole moment errors for new functional and other models.
| Dipole moments | Our previous functional27 | new functional | B3LYP | Chachiyo |
|---|---|---|---|---|
| ME | −0.02 | −0.001 | −0.02 | −0.03 |
| MAE | 0.09 | 0.06 | 0.05 | 0.11 |
The unit of every dipole moments is Debye.
As demonstrated in Fig. 4 and Table 6, our new functional has a lower MAE than our earlier work, the Chachiyo model, and B3LYP.
Lastly, we have determined the zero-point energy of molecules, which takes into consideration the quantum mechanical vibrations of atoms and molecules and accounts for the lowest possible energy state of a system. To ensure accuracy in DFT calculations, zero-point energy is crucial for molecular vibrations, thermal corrections, stability, and chemical reactivity. Equation (15) can be used to evaluate it as follows38:
| 15 |
where h is the Planck constant and i represents the frequency of a particular molecule. We plotted the zero-point energy error for each of the four models indicated in Fig. 5, demonstrating that our new functional is more accurate than the others. The MAE of zero-point energy for the models mentioned is summarized in Table 7.
Figure 5.
The zero-point energy mean absolute error (kcal/mol) obtained by various DFT approaches compared to the experimental values.
Table 7.
The comparison of the zero-point energy errors for new functional and other models.
| Zero point energy | Our previous functional27 | New functional | B3LYP | Chachiyo |
|---|---|---|---|---|
| ME | 0.11 | 0.02 | −0.07 | −0.003 |
| MAE | 0.11 | 0.11 | 0.16 | 0.12 |
The unit of every energy is kcal/mol.
Table 8 provides a comparison between our new functional results and the reference values of zero-point energies and dipole moments of 62 molecules34,39–41. This article computes total energy, bond energy, and dipole moments using the QZP-gh basis set. We have also computed zero point energy using the 631-g* basis set. It is important to note that the QZP-gh basis set was selected since its error was less than that of other base sets (TZP, pcseg).
Table 8.
The references and new functional estimated dipole moments and zero-point energies of molecules34,39–41.
| Molecules | Ezp (kcal/mol) | Dipole moment (Debye) | ||||
|---|---|---|---|---|---|---|
| Reference Ezp | New functional estimated Ezp | Errors | Experimental | Our calc (Debye) | Errors | |
| H2 | 6.2 | 6.4 | 0.2 | 0.0 | 0.0 | 0.0 |
| LiH | 2.0 | 2.1 | 0.1 | 5.87 | 5.67 | −0.2 |
| BeH | 2.9 | 2.9 | 0.0 | 0.25 | 0.28 | 0.03 |
| Li2 | 0.5 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| CH | 4.0 | 4.1 | 0.1 | 1.45 | 1.39 | −0.06 |
| NH | 4.6 | 4.7 | 0.1 | 1.39 | 1.46 | 0.07 |
| NH2 | 11.5 | 12.0 | 0.5 | 1.76 | 1.72 | −0.04 |
| OH | 5.3 | 5.3 | 0.0 | 1.65 | 1.60 | −0.05 |
| CH4 | 27.1 | 27.4 | 0.3 | 0.0 | 0.0 | 0.0 |
| H2O | 12.9 | 13.1 | 0.2 | 1.85 | 1.81 | −0.04 |
| HF | 5.9 | 5.9 | 0.0 | 1.82 | 1.75 | −0.07 |
| NH3 | 20.6 | 21.2 | 0.6 | 1.47 | 1.53 | 0.06 |
| LiF | 1.3 | 1.4 | 0.1 | 6.28 | 6.15 | −0.13 |
| CN | 2.9 | 3.0 | 0.1 | 1.14 | 1.23 | 0.09 |
| C2H2 | 15.3 | 15.2 | −0.1 | 0.0 | 0.0 | 0.0 |
| CO | 3.1 | 3.0 | −0.1 | 0.11 | 0.15 | 0.04 |
| HCN | 8.7 | 9.0 | 0.3 | 2.98 | 2.90 | −0.08 |
| N2 | 3.4 | 3.3 | −0.1 | 0.0 | 0.0 | 0.0 |
| HCO | 7.8 | 7.7 | −0.1 | 1.39 | 1.39 | 0.0 |
| NO | 2.7 | 2.8 | 0.1 | 0.16 | 0.17 | 0.01 |
