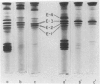
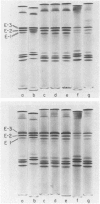
Abstract
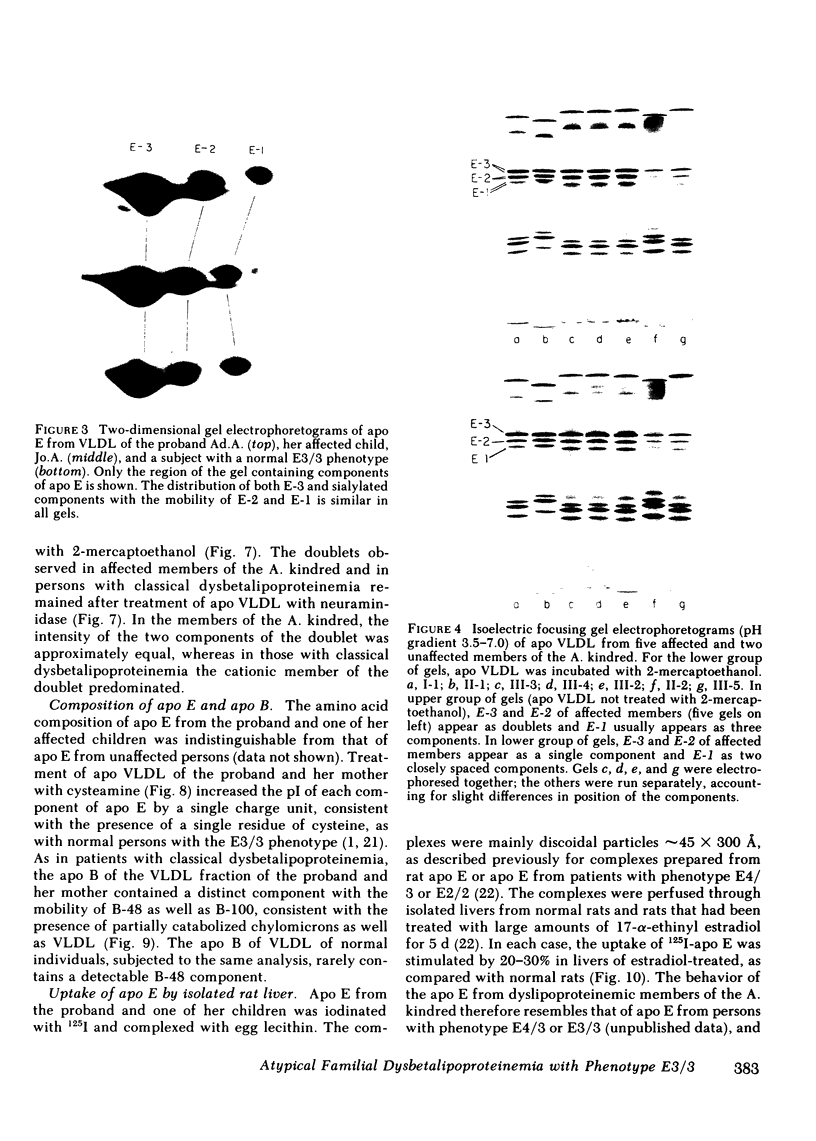
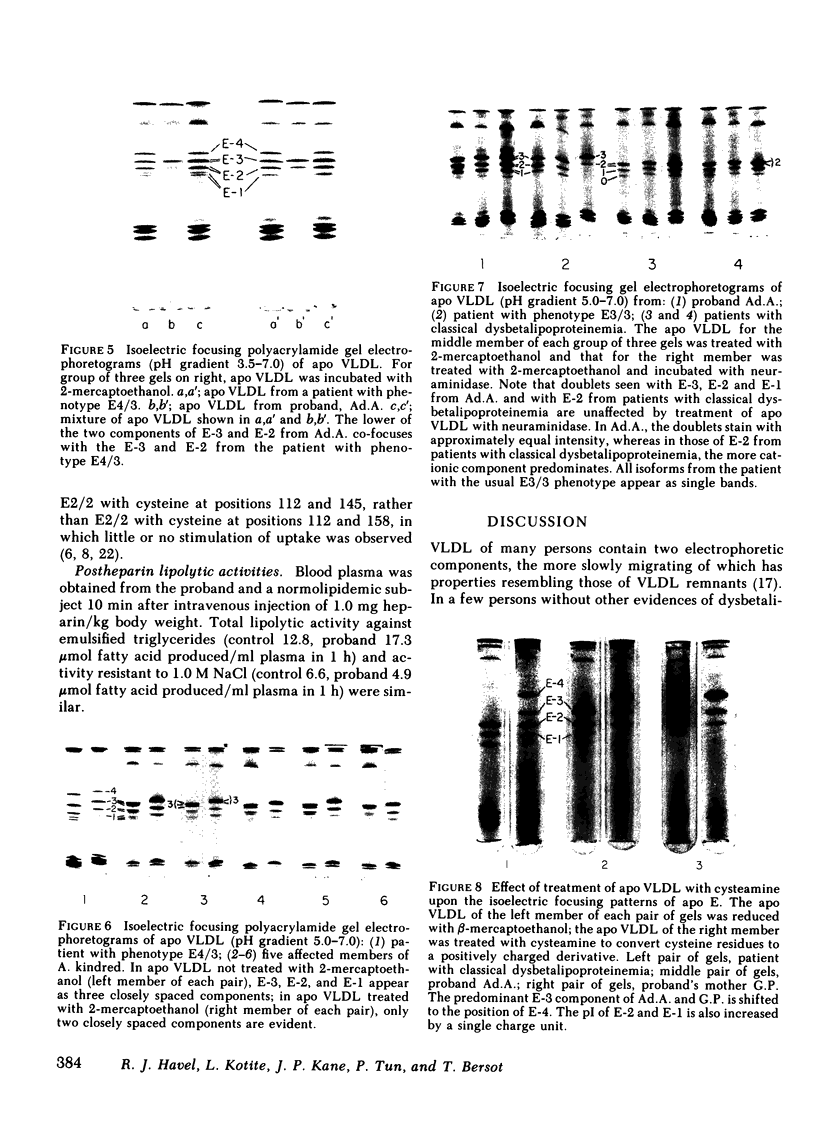
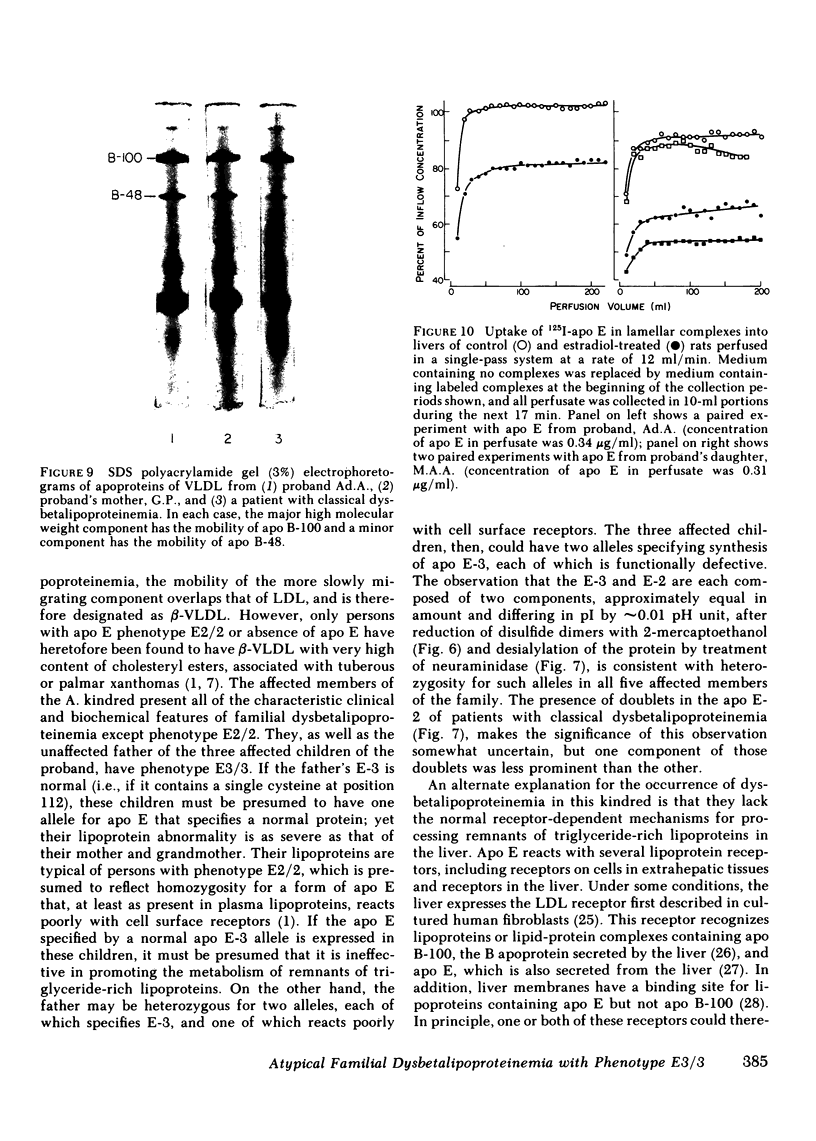
Familial dysbetalipoproteinemia has been reported to be associated uniquely with an apolipoprotein E phenotype (E2/2) that occurs in approximately 1% of all persons. We have observed the typical clinical and biochemical characteristics of this disorder in five members of a family, in all of whom the apolipoprotein E phenotype, as determined by isoelectric focusing electrophoresis, is E3/3. The disorder is present in three generations of the family: the proband, her mother, and three of the proband's five children. The proband's husband, father of all five children, also has apolipoprotein E phenotype E3/3, as do his two unaffected children. As in normal persons with phenotype E3/3, the apolipoprotein E of affected members appears to have a single residue of cysteine. When incorporated with egg lecithin into discoidal complexes, the apolipoprotein E from affected members was taken up normally into perfused livers of estradiol-treated rats, in which a high level of LDL receptors is expressed. When isoelectric focusing electrophoresis was carried out over a narrow range of pH (5-7), each of the apolipoprotein E isoforms of affected members was observed as a doublet, even after reduction of dimers of the protein with 2-mercaptoethanol and treatment with neuraminidase to minimize the content of sialylated forms of the protein. Doublets were also observed in the