Abstract
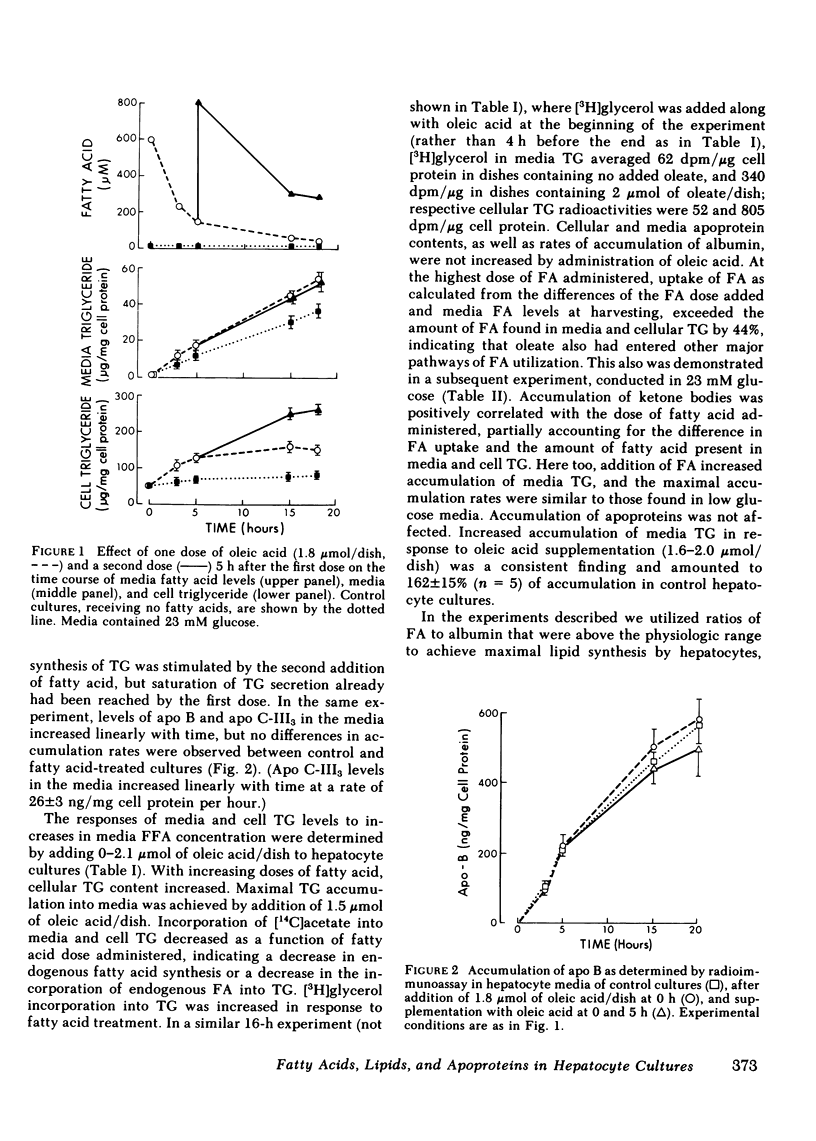
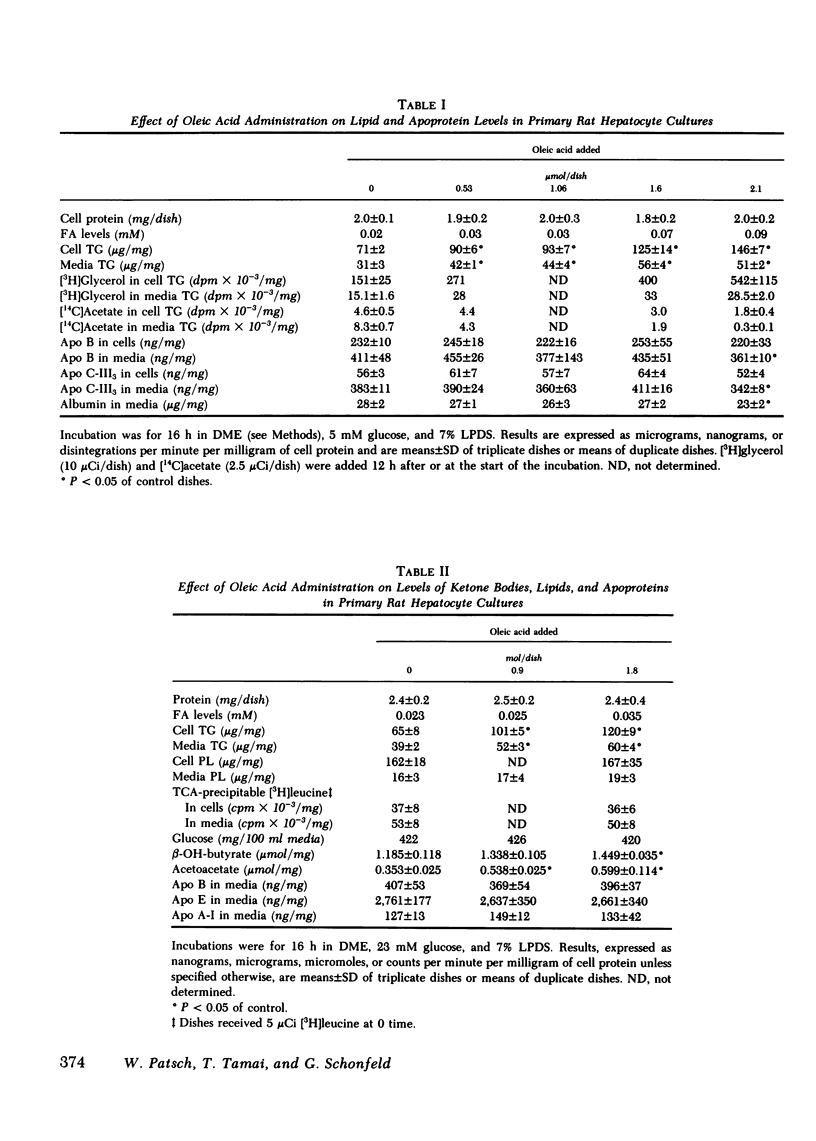
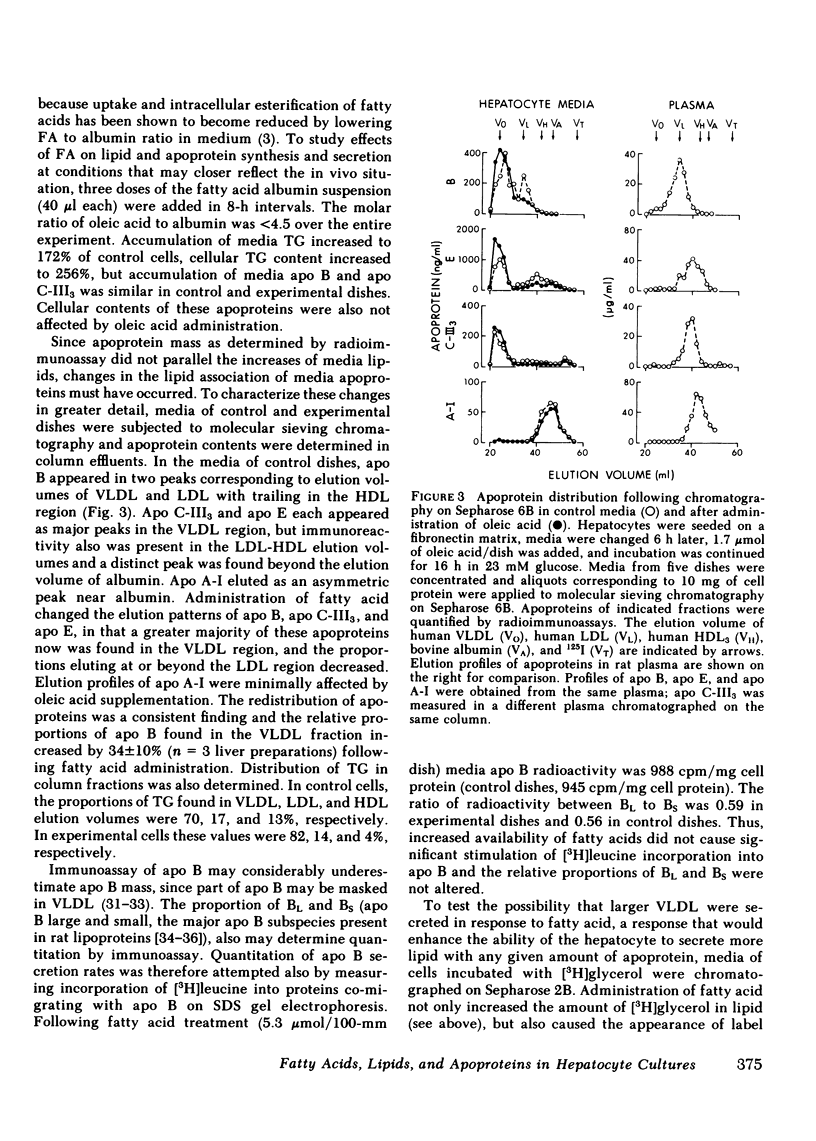
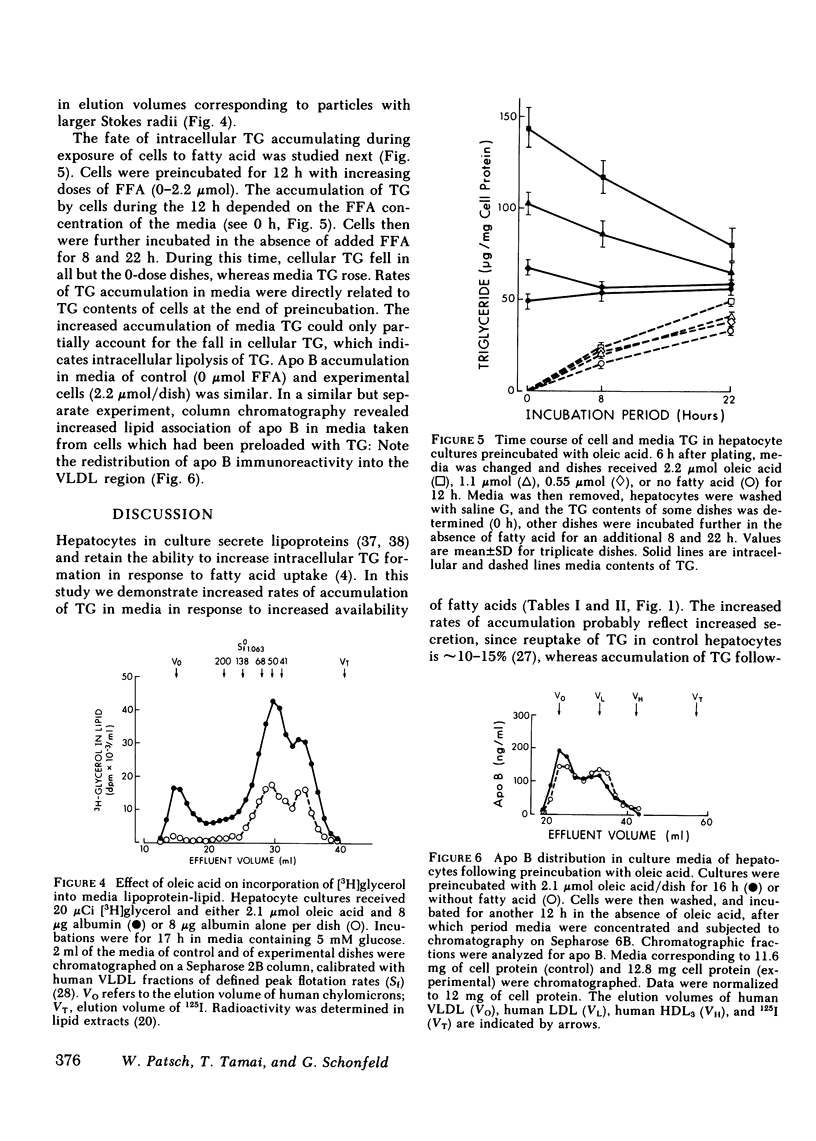
Increasing availability of free fatty acids (FFA) to liver results in enhanced rates of secretion of triglycerides in lipoproteins. However, as FFA uptake increases, triglyceride secretory rates reach a plateau and esterified fatty acids accumulate intracellularly, suggesting that something is limiting lipid transport out of the liver. One possibility could be the limited availability of apoproteins. To test this hypothesis, primary rat hepatocytes in culture were incubated with increasing amounts of FFA (0-2.1 mumol/dish) and the amounts of lipids and apoproteins inside the cells and in culture media were measured; the latter by specific radioimmunoassays. Media also were fractionated on Sepharose 2B and 6B columns and the elution profiles of apoproteins were obtained. With exposure to increasing amounts of free fatty acids, hepatocytes took up more fatty acids and intracellular levels of triglycerides rose (from 71 to 146 micrograms/mg cell protein). Concomitantly, media triglycerides nearly doubled (31 to 51 micrograms/mg). Incorporation of [3H]glyceride into cellular and media triglyceride also rose. However, levels of apoproteins A-I, B, C-III3, and E in cells and media were unchanged. The increasing amounts of triglycerides in media were present in larger particles, as demonstrated on gel permeation chromatography. The elution profiles of apoproteins B, C-III3, and