| H2CO | 16.1 | 16.4 | 0.3 | 2.32 | 2.22 | −0.1 |
| O2 | 2.3 | 2.2 | −0.1 | 0.0 | 0.0 | 0.0 |
| F2 | 1.3 | 1.4 | 0.1 | 0.0 | 0.0 | 0.0 |
| H2O2 | – | 16.0 | – | 1.57 | 1.66 | 0.09 |
| CH4O | – | 31.5 | – | 1.69 | 1.61 | -0.08 |
| N2H4 | – | 32.1 | – | 1.75 | 1.90 | 0.15 |
| CO2 | 6.2 | 6.1 | -0.1 | 0.0 | 0.0 | 0.0 |
| SiH2 | 7.2 | 7.3 | 0.1 | 0.26 | 0.27 | 0.01 |
| H2S | 9.2 | 9.4 | 0.2 | 0.98 | 1.01 | 0.03 |
| HCl | 4.2 | 4.3 | 0.1 | 1.11 | 1.15 | 0.04 |
| PH3 | 14.6 | 14.8 | 0.2 | 0.57 | 0.65 | 0.08 |
| SiH4 | 19.2 | 19.0 | −0.2 | 0.0 | 0.0 | 0.0 |
| CS | 1.8 | 1.8 | 0.0 | 1.95 | 1.97 | 0.02 |
| SiO | 3.5 | 3.6 | 0.1 | 3.09 | 2.94 | −0.15 |
| SO | 1.6 | 1.7 | 0.1 | 1.54 | 1.48 | −0.06 |
| ClO | 1.2 | 1.2 | 0.0 | 1.3 | 1.38 | 0.08 |
| CH3Cl | – | 23.4 | – | 1.88 | 1.95 | 0.07 |
| ClF | 1.1 | 1.1 | 0.0 | 0.88 | 0.94 | 0.06 |
| CSH4 | - | 28.3 | – | 1.66 | 1.7 | 0.04 |
| HOCl | 8.0 | 8.2 | 0.2 | 1.40 | 1.49 | 0.09 |
| SO2 | 4.3 | 4.5 | 0.2 | 1.62 | 1.55 | −0.07 |
| Na2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| NaCl | 0.5 | 0.5 | 0.0 | 8.99 | 8.74 | −0.25 |
| Si2 | 0.7 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| P2 | 1.1 | 1.2 | −0.1 | 0.0 | 0.0 | 0.0 |
| S2 | 1.0 | 1.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| Cl2 | 0.8 | 0.7 | −0.1 | 0.8 | 0.0 | 0.0 |
| BeCl2 | – | 3.2 | – | 0.0 | 0.0 | 0.0 |
| BeO | 2.0 | 1.9 | −0.1 | – | 6.04 | – |
| Na2S | – | 0.5 | – | 0.0 | 0.0 | 0.0 |
| MgF2 | 2.4 | 2.2 | −0.2 | 0.0 | 0.0 | 0.0 |
| Thiadiazole | 27.4 | 27.1 | −0.3 | 1.57 | 1.87 | 0.3 |
| Thiophene | 43.6 | 43.0 | −0.6 | 0.55 | 0.51 | −0.04 |
| MAE | 0.11 | MAE | 0.06 | |||
| ME | 0.02 | ME | −0.001 | |||
The initial circumstances, crystal structure, and atom count of the system all affect how much time elapses for each run. We compared the mean computation times for the old and new functions in the accompanying table. As the Table 9 illustrates, the average execution time for the new correlation was 54% longer than the old functional, despite Siam-Quantum software's extremely quick calculations. This demonstrates that while the total energy is predicted more precisely by the improved correlation functional, the computation time for it also increases.
Table 9.
The percentage of modifications between the old and new functional calculations.
| The quantity of atoms that each molecule is composed of | The percentage of modifications |
|---|---|
| 1 | 33 |
| 2 | 70 |
| 3 | 33 |
| 4 | 50 |
| 5 | 45 |
| 6 | 35 |
| 7 | 64 |
| 8 | 78 |
| 9 | 66 |
| 14 | 71 |
Conclusion
We proposed an accurate and straightforward correlation functional that could be utilized in DFT. After conducting detailed computations using 62 molecules and single atoms from the first, second, and third rows of the periodic table, new correlation functional demonstrates remarkable precision in estimating bond energies, total energy, dipole moments, and zero-point energies. The examined molecules' mean absolute error in total energy calculations was 2.5 kcal/mol, which outperformed the results of the Quantum Monte Carlo (QMC) and B3LYP methods. Interestingly, new functional for third-row atoms is more accurate than for first- and second-row atoms, indicating the important role of correlation energy in heavier elements. Moreover, new functional predicts single atom characteristics better than QMC and B3LYP.