apolipoprotein E-2 of patients with classical dysbetalipoproteinemia, but only in the affected members of the family with atypical dysbetalipoproteinemia were the components of the doublets equally prominent. As in classical dysbetalipoproteinemia, both apolipoprotein B-100 and B-48 were present in the very low density lipoprotein fraction of plasma obtained in the postabsorptive state, indicating that remnantlike lipoproteins of both hepatic and intestinal origin accumulate. This observation, together with available evidence on the structural and functional heterogeneity of human apolipoprotein E, lead us to suggest that the disorder in this family is caused by one or two structurally abnormal forms of apolipoprotein E that contain a single residue of cysteine.
Full text
PDF

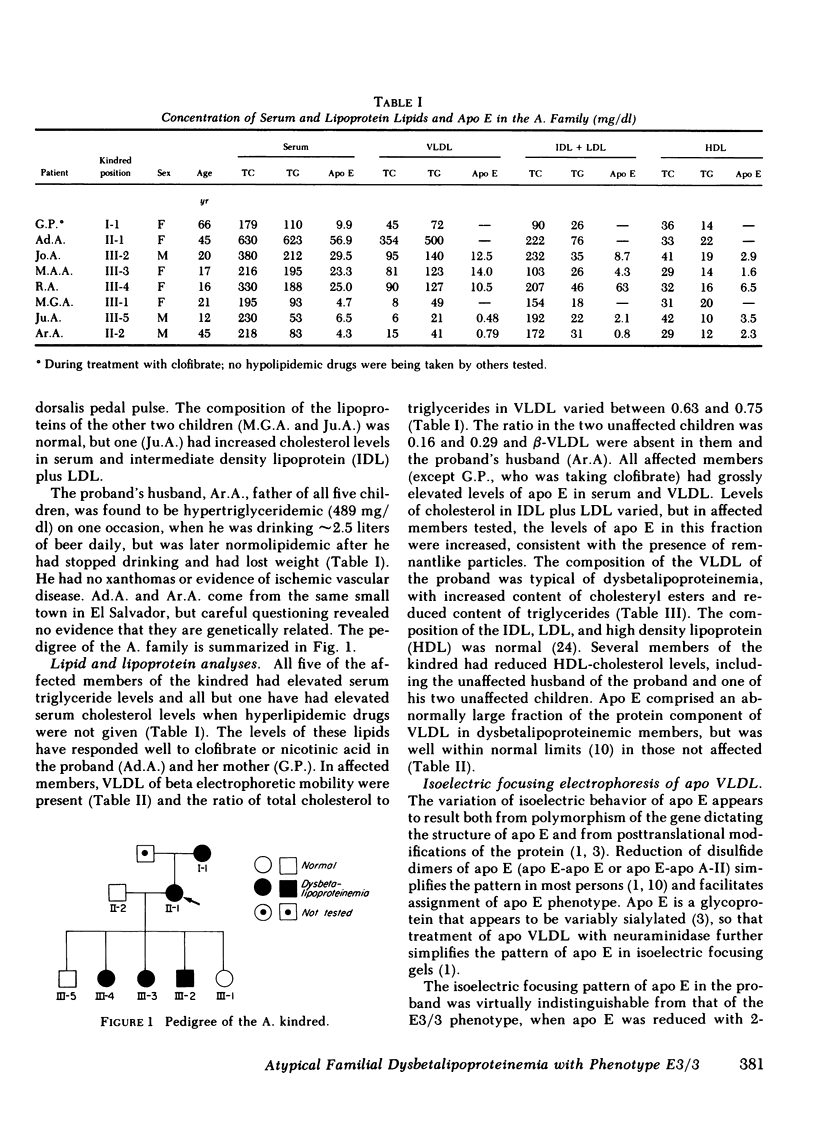
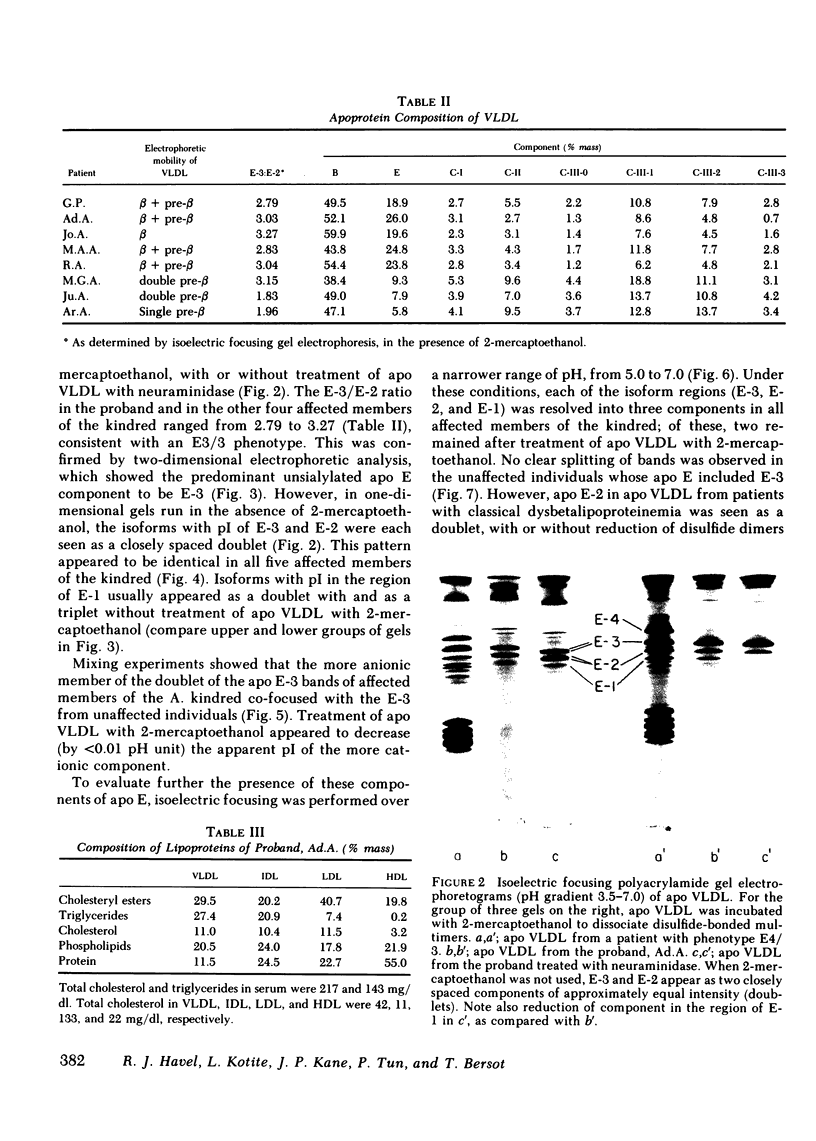


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Fainaru M., Havel R. J., Imaizumi K. Radioimmunoassay of arginine-rich apolipoprotein of rat serum. Biochim Biophys Acta. 1977 Jan 25;490(1):144–155. doi: 10.1016/0005-2795(77)90114-3. [DOI] [PubMed] [Google Scholar]
- Fainaru M., Mahley R. W., Hamilton R. L., Innerarity T. L. Structural and metabolic heterogeneity of beta-very low density lipoproteins from cholesterol-fed dogs and from humans with type III hyperlipoproteinemia. J Lipid Res. 1982 Jul;23(5):702–714. [PubMed] [Google Scholar]
- Frost P. H., Shore V. G., Havel R. J. Purification of canine post-heparin hepatic lipase. Biochim Biophys Acta. 1982 Jul 20;712(1):71–78. doi: 10.1016/0005-2760(82)90086-8. [DOI] [PubMed] [Google Scholar]