E were altered in that a greater proportion of the apoproteins eluted with larger particles. Similar results were obtained when hepatocytes were preloaded with increasing amounts of FFA over 12 h and analyses of cells and media were carried out 8 and 22 h after removal of fatty acids from the media. During loading of cells, accumulation of cellular triglycerides was directly related to media FFA concentrations. During unloading, triglyceride secretory rates were related to cellular triglyceride levels. At higher triglyceride secretory rates larger particles were secreted and a greater proportion of apoproteins was associated with the larger particles, but total amounts of apoproteins in the system did not change. These data lead us to suggest that enhanced rates of apoprotein synthesis need not occur in the response to acute changes in hepatic lipid transport, rather, increased secretion of lipid is brought about by augmented intracellular lipid apoprotein association.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R. Intrahepatic assembly of very low density lipoproteins. Effect of fatty acids on triacylglycerol and apolipoprotein synthesis. J Biol Chem. 1982 Sep 25;257(18):10908–10913. [PubMed] [Google Scholar]
- Davis R. A., Engelhorn S. C., Pangburn S. H., Weinstein D. B., Steinberg D. Very low density lipoprotein synthesis and secretion by cultured rat hepatocytes. J Biol Chem. 1979 Mar 25;254(6):2010–2016. [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovson J., Huang Y. O., Baker N., Kannan R. Apolipoprotein B is structurally and metabolically heterogeneous in the rat. Proc Natl Acad Sci U S A. 1981 Jan;78(1):157–161. doi: 10.1073/pnas.78.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr Transport of fatty acids. Physiol Rev. 1958 Oct;38(4):585–630. doi: 10.1152/physrev.1958.38.4.585. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg K. M., Pindyck J., Mosesson M. W., Grieninger G. Thyroid hormone stimulation of plasma protein synthesis in cultured hepatocytes. J Biol Chem. 1981 Jan 25;256(2):563–566. [PubMed] [Google Scholar]
- Kohout M., Kohoutova B., Heimberg M. The regulation of hepatic triglyceride metabolism by free fatty acids. J Biol Chem. 1971 Aug 25;246(16):5067–5074. [PubMed] [Google Scholar]
- Krishnaiah K. V., Walker L. F., Borensztajn J., Schonfeld G., Getz G. S. Apolipoprotein B variant derived from rat intestine. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3806–3810. doi: 10.1073/pnas.77.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Guest M. J., Foster D. W. Ketone body metabolism in the ketosis of starvation and alloxan diabetes. J Biol Chem. 1970 Sep 10;245(17):4382–4390. [PubMed] [Google Scholar]
- Mooney R. A., Lane M. D. Formation and turnover of triglyceride-rich vesicles in the chick liver cell. Effects of cAMP and carnitine on triglyceride mobilization and conversion to ketones. J Biol Chem. 1981 Nov 25;256(22):11724–11733. [PubMed] [Google Scholar]
- Ontko J. A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J Biol Chem. 1972 Mar 25;247(6):1788–1800. [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Patsch J. R., Kostner G. M., Sailer S., Braunsteiner H. Isolation of subfractions of human very low density lipoproteins by zonal ultracentrifugation. J Biol Chem. 1978 Jul 25;253(14):4911–4915. [PubMed] [Google Scholar]
- Patsch W., Schonfeld G. The degree of sialylation of ApoC-III is altered by diet. Diabetes. 1981 Jun;30(6):530–534. doi: 10.2337/diab.30.6.530. [DOI] [PubMed] [Google Scholar]
- ROSE H., VAUGHAN M., STEINBERG D. UTILIZATION OF FATTY ACIDS BY RAT LIVER SLICES AS A FUNCTION OF MEDIUM CONCENTRATION. Am J Physiol. 1964 Feb;206:345–350. doi: 10.1152/ajplegacy.1964.206.2.345. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Frick M. S., Bailey A. P. Measurement of apolipoprotein A-I in rat high density lipoprotein and in rat plasma by radioimmunoassay. J Lipid Res. 1976 Jan;17(1):25–29. [PubMed] [Google Scholar]
- Schonfeld G., Grimme N., Alpers D. Detection of apolipoprotein C in human and rat enterocytes. J Cell Biol. 1980 Aug;86(2):562–567. doi: 10.1083/jcb.86.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld G., Patsch W., Pfleger B., Witztum J. L., Weidman S. W. Lipolysis produces changes in the immunoreactivity and cell reactivity of very low density lipoproteins. J Clin Invest. 1979 Nov;64(5):1288–1297. doi: 10.1172/JCI109584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld G., Pfleger B. Utilization of exogenous free fatty acids for the production of very low density lipoprotein triglyceride by livers of carbohydrate-fed rats. J Lipid Res. 1971 Sep;12(5):614–621. [PubMed] [Google Scholar]
- Siuta-Mangano P., Janero D. R., Lane M. D. Association and assembly of triglyceride and phospholipid with glycosylated and unglycosylated apoproteins of very low density lipoprotein in the intact liver cell. J Biol Chem. 1982 Oct 10;257(19):11463–11467. [PubMed] [Google Scholar]
- Stajner A., Sůva J. The determination of nonesterified fatty acids in blood serum using a stable cupric reagent. J Clin Chem Clin Biochem. 1977 Sep;15(9):513–514. doi: 10.1515/cclm.1977.15.1-12.513. [DOI] [PubMed] [Google Scholar]
- Tarlow D. M., Watkins P. A., Reed R. E., Miller R. S., Zwergel E. E., Lane M. D. Lipogenesis and the synthesis and secretion of very low density lipoprotein by avian liver cells in nonproliferating monolayer culture. Hormonal effects. J Cell Biol. 1977 May;73(2):332–353. doi: 10.1083/jcb.73.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Schonfeld G. Carbohydrate diet-induced changes in very low density lipoprotein composition and structure. Diabetes. 1978 Dec;27(12):1215–1229. doi: 10.2337/diab.27.12.1215. [DOI] [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Variant forms of plasma apolipoprotein B. Hepatic and intestinal biosynthesis and heterogeneous metabolism in the rat. J Biol Chem. 1981 Apr 25;256(8):3615–3618. [PubMed] [Google Scholar]
- Yedgar S., Weinstein D. B., Patsch W., Schonfeld G., Casanada F. E., Steinberg D. Viscosity of culture medium as a regulator of synthesis and secretion of very low density lipoproteins by cultured hepatocytes. J Biol Chem. 1982 Mar 10;257(5):2188–2192. [PubMed] [Google Scholar]