Author contributions
All authors participated on the introducing new correlation functional. All authors wrote and reviewed the manuscript. Asghar Esmaeili supervised the work. Esmaeil Rahmatpour prepared Figures and Tables.
Data availability
All data generated or analyzed during this study are included in this article. If required, any data are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev.140(4A), A1133–A1138 (1965). 10.1103/PhysRev.140.A1133 [DOI] [Google Scholar]
- 2.Calais, J.-L. Density-functional theory of atoms and molecules. R.G. Parr and W. Yang, Oxford University Press, New York, Oxford, 1989. IX + 333 pp. Price £45.00. Int. J. Quantum Chem. 47(1), 101 (1993).
- 3.Engel, T. Quantum Chemistry and Spectroscopy. 3rd ed. (Pearson, 2012).
- 4.Friedman, P.W.A.R.S. Molecular Quantum Mechanics (Oxford University, 2010).
- 5.Szabó, A. & Ostlund, N.S. Modern Quantum Chemistry : Introduction to Advanced Electronic Structure Theory. (Dover Publications, 1996).
- 6.Anderson, L. N., Oviedo, M. B. & Wong, B. M. Accurate electron affinities and orbital energies of anions from a nonempirically tuned range-separated density functional theory approach. J. Chem. Theory Comput.13(4), 1656–1666 (2017). 10.1021/acs.jctc.6b01249 [DOI] [PubMed] [Google Scholar]
- 7.Vosko, S. H., Wilk, L. & Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys.58(8), 1200–1211 (1980). 10.1139/p80-159 [DOI] [Google Scholar]
- 8.Vosko, S. & Wilk, L. Influence of an improved local-spin-density correlation-energy functional on the cohesive energy of alkali metals. Phys. Rev. B22(8), 3812 (1980). 10.1103/PhysRevB.22.3812 [DOI] [Google Scholar]
- 9.Sun, J., Ruzsinszky, A. & Perdew, J. P. Strongly constrained and appropriately normed semilocal density functional. Phys. Rev. Lett.115(3), 036402 (2015). 10.1103/PhysRevLett.115.036402 [DOI] [PubMed] [Google Scholar]
- 10.Sun, J., Perdew, J. P., Yang, Z. & Peng, H. Communication: Near-locality of exchange and correlation density functionals for 1- and 2-electron systems. J. Chem. Phys.144(19), 3 (2016). 10.1063/1.4950845 [DOI] [PubMed] [Google Scholar]
- 11.Becke, A. D. Density-functional thermochemistry. II. The effect of the Perdew–Wang generalized-gradient correlation correction. J. Chem. Phys.97(12), 9173–9177 (1992). 10.1063/1.463343 [DOI] [Google Scholar]
- 12.Perdew, J. P. & Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B45(23), 13244 (1992). 10.1103/PhysRevB.45.13244 [DOI] [PubMed] [Google Scholar]
- 13.Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B46(11), 6671 (1992). 10.1103/PhysRevB.46.6671 [DOI] [PubMed] [Google Scholar]
- 14.Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett.77(18), 3865–3868 (1996). 10.1103/PhysRevLett.77.3865 [DOI] [PubMed] [Google Scholar]
- 15.Slater, J.C. & Phillips, J.C. Quantum theory of molecules and solids. Vol. 4. In The Self‐Consistent Field for Molecules and Solids. Physics Today. Vol. 27(12). 49–50 (1974).
- 16.Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A38(6), 3098 (1988). 10.1103/PhysRevA.38.3098 [DOI] [PubMed] [Google Scholar]
- 17.Jacquemin, D. et al. On the performances of the M06 family of density functionals for electronic excitation energies. J. Chem. Theory Comput.6(7), 2071–2085 (2010). 10.1021/ct100119e [DOI] [PubMed] [Google Scholar]
- 18.Zhao, Y. & Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res.41(2), 157–167 (2008). 10.1021/ar700111a [DOI] [PubMed] [Google Scholar]
- 19.Zhao, Y. & Truhlar, D. G. Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions. J. Chem. Theory Comput.4(11), 1849–1868 (2008). 10.1021/ct800246v [DOI] [PubMed] [Google Scholar]
- 20.Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc.120(1), 215–241 (2008). 10.1007/s00214-007-0310-x [DOI] [Google Scholar]
- 21.Goerigk, L. & Grimme, S. A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys. Chem. Chem. Phys. PCCP13(14), 6670–6688 (2011). 10.1039/c0cp02984j [DOI] [PubMed] [Google Scholar]
- 22.Vydrov, O. A. & Van Voorhis, T. Nonlocal van der Waals density functional: The simpler the better. J. Chem. Phys.133(24), 244103 (2010). 10.1063/1.3521275 [DOI] [PubMed] [Google Scholar]
- 23.Chachiyo, T. & Chachiyo, H. Understanding electron correlation energy through density functional theory. Comput. Theor. Chem.1172, 112669 (2020). 10.1016/j.comptc.2019.112669 [DOI] [Google Scholar]
- 24.Chachiyo, T. & Chachiyo, H. Simple and accurate exchange energy for density functional theory. Molecules.25(15), 3485 (2020). 10.3390/molecules25153485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Quest for Approximate Exchange-Correlation Functionals. A Chemist's Guide to Density Functional Theory. 65–91 (2001).