- Ghiselli G., Schaefer E. J., Gascon P., Breser H. B., Jr Type III hyperlipoproteinemia associated with apolipoprotein E deficiency. Science. 1981 Dec 11;214(4526):1239–1241. doi: 10.1126/science.6795720. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Chao Y., Windler E. E., Kotite L., Guo L. S. Isoprotein specificity in the hepatic uptake of apolipoprotein E and the pathogenesis of familial dysbetalipoproteinemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4349–4353. doi: 10.1073/pnas.77.7.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J. Familial dysbetalipoproteinemia. New aspects of pathogenesis and diagnosis. Med Clin North Am. 1982 Mar;66(2):441–454. doi: 10.1016/s0025-7125(16)31429-8. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kita T., Kotite L., Kane J. P., Hamilton R. L., Goldstein J. L., Brown M. S. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis. 1982 Nov-Dec;2(6):467–474. doi: 10.1161/01.atv.2.6.467. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kotite L., Vigne J. L., Kane J. P., Tun P., Phillips N., Chen G. C. Radioimmunoassay of human arginine-rich apolipoprotein, apoprotein E. Concentration in blood plasma and lipoproteins as affected by apoprotein E-3 deficiency. J Clin Invest. 1980 Dec;66(6):1351–1362. doi: 10.1172/JCI109988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J. Lipoprotein biosynthesis and metabolism. Ann N Y Acad Sci. 1980;348:16–29. doi: 10.1111/j.1749-6632.1980.tb21288.x. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Chen G. C., Hamilton R. L., Hardman D. A., Malloy M. J., Havel R. J. Remnants of lipoproteins of intestinal and hepatic origin in familial dysbetalipoproteinemia. Arteriosclerosis. 1983 Jan-Feb;3(1):47–56. doi: 10.1161/01.atv.3.1.47. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. P., Sata T., Hamilton R. L., Havel R. J. Apoprotein composition of very low density lipoproteins of human serum. J Clin Invest. 1975 Dec;56(6):1622–1634. doi: 10.1172/JCI108245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Brown M. S., Bilheimer D. W., Goldstein J. L. Delayed clearance of very low density and intermediate density lipoproteins with enhanced conversion to low density lipoprotein in WHHL rabbits. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5693–5697. doi: 10.1073/pnas.79.18.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Goldstein J. L., Brown M. S., Watanabe Y., Hornick C. A., Havel R. J. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P., Hardman D. A., Hamilton R. L., Dalal K. B. Normotriglyceridemic abetalipoproteinemia. absence of the B-100 apolipoprotein. J Clin Invest. 1981 May;67(5):1441–1450. doi: 10.1172/JCI110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel H. J., Kladetzky R. G., Assmann G. One-step screening method for the polymorphism of apolipoproteins A-I, A-II, and A-IV. J Lipid Res. 1982 Aug;23(6):915–922. [PubMed] [Google Scholar]
- Myers L. H., Phillips N. R., Havel R. J. Mathematical evaluation of methods for estimation of the concentration of the major lipid components of human serum lipoproteins. J Lab Clin Med. 1976 Sep;88(3):491–505. [PubMed] [Google Scholar]
- Pagnan A., Havel R. J., Kane J. P., Kotite L. Characterization of human very low density lipoproteins containing two electrophoretic populations: double pre-beta lipoproteinemia and primary dysbetalipoproteinemia. J Lipid Res. 1977 Sep;18(5):613–622. [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Innerarity T. L., Mahley R. W. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Mahley R. W. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982 Apr 25;257(8):4171–4178. [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Schneider W. J., Kovanen P. T., Brown M. S., Goldstein J. L., Utermann G., Weber W., Havel R. J., Kotite L., Kane J. P., Innerarity T. L. Familial dysbetalipoproteinemia. Abnormal binding of mutant apoprotein E to low density lipoprotein receptors of human fibroblasts and membranes from liver and adrenal of rats, rabbits, and cows. J Clin Invest. 1981 Oct;68(4):1075–1085. doi: 10.1172/JCI110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982 Mar 10;257(5):2518–2521. [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Mahley R. W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981 Sep 10;256(17):9077–9083. [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L. Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry. 1981 Feb 17;20(4):1033–1041. doi: 10.1021/bi00507a059. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., Utermann G., Mahley R. W., Weisgraber K. H., Havel R. J., Goldstein J. L., Brown M. S., Schonfeld G., Hazzard W. R. Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J Lipid Res. 1982 Aug;23(6):911–914. [PubMed] [Google Scholar]