- 26.Martin, R. M. Electronic Structure: Basic Theory and Practical Methods (Cambridge University Press, 2004). [Google Scholar]
- 27.Rahmatpour, E. & Esmaeili, A. Introducing a new exchange functional by altering the electron density’s ionization dependency in density functional theory. Sci. Rep.14(1), 3226 (2024). 10.1038/s41598-024-53341-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chachiyo, T. Communication: Simple and accurate uniform electron gas correlation energy for the full range of densities. J. Chem. Phys.145(2), 4 (2016). 10.1063/1.4958669 [DOI] [PubMed] [Google Scholar]
- 29.Katriel, J. & Davidson, E. R. Asymptotic behavior of atomic and molecular wave functions. Proc. Natl. Acad. Sci.77(8), 4403–4406 (1980). 10.1073/pnas.77.8.4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Politzer, P., Parr, R. G. & Murphy, D. R. Approximate determination of Wigner–Seitz radii from free-atom wave functions. Phys. Rev. B31(10), 6809–6810 (1985). 10.1103/PhysRevB.31.6809 [DOI] [PubMed] [Google Scholar]
- 31.Wang, Y. & Perdew, J. P. Spin scaling of the electron-gas correlation energy in the high-density limit. Phys. Rev. B43(11), 8911–8916 (1991). 10.1103/PhysRevB.43.8911 [DOI] [PubMed] [Google Scholar]
- 32.Veberič, D. Lambert W function for applications in physics. Comput. Phys. Commun.183(12), 2622–2628 (2012). 10.1016/j.cpc.2012.07.008 [DOI] [Google Scholar]
- 33.Chachiyo, T. A compact open-source quantum simulation software for molecules. SIAM Quantum (2020).
- 34.III RDJ. NIST Computational Chemistry Comparison and Benchmark Database. http://cccbdb.nist.gov/ (2022).
- 35.Ma, A., Drummond, N. D., Towler, M. D. & Needs, R. J. All-electron quantum Monte Carlo calculations for the noble gas atoms He to Xe. Phys. Rev. E71(6), 066704 (2005). 10.1103/PhysRevE.71.066704 [DOI] [PubMed] [Google Scholar]
- 36.Nemec, N., Towler, M. D. & Needs, R. J. Benchmark all-electron ab initio quantum Monte Carlo calculations for small molecules. J. Chem. Phys.132(3), 8 (2010). 10.1063/1.3288054 [DOI] [PubMed] [Google Scholar]
- 37.O’eill, D. P. & Gill, P. M. W. Benchmark correlation energies for small molecules. Mol. Phys.103(6–8), 763–766 (2005). 10.1080/00268970512331339323 [DOI] [Google Scholar]
- 38.Rahal, M., Hilali, M., El Hammadi, A., El Mouhtadi, M. & El Hajbi, A. Calculation of vibrational zero-point energy. J. Mol. Struct. Theochem.572(1), 73–80 (2001). 10.1016/S0166-1280(01)00565-6 [DOI] [Google Scholar]
- 39.Dobyns, V. & Pierce, L. Microwave spectrum, structure, dipole moment, and quadrupole coupling constants of 1,2,5-thiadiazole. J. Am. Chem. Soc.85(22), 3553–3556 (1963). 10.1021/ja00905a005 [DOI] [Google Scholar]
- 40.McClellan, A.L. Tables of Experimental Dipole Moments. (Rahara Enterprises El Cerrito).
- 41.Adhikari, S. et al. The Fermi–Löwdin self-interaction correction for ionization energies of organic molecules. J. Chem. Phys.153(18), 4 (2020). 10.1063/5.0024776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. If required, any data are available from the corresponding author on reasonable